Abstract
This study was performed in order to assess the cytotoxic activity, both natural (NK) and antibody-dependent (ADCC), of PBMC from 38 IBD patients and correlate it with their clinical features. Cytotoxicity assays were performed using sensitive target cells for NK and ADCC activities. In some experiments, highly purified NK cells, obtained both by Percoll density gradient and by co-culturing non-adherent PBMC with RPMI 8866 feeder cells, were used as effector cells. Furthermore, we evaluated NK cell parameters such as number, surface expression of adhesion molecules (CD11a/CD18, CD49d and CD54) and response to different stimuli. We observed a decreased NK cytotoxicity of PBMC from IBD patients, both in ulcerative colitis (UC) and Crohn's disease (CD), independently of the clinical activity of disease. In contrast, the ADCC lytic activity was within normal range. The lower NK cytotoxic activity observed in our IBD patients cannot be related to a decreased number of NK cells, surface expression of adhesion molecules, defective response to IL-2 and maturative defect. Decreased NK activity was induced in PBMC of controls when serum of patients was added and this was unrelated to monocyte-derived modulating factor(s). Our data show a decreased natural killing by fresh PBMC from IBD patients. This lower activity seems to be unrelated to a primary NK cell defect, since purified NK cells exhibited normal levels of killing. It might be hypothesized that serum factors, possibly derived from lymphocytes, with inhibitory properties on NK activity, might be functionally active in the blood of IBD patients, thus modulating NK activity.
Keywords: inflammatory bowel disease, natural killer cells, NK activity, antibody-dependent cell-mediated cytotoxicity
INTRODUCTION
IBD is a term which includes two forms of chronic intestinal inflammation, ulcerative colitis (UC) and Crohn's disease (CD).
Although the underlying mechanisms responsible for initiation and perpetuation of the inflammatory reaction remain unknown, there is strong evidence that the immune system plays an important role in the pathogenesis of IBD. The number of both peripheral blood and lamina propria mononuclear cells (PBMC and LPMC, respectively) bearing activation markers is increased in CD patients [1,2] and several data show that a polyclonal B cell activation can occur in IBD patients [3,4]. In a previous study we observed an imbalance in the cytotoxic phenotype of PBMC of IBD patients and suggested that this alteration might mirror an alteration in cytotoxic activity [5]. The role of non-MHC-restricted cytotoxicity as well as the role of its effector cells, NK cells, in the pathogenesis of IBD is not yet completely understood. In fact, both enhanced and decreased non-MHC-restricted cytotoxicity by PBMC from IBD patients has been reported [6–12]. NK cells can be defined as CD3− CD56+ CD16+ or CD3−CD56+ CD16− lymphocytes [13,14]. NK cells are capable of a dual cytolytic activity: in addition to spontaneous antibody-independent non-MHC-restricted cytotoxicity (NK activity), they can mediate antibody-dependent cell-mediated cytotoxicity (ADCC) through the expression of low-affinity IgG-specific Fc receptors (CD16) [15]. NK cells play a major role in immunosurveillance towards tumour cells or virus-infected cells and in the regulation of haematopoiesis and immunoglobulin secretion [15]. Recently, an experimental model of mice colitis has been proposed in which a pathogenic role of both NK and tumour necrosis factor (TNF) activities was suggested [16]. Thus, in this study we explore both NK and ADCC activities of PBMC from IBD patients against sensitive target cells, evaluating their possible relationship with some NK cell parameters such as number, surface expression of adhesion molecules and response to activation and proliferation stimuli. Furthermore, we study the possible evidence of soluble factors inhibiting NK activity. The findings are correlated with disease subsets and clinical features of IBD patients.
PATIENTS AND METHODS
Patients and controls
After informed consent, 38 consecutive patients, 21 UC and 17 CD, 22 men and 16 women, with IBD diagnosed by usual clinical, radiological, endoscopical and histological criteria were studied. Disease activity was assessed for all patients at the time of immunological studies. In UC, disease activity was evaluated by Truelove & Witts' criteria [17]; in CD by Best's index (CDAI) and in patients with colonic involvement by endoscopy [18]. UC patients had a median age of 35 years (range 18–65 years), and the duration of disease ranged from 1 to 26 years. Two patients had proctitis, 11 had proctosigmoiditis and eight had pancolitis. Thirteen patients had active, eight quiescent disease. CD patients had a median age of 39 years (range 20–70 years) and the disease had been diagnosed from 6 months to 24 years. Ten patients had ileal involvement, five had ileocolitis and two colitis. Ten patients had active, seven quiescent disease. Three patients had been previously operated by ileocaecal resection. Steroid-treated patients discontinued therapy 3 months before initiation of the study. Twenty-nine patients were receiving oral salazopyrine or controlled-release 5-ASA tablets and nine patients were receiving no specific treatment. Forty age- and sex-matched healthy volunteers were enrolled as controls.
Isolation of PBMC
Mononuclear cells were separated from patients' and controls' heparinized venous peripheral blood by standard Ficoll–Hypaque density gradient centrifugation.
Purification of NK cells
PBMC were further purified by: (i) passage through a nylon wool column (Cellular Products, Buffalo, NY); (ii) plastic adherence to remove monocytes; (iii) Percoll (Pharmacia, Uppsala, Sweden) density gradient fractionation. Cell recovery was routinely 85–95% and viability, as detected by trypan blue exclusion assay, was always > 95%. Contaminating T cells were eliminated from large granular lymphocyte (LGL) fractions by one round of panning on plastic dishes (Falcon, Becton Dickinson, Mountain View, CA) coated with affinity-purified goat F(ab′)2 fragment anti-mouse IgG (10 μg/ml). Cells (10–20 × 106) from LGL-enriched fraction, pretreated with OKT3, were added and incubated at 4°C for 2 h. The non-adherent cells were then gently poured off. Analysis of LGL morphology was performed by 10% Giemsa staining (Fisher Scientific Co., Fairlawn, NJ) in PBS pH 7.4 on cytocentrifuged slides (Cytospin 2; Shandon Southern Instruments, Inc., Sewickey, PA). At least 200 cells/slide were counted and the LGL percentage was determined.
Long-term activated NK cells
NK cells were obtained by co-culturing nylon non-adherent human PBMC from buffy coats (4 × 105/ml) with irradiated (30 Gy) RPMI 8866 cells (1 × 105/ml) at 37°C in a humidified 5% CO2 atmosphere for 10 days, as previously described [19,20]. On day 10, the cell population was routinely 80–90% CD56+, CD16+, CD3−, CD14−, as assessed by cytofluorimetric analysis. In some experiments, contaminating T cells were eliminated by negative panning on plastic dishes. Ten to 20 × 106 anti-CD5-pretreated cells were added to plastic Petri dishes coated with affinity-purified F(ab′)2 fragments of goat anti-mouse (10 mg/ml) and incubated at 4°C for 2 h. The non-adherent cells were gently poured off. The resulting NK cell population was > 90% CD56+, CD16+ CD3−, CD14−, as assessed by immunofluorescence and cytofluorimetric analysis.
Cytotoxic activity
Cytotoxicity assays were performed by incubating serial dilutions of effector cells with 5 × 10351Cr-labelled (Na251CrO4; New England Nuclear, Dreiech, Germany) K562 (human erythroleukaemia) or anti-P815-coated P815 target cells (a chemically induced NK-resistant murine mastocytoma) in triplicate wells of round-bottomed microtitre plates (Sterilin, Teddington, UK) in a final volume of 0.2 ml. PBMC were used routinely as effector at different effector:target (E:T) ratios. In some experiments NK purified cells were used as effector cells. Where indicated, PBMC, previously cultured at 2 × 106/ml with IL-2 (1000 U/ml) for 24 h at 37°C, were used as effector cells. Moreover, the supernatants from peripheral blood monocytes, isolated by plastic adherence and cultured for 24 h at 37°C in complete medium alone or collected at 72 h after lipopolysaccharide (LPS) stimulation, were tested for their possible influence on cytotoxic activity of monocyte-depleted peripheral blood lymphocytes (PBL). After 4 h of incubation the plates were centrifuged and 0.1 ml supernatant was removed and counted. Furthermore, we evaluated the effect of IBD sera, at different dilutions, on NK activity of controls. The percentage of specific 51Cr-release was calculated as follows: 100 × (experimental release — spontaneous release)/(maximum release — spontaneous release).
Surface phenotype of PBMC and purified NK cells
The phenotype of PBMC and purified NK cells was determined by single- and two-colour immunofluorescence with the following MoAbs: anti-Leu-11a (CD16, FITC-conjugated); anti-Leu-19 (CD56, PE-conjugated); anti-Leu-54 (intercellular adhesion molecule-1 (ICAM-1), CD54, PE-conjugated); anti-α-chain of very late antigen-4 (VLA-4, CD49d, PE-conjugated); all these MoAbs were from Becton Dickinson; anti-α L chain of the lymphocyte function-associated antigen 1 (LFA-1, CD11a, PE-conjugated, courtesy of Professor A. Pavan, University of L'Aquila, L'Aquila, Spain). Phenotypic analysis was performed by flow cytometry (FACScan; Becton Dickinson).
Serum samples
Serum samples were obtained from IBD patients and controls. All samples were aliquoted and stored at −80°C until use.
Assay for TNF-α in sera
TNF-α was measured by ELISA test kits (Biotrak Tumor Necrosis Factor-alfa ((h)TNFα) human, ELISA System; Amersham, Aylesbury, UK). The assay is based on the dual immunometric sandwich principle and was performed according to the manufacturer's instructions.
Statistical analysis
The Mann–Whitney U-test for non-paired samples, Wilcoxon's signed rank test for paired data, Spearman's correlation coefficient and simple linear regression were used for statistical analysis of data.
RESULTS
Cytotoxic activity of PBMC
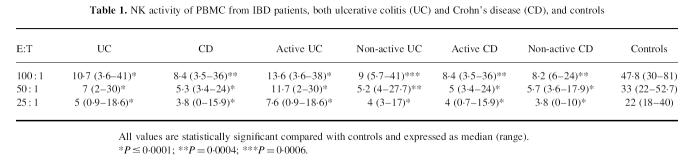
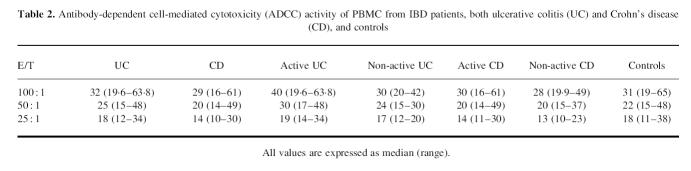
As shown in Table 1, NK cytotoxic activity of PBMC was significantly reduced in IBD patients, both UC and CD, compared with controls, at every E:T ratio studied, independently of disease activity. In contrast, the ADCC lytic activity fell within normal range in IBD patients, both UC and CD, compared with controls, at every E:T ratio (Table 2).
Table 1.
NK activity of PBMC from IBD patients, both ulcerative colitis (UC) and Crohn's disease (CD), and controls
Table 2.
Antibody-dependent cell-mediated cytotoxicity (ADCC) activity of PBMC from IBD patients, both ulcerative colitis (UC) and Crohn's disease (CD), and controls
Surface phenotype of PBMC and NK cells
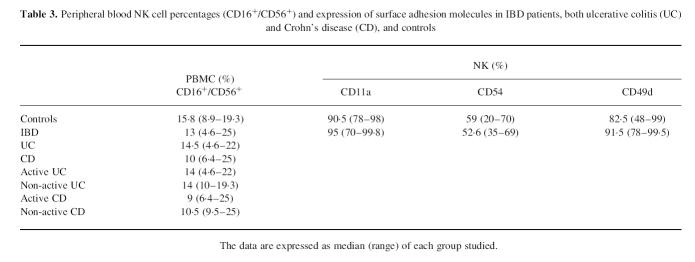
In an attempt to explain the reduced NK activity we evaluated the NK (CD16+/CD56+) cell percentages and absolute numbers of PBMC of IBD patients and controls. The reduced cytotoxic activity cannot be ascribed to a decreased number of CD16+/CD56+ lymphocytes, since NK cell percentages were similar in our patients and controls (Table 3).
Table 3.
Peripheral blood NK cell percentages (CD16+/CD56+) and expression of surface adhesion molecules in IBD patients, both ulcerative colitis (UC) and Crohn's disease (CD), and controls
The surface expression of adhesion molecules such as CD11a/CD18, CD54 and CD49d in Percoll-purified NK cells was comparable to healthy controls (Table 3).
Effect of IL-2 on NK activity
Since it has been shown that incubation with IL-2 can enhance the lytic activity of normal NK cells [21], we tested the effect of rIL-2 on NK cytotoxicity of PBMC from six patients after 24 h stimulation. In such experimental conditions, IL-2 restored NK activity (Fig. 1).
Fig. 1.
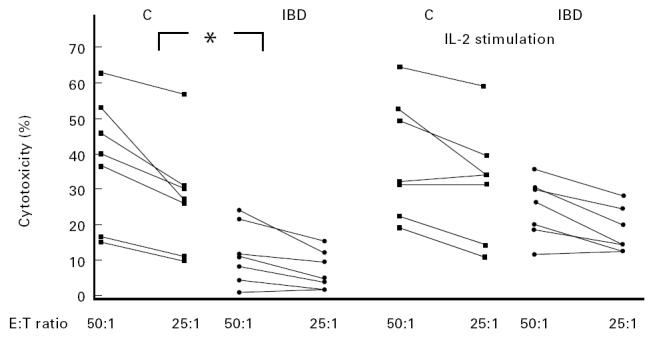
Ability of IL-2 to restore NK activity of PBMC against K562 target cells in IBD patients. The points represent the means of triplicate determinations. The differences between patients and controls (C) before IL-2 stimulation were statistically significant at every considered E:T ratio (*P = 0.007 and *P = 0.004 at E:T ratios 50:1 and 25:1, respectively). No statistically significant differences between the two groups could be observed after stimulation of effector cells with IL-2 (1000 U/ml) for 24 h.
Cytotoxic activity of purified NK cells
In order to explain the discrepancy between the normal percentage of NK cells and their lower activity, we performed further functional tests. Intriguingly, we observed that in nine patients with lower NK activity of PBMC, the cytotoxic activity of Percoll-purified NK cells was normal (Fig. 2a). Furthermore, we investigated the NK activity after long-term activation, as previously reported [17], in six patients. To this purpose, PBMC were cultured in the presence of RPMI 8866, an Epstein–Barr virus (EBV)-transformed B cell line, resulting in a preferential proliferation of NK cells. In this assay, the lytic activity of NK cells of IBD patients was similar to controls (Fig. 2b).
Fig. 2.
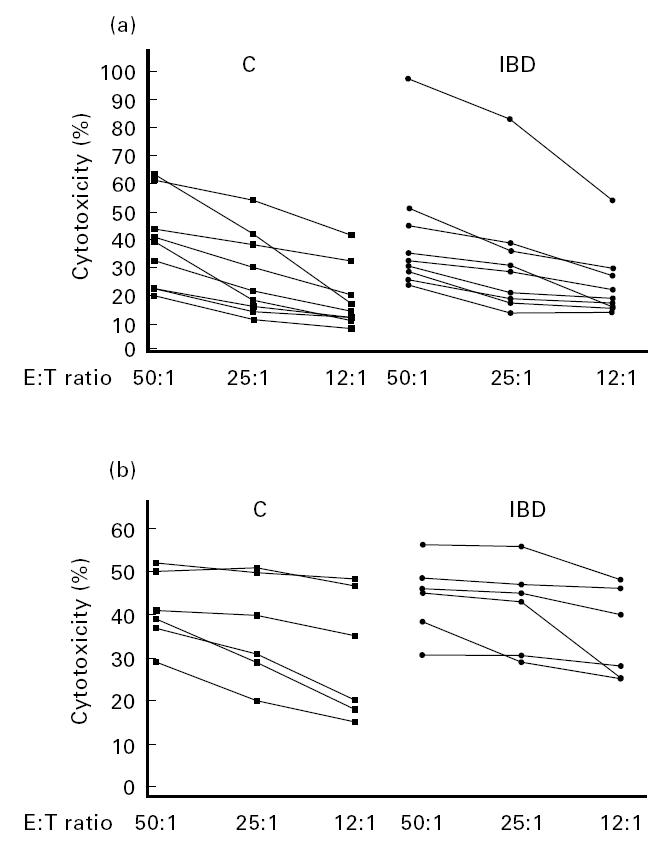
(a) Cytotoxic activity of highly enriched NK cells from IBD patients and controls (C). Cytotoxic assays were performed by incubating serial dilutions of Percoll-purified NK cells with 5 × 103 51Cr-labelled K562 target cells. The points represent the means of triplicate determinations. No statistically significant differences were present between patients and controls at any considered E:T ratio. (b) Levels of killing of NK cells from patients (IBD) and C after co-culture with RPMI 8866 feeder cells. NK cells were obtained by co-culturing non-adherent PBMC from buffy coats (4 × 105/ml) with irradiated RPMI 8866 cells (1 × 105/ml) for 10 days. 51Cr-labelled K562 were used as target cells. The points represent the means of triplicate determinations. In this assay, the lytic activity of NK cells of patients was similar to controls.
Effect of IBD serum on NK activity
To assess the possible presence of a circulating soluble factor able to induce the inhibition of NK cell function, we added sera of patients to cytotoxicity tests performed using PBMC of controls as effector cells. Decreased NK activity was observed in PBMC of controls when sera of patients were added. This finding was dose-dependent, as confirmed using serum serial dilutions (Fig. 3).
Fig. 3.
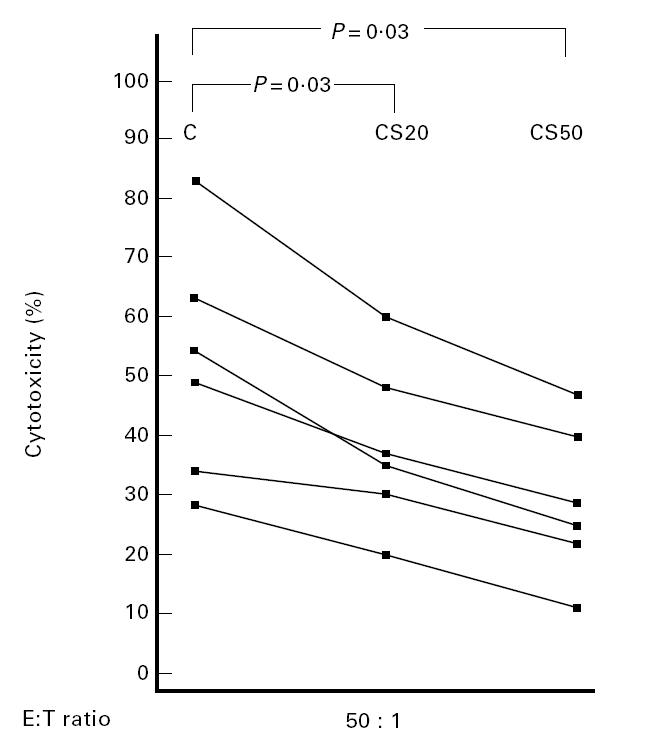
Dose-dependent effect of patient sera (S) on NK activity of PBMC from control subjects (C). Sera of patients were added to cytotoxicity tests performed using PBMC of controls as effector cells and 5 × 103 51Cr-labelled K562 as target cells. CS20 and CS50: the assay test was performed in the presence of 20% or 50% serum from patients, respectively. The points represent the means of triplicate determinations.
Effect of monocyte depletion on NK activity
In seven patients we assessed natural killing of PBL in presence or absence of monocytes or supernatants from monocytes previously cultured in the presence or absence of LPS. The low lytic activity of PBMC did not vary in the absence of monocytes, nor after incubation with LPS-treated or untreated monocyte supernatants (data not shown).
TNF-α serum levels
Recently, using an experimental murine model of IBD, it has been suggested that the TNF-α plays a key role in the inhibition of NK cells. With this in mind, we assayed the serum levels of this cytokine. Serum TNF-α was undetectable in IBD patients, both UC and CD, and in controls.
In our patients, the localization and the extent of lesions, and drug treatments did not correlate with immunological parameters.
DISCUSSION
In this study we observed a decreased NK cytotoxicity of PBMC from IBD patients, both in UC and CD, and this finding was unrelated to the clinical activity of disease. In contrast, ADCC lytic activity was within normal range in IBD patients. The lower NK cytotoxic activity observed in our IBD patients cannot be related to a decreased number of NK cells, since normal percentages and absolute numbers of CD16+/CD56+ NK cells were found in their PBMC.
This lack of correlation between NK and ADCC activities has been already described in several diseases of the immune system [22,23] and could be related to the fact that these two forms of cytotoxicity are mediated by distinct cell subsets, or by overlapping populations of LGL with distinct biological characteristics [24–26].
A defect of NK cytotoxicity has been found in several autoimmune disorders of the immune system such as systemic lupus erythematosus (SLE), multiple sclerosis, chronic autoimmune thrombocytopenic purpura, and Graves' disease, although the mechanisms underlying this defect remain obscure [27–30]. A functional defect in NK cell maturation from non-cytotoxic precursors to mature cells and/or in the activation of these lytic cells has been previously suggested to be present in IBD [11]. Our data do not support this hypothesis. In fact, in our experiments short-term in vitro incubation of PBMC with rIL-2, a cytokine which exerts fundamental regulatory functions for NK cells, reversed the decreased NK activity. Thus, we can suppose that the functional abnormality of PBMC observed in our patients was not associated with a defective response of NK cells to IL-2. Moreover, normal levels of killing of targets were observed when Percoll-enriched NK cells from IBD patients were used as effector cells. Furthermore, the lytic activity of NK cells purified by co-culture with NK-RPMI 8866 feeder cells did not show statistically significant differences from controls. This latter technique, inducing proliferation and activation of NK cells [19,20,31], proves that normal levels of NK activity were maintained in the progeny either in patients or in controls. Taken together, this findings suggest that an inhibiting factor(s) could be present in the blood of patients. With this in mind we assayed the effect of IBD sera on NK activity of control PBMC. We found a decreased cytotoxicity of PBMC of controls when the sera of patients were added. The decrease was dose-dependent, as confirmed by using serial dilutions of the sera. Several mediators produced by other cells, such as cytokines [32], can modulate the NK cell functions. On these grounds we evaluated the possible influence of monocyte-derived factor(s) on PBL-mediated lysis. In our experiments, the natural killing of monocyte-depleted PBL of IBD patients were lower than in controls and did not change after incubation with supernatants of both unstimulated and stimulated monocytes.
The binding of NK cells with their targets as well as the activation of the effector cells' killing machinery are related to the normal kinetics of different adhesion molecules. In order to evaluate the presence of these molecules on NK cell surfaces and correlate them to the decreased cytotoxic activity of PBMC observed in our patients, we assessed the expression, on freshly isolated NK cells, of CD11a/CD18, CD49d and CD54. It is well known that in NK cells these adhesion molecules play different roles. The CD11a/CD18 antigens participate in many adhesion-dependent functions such as binding to endothelial cells, migration and cytotoxicity [33]; the CD49d integrin is constitutively expressed on NK cells and is essential for the cell–cell and cell–extracellular matrix interactions [34]; similarly, CD54 may be involved in both the cell–cell interactions and the adhesion to target cells [15]. In our experiments, decreased NK activity of fresh PBMC seemed to be unrelated to surface expression of these adhesion molecules, since their expression on NK cells did not differ from controls. Furthermore, we observed by light microscopy the presence of a normal binding of effector–target cell (data not shown), confirming the normal physiology of the adhesive phase of the killing machinery.
In conclusion, in this study we demonstrated a decreased natural killing by fresh PBMC from IBD patients. This lower activity seems to be unrelated to a primary NK cell defect, since purified NK cells exhibited normal levels of killing, as well as to the clinical features of the disease. It might be hypothesized that serum factors, possibly derived from lymphocytes, with inhibitory properties on NK activity are functionally active in the blood of IBD patients, thus modulating NK activity. This hypothesis seems supported by recent studies in an experimental murine model of IBD, lacking both T and NK cells, in which a severe disease develops after bone marrow transplantation, related to donor's T lymphocytes [35]. This colitis can be transferred in another mouse strain, using these T cells, which are able to inhibit the NK activity, thus suggesting a protective role of NK cells [16]. High serum levels of TNF-α were detected in this experimental model and therapy with anti-TNF-α MoAbs, preserving NK activity, seemed to protect mice from the disease, suggesting a relationship between this cytokine and the inhibition of NK cells. Available literature concerning the serum TNF-α levels in IBD shows conflicting results, although anti-TNF-α MoAb treatment of CD has been reported to be helpful [36]. In our patients, as well as in normal controls, we did not detect any circulating levels of TNF-α, suggesting that in humans, differently from mice, this cytokine could be relatively involved in the development of NK disfunction.
Taken together, these data seem to indicate that in human IBD, serum factors capable of inducing an NK cell malfunction may play a pathogenic role. Further studies are in progress in our laboratory in order to verify this hypothesis.
Acknowledgments
The authors wish to thank M. Termine for technical assistance. This work was supported by MURST 40% and MURST 60% grants.
REFERENCES
- 1.Raedler A, Schreiber S, Weerth A, Voss A, Peters S, Greten H. Assessment of in vivo activated T cells in patients with Crohn's disease. Hepato Gastroenterol. 1990;37:67–71. [PubMed] [Google Scholar]
- 2.Pallone F, Fais S, Squarcia O, Biancone L, Pozzilli P, Boirivant M. Activation of peripheral blood and lamina propria lymphocytes in Crohn's disease In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28:745–53. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baklien K, Brandtzaeg P. Comparative mapping of local distribution immunoglobulin-forming cells in ulcerative colitis and Crohn's disease of the colon. Clin Exp Immunol. 1975;22:197–209. [PMC free article] [PubMed] [Google Scholar]
- 4.Sieber G, Herrmann F, Ruhl H. Abnormalities of B-cell activation and immunoregulation in patients with Crohn's disease. Gut. 1984;25:1255–61. doi: 10.1136/gut.25.11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacomelli R, Parzanese I, Frieri G, et al. Increase of circulating γ/δ T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin Exp Immunol. 1994;98:83–88. doi: 10.1111/j.1365-2249.1994.tb06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson DW, Quigley A, Bolt RJ. Effects of lymphocytes from patients with ulcerative colitis on human adult colon epithelial cells. Gastroenterology. 1966;51:985–93. [PubMed] [Google Scholar]
- 7.Shorter RG, McGill DB, Bahn RC. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984;86:13–22. [PubMed] [Google Scholar]
- 8.Auer IO, Ziemer E. Immune status in Crohn's disease. In vitro antibody dependent cell mediated cytotoxicity in peripheral blood. Klin Wochenschr. 1980;50:779–87. doi: 10.1007/BF01478286. [DOI] [PubMed] [Google Scholar]
- 9.MacDermott RP, Bragdon MJ, Kodner IJ, Bertovich MJ. Deficient cell-mediated cytotoxicity and hyporesponsiveness to interferon and mitogenic lectin activation by inflammatory bowel disease peripheral blood and intestinal mononuclear cells. Gastroenterology. 1986;90:6–11. doi: 10.1016/0016-5085(86)90067-3. [DOI] [PubMed] [Google Scholar]
- 10.Gibson PR, Pol EDV, Pullman W, Doe WF. Lysis of colonic epithelial cells by allogeneic mononuclear and lymphokine activated killer cells derived from peripheral blood and intestinal mucosa: evidence against a pathogenic role in inflammatory bowel disease. Gut. 1988;29:1076–84. doi: 10.1136/gut.29.8.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzano L, Alvarez-Mon M, Abreu L, et al. Functional impairment of natural killer cells in active ulcerative colitis: reversion of the defective natural killer activity by interleukin 2. Gut. 1992;33:246–51. doi: 10.1136/gut.33.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auer IO, Ziemer E, Sommer H. Immune status in Crohn's disease. Decreased in vitro natural killer cell activity in peripheral blood. Clin Exp Immunol. 1980;42:41–49. [PMC free article] [PubMed] [Google Scholar]
- 13.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human fcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–91. [PubMed] [Google Scholar]
- 15.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 16.Wang B, Shah SA, Simpson SJ, et al. Protective role of natural killer cells in a mouse model of inflammatory bowel disease. Gastroenterology. 1996;110(A1042) [Google Scholar]
- 17.Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron JH, Connel AM, Lennard-Jones JE. Variation between observer in describing mucosal appearances in proctocolitis. Br Med J. 1964;1:89–92. doi: 10.1136/bmj.1.5375.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171–88. [PubMed] [Google Scholar]
- 20.Milella M, Gismondi A, Roncaioli P, et al. CD16 cross-linking induces both secretory and extracellular signal-regulated kinase (ERK)-dependent cytosolic phosholipase A2 (PLA2) activity in human natural killer cells. J Immunol. 1997;158:3148–54. [PubMed] [Google Scholar]
- 21.Trinchieri G, Matumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting of peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160:1147–69. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren HS, Amos DB, Buckley RH. Natural killing in immunodeficient patients. J Immunol. 1978;120:796–9. [PubMed] [Google Scholar]
- 23.Cifone MG, Giacomelli R, Famularo G, et al. Natural killer activity and antibody-dependent cellular cytotoxicity in progressive systemic sclerosis. Clin Exp Immunol. 1990;80:360–5. doi: 10.1111/j.1365-2249.1990.tb03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neville ME. Human killer cells and natural killer cells: distinct subpopulations of Fc receptor-bearing lymphocytes. J Immunol. 1980;125:2604–8. [PubMed] [Google Scholar]
- 25.Wahilin B, Alsheikhly A, Perlmann P, Schreiber RD, Muller-Eberhard HJ. Enumeration and characterization of human killer and natural killer cells by a modified single-cell assay. Scand J Immunol. 1984;12:529–32. doi: 10.1111/j.1365-3083.1984.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 26.Fukui H, Overton WR, Herberman RB, Reynolds CW. Natural killer cell activity in the rat. VI. Characterization of rat large granular lymphocytes as effector cells in natural killer and antibody-dependent cellular cytotoxic activities. J Leuk Biol. 1987;41:130–6. doi: 10.1002/jlb.41.2.130. [DOI] [PubMed] [Google Scholar]
- 27.Gaspar ML, Alvarez-Mon M, Gutierrez C. Role of interleukin 2 in inducing normalization of natural killer activity in systemic lupus erythematosus. Clin Immunol Immunopathol. 1988;49:204–14. doi: 10.1016/0090-1229(88)90110-9. [DOI] [PubMed] [Google Scholar]
- 28.Hauser SL, Ault KA, Levin MJ, Garovoy MR, Weiner HL. Natural killer cell activity in multiple sclerosis. J Immunol. 1981;127:1114–7. [PubMed] [Google Scholar]
- 29.Semple JW, Bruce S, Freedman J. Suppressed natural killer activity in patients with chronic autoimmune thrombocytopenic purpura. Am J Hematol. 1991;37:258–61. doi: 10.1002/ajh.2830370409. [DOI] [PubMed] [Google Scholar]
- 30.Marazuela M, Vargas JA, Alvarez-Mon M, Albarran F, Lucas T, Durantez A. Impaired natural killer cytotoxicity in peripheral blood mononuclear cells in Graves' disease. Eur J Endocrinol. 1995;132:175–80. doi: 10.1530/eje.0.1320175. [DOI] [PubMed] [Google Scholar]
- 31.Valiante NM, Rengaraju M, Trinchieri G. Role of production of natural killer cell stimulating factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell Immunol. 1992;145:187–98. doi: 10.1016/0008-8749(92)90322-g. [DOI] [PubMed] [Google Scholar]
- 32.Naume B, Espevik T. Immunoregulatory effects of cytokines on natural killer cells. Scand J Immunol. 1994;40:128–34. doi: 10.1111/j.1365-3083.1994.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 33.Somersalo K, Carpén O, Saksela E, Gahmberg CG, Nortamo P, Timonen T. Activation of natural killer cell migration by leukocyte integrin-binding peptide from intracellular adhesion molecule-2 (ICAM-2) J Biol Chem. 1995;270:8629–36. doi: 10.1074/jbc.270.15.8629. [DOI] [PubMed] [Google Scholar]
- 34.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Response of human NK cells to IL-6 alterations of the cell surface phenotype, adhesion to fibronectin and laminin, and tumor necrosis factor-α/β secretion. J Immunol. 1993;150:4844–53. [PubMed] [Google Scholar]
- 35.Hollander GA, Simpson SJ, Mizoguchi E, et al. Severe colitis in mice with aberrant thymic selection. Immunity. 1995;3:27–38. doi: 10.1016/1074-7613(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 36.Van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]