Abstract
Linomide (quinoline-3-carboxamide) is a synthetic immunomodulator that suppresses several experimental autoimmune diseases. Here we report the effects of Linomide on experimental autoimmune neuritis (EAN), a CD4+ T cell-mediated animal model of acute Guillain–Barré syndrome (GBS) in humans. EAN induced in Lewis rats by inoculation with bovine peripheral nervous system (PNS) myelin and Freund's complete adjuvant was strongly suppressed by Linomide administered daily subcutaneously from the day of inoculation. Linomide dose-dependently delayed the interval between immunization and onset of clinical EAN, as well as the severity of EAN symptoms. These clinical effects were associated with dose-dependent down-modulation of PNS antigen-induced T and B cell responses and with suppression of the proinflammatory cytokines IL-12, interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) mRNA. In PNS sections, Linomide suppressed IL-12 and TNF-α, and up-regulated IL-10 mRNA expression. These findings suggest that Linomide could be useful in certain T cell-dependent autoimmune diseases.
Keywords: experimental autoimmune neuritis, Linomide, myelin antigen, cytokine, autoimmunity
INTRODUCTION
Acute experimental autoimmune neuritis (EAN) can be induced by inoculation of peripheral nervous system (PNS) myelin antigens and adjuvants into susceptible animals [1]. Antigens useful for induction of EAN include, besides whole PNS myelin, the PNS myelin components P2 protein and P0 protein as well as certain synthetic P2 peptides [2,3]. EAN can be transferred to naive recipients by syngeneic P2 or P0 protein-specific CD4+ T cell lines [4,5]. The pathogenesis of EAN comprises breakdown of blood–nerve barrier, immunoglobulin leakage, infiltration of the nerve roots and peripheral nerves with macrophages and activated T lymphocytes, and focal demyelination of the nerve roots predominantly around venules [3]. The pathogenesis of EAN involves an integrated attack by T cells, B cells and macrophages [2,6,7]. Levels of interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF- α) and IL-12 mRNA-expressing cells obtained from lymph nodes and in sciatic nerve tissue sections roughly correlate with clinical EAN, consistent with a disease-promoting role for these cytokines. In contrast, levels of IL-4, IL-10 and transforming growth factor-beta (TGF-β) mRNA-expressing cells are highest during recovery from EAN. These cytokines may thus act by limiting PNS inflammation [8–10].
In its acute form, EAN serves as a model for the human disease Guillain–Barré syndrome (GBS; acute inflammatory demyelinating polyradiculoneuropathy). The pathogenesis of GBS remains poorly understood. The majority of cells infiltrating the peripheral nerves in GBS are macrophages, although T lymphocytes are also observed in biopsied nerve tissue [11]. Some patients with GBS exhibit abnormal T and B cell responses to the P2 and P0 proteins [3]. Systemically, IFN-γ is up-regulated in the acute stage of GBS and IL-4 during convalescence, indicating a role for the balance between Th1 and Th2 cytokines in disease development [12]. There is evidence that IL-2, TNF-α, IL-6 and leucocyte adhesion molecules are also involved in GBS [3]. The immunomodulatory treatment currently used in GBS consists of plasmapheresis or i.v. immunoglobulins. However, these measures reduce the length and severity of the paralytic phase in only one third of patients with GBS [2]. New treatments to improve the prognosis of GBS are greatly needed.
Linomide (roquinimex, PNU-212616), an immunomodulatory quinoline-3-carboxamide, is effective in inhibiting clinical manifestations of EAN [13], acute and chronic-relapsing experimental autoimmune encephalomyelitis (EAE), which is another CD4+ T cell-mediated autoimmune disease that serves as an animal model for multiple sclerosis (MS) [14–16], and in experimental autoimmune myasthenia gravis (EAMG), which is a classical antibody-mediated autoimmune disease that serves as an animal model for myasthenia gravis [17,18]. However, the immunomodulatory effects of Linomide are only incompletely defined.
In a previous study, we showed that Linomide suppresses clinical EAN through down-regulation of macrophage activity, decreasing IL-1β, TNF-α and IL-6 mRNA expression in response to IFN-γ and lipopolysaccharide (LPS) at the height of clinical EAN [13]. In the present study, the effects of different doses of Linomide on PNS antigen-induced T and B cell responses and cytokine production during the recovery phase of EAN are examined.
MATERIALS AND METHODS
Induction of EAN and assessment of clinical signs
Male Lewis rats, Hannover strain, were purchased from Harlan (Posbus Zeist, The Netherlands). In two separate experiments, 64 rats weighing 200–230 g were immunized by injection into both hind footpads with altogether 200 μl of inoculum containing 4 mg of bovine PNS myelin (BPM) and 2 mg Mycobacterium tuberculosis (strain H37RA; Difco, Detroit, MI) emulsified in 100 μl saline and 100 μl Freund's incomplete adjuvant (FIA; Difco). The FIA +M. tuberculosis mixture is referred to as Freund's complete adjuvant (FCA). Body weight and symptoms of paresis were assessed immediately before immunization (day 0), on day 3 post-immunization (p.i.) and thereafter every second day p.i. to day 35. Severity of paresis was graded as follows: 0 = no illness, 1 = flaccid tail, 2 = moderate paraparesis, 3 = severe paraparesis, 4 = tetraparesis.
In vivo treatment with Linomide
Linomide (Pharmacia & Upjohn, Lund, Sweden) was dissolved in distilled water and administered by a daily subcutaneous injection from the day of immunization until termination of the experiment on day 35 p.i. Groups of 16 rats received Linomide at 1.6, 16 or 160 mg/kg per day, respectively, while 16 control rats received PBS daily via subcutaneous injection. The concentration of 1.6 mg/kg per day is close to the concentration of 2.5 mg p.o. in daily use in a phase III trial in MS.
Isolation of mononuclear cells from lymph nodes
In each of two separate experiments, groups of eight rats were treated with Linomide (1.6, 16 and 160 mg/kg per day, respectively) or PBS. Thirty-two rats were killed on day 27 p.i. and examined for T cell reactivities and cytokine profiles as well as B cell responses. An additional 4 × 8 rats were kept until day 35 p.i. for follow up of clinical scores. Popliteal and inguinal lymph nodes were removed under aseptic conditions. Single-cell suspensions of mononuclear cells (MNC) from individual rats were prepared. The cells were washed three times in culture medium before being suspended to 2 × 106 cells/ml. The culture medium consisted of Iscove's modification of Dulbecco's medium (Flow Labs, Irvine, UK) supplemented with 1% (v/v) minimum essential medium (Flow), 50 U/ml penicillin, 60 μg/ml streptomycin (Gibco, Paisley, UK), 2 mm glutamine (Flow), and 3% normal human AB+ serum without mercaptoethanol.
Antigens and immunoreagents
BPM was prepared from bovine lumbosacral plexus [19]. The bovine P2 and P0 proteins were prepared according to Kadlubowski et al. [20] and Milner et al. [21]. The P2 peptide, corresponding to the aa 57–81 of the bovine P2 protein [22], was synthesized on solid phase by stepwise elongation using a Tecan peptide synthesizer (MultiSyntech, Bochum, Germany). Mass spectrometry confirmed the expected masses as major components in the spectra. Purified protein derivative (PPD) of M. tuberculosis was from Statens Serum Institut (Copenhagen, Denmark). As mitogen, phytohaemagglutinin (PHA; Difco) was used.
Lymphocyte proliferation assays
Two hundred-microlitre aliquots of MNC suspensions were cultured in triplicate in round-bottomed 96-well polystyrene microtitre plates (Nunc, Copenhagen, Denmark) at a cell density of 2 × 106 cells/ml in culture medium. For lymphocyte stimulation 10-μl aliquots of BPM, P0 protein or P2 peptide were added to cultures to a final concentration of 10 μg/ml, or 10 μl of PHA at a final dilution of 1:1000 (v/v). These concentrations had high stimulatory effects as assessed in preliminary experiments. Triplicate wells without antigen or lectin served as background controls. After 60 h of incubation, the cells were pulsed with 3H-methyl-thymidine (1 mCi/well; Amersham, Aylesbury, UK) and cultured for an additional 12 h. Cells were harvested onto glass fibre filters (Titertek; Skatron A/S, Lierbyen, Norway), and 3H-thymidine incorporation was measured in a liquid β-scintillation counter. The results were expressed as ct/min per culture.
Assay of antigen-reactive single cells secreting IFN-γ
An enzyme-linked immunospot (ELISPOT) assay was used to detect and enumerate single cells responding by IFN-γ secretion upon culture of MNC without antigen as well as in the presence of antigen or mitogen. Briefly, nitrocellulose-bottomed wells of microtitre plates (Millititre-HAM plates; Millipore Co., Bedford, MA) were coated overnight at 4°C with 100 μl of a mouse anti-rat IFN-γ MoAb (DB1; Innogenetics, Ghent, Belgium). After washing with PBS pH 7.4, 200-μl aliquots containing 4 × 105 regional lymph node MNC were added to individual wells in duplicate with either medium alone (control cultures without antigen or mitogen) or 10-μl aliquots of BPM, P0 protein, P2 peptide or PHA at the same concentrations as used in the proliferation assays. After 48 h of culture, the plates were washed with PBS. Secreted and bound IFN-γ was visualized by sequential application of polyclonal rabbit anti-rat IFN-γ (Innogenetics), biotinylated swine anti-rabbit IgG and ABC (both from Dakopatts, Copenhagen, Denmark). Unbound ABC was removed by immersions in PBS, and 100 μl/well of peroxidase-substrate solution were added. The developed red-brown spots, which corresponded to cells that had secreted IFN-γ, were enumerated in a dissection microscope under low magnification (×25). Results were expressed as numbers of spots per 105 MNC. Variations within duplicates were < 10%.
Antibody measurements
The serum concentrations of IgG antibodies against BPM, P2 protein, P0 protein and PPD were measured by ELISA. Microtitre plates were coated with BPM (20 μg/ml), P2 protein, P0 protein or PPD (10 μg/ml). The diluted sera (1:100) were incubated for 2 h. After extensive washing, alkaline phosphatase-labelled goat anti-rat IgG (Sigma, St Louis, MO) diluted 1:2000 was added for 2 h. Unbound conjugate was removed by four consecutive washings with PBS–Tween buffer, and 100 μl/well of phosphatase substrate solution was added. Absorbances were measured at 405 nm. Antibody levels were expressed as the optical density (OD).
In situ hybridisation to detect cytokine mRNA expression
Two hundred-microlitre aliquots of lymph node MNC suspensions were applied to 96-well round-bottomed microtitre plates and cultures received either no exogenous antigen or PHA, BPM or P2 peptide at the same concentration as in lymphocyte proliferation assays. After 24 h of culture (optimized in preliminary experiments) at 37°C and 7% CO2, the cells were washed in sterile PBS. MNC (105) from each culture were dried onto restricted areas of electrically charged microscope slides (ProbeOn slides; Fisher Scientific, Pittsburgh, PA) at room temperature. The cells were dried at 55°C for 5 min, fixed for 1 min in 1% formaldehyde and rinsed in PBS. Slides were stored with silica in sealed boxes at −20°C until hybridization.
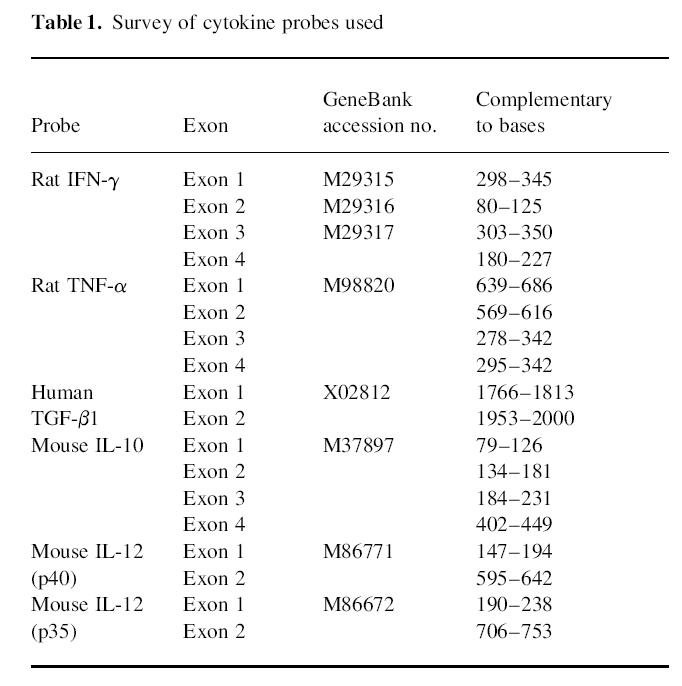
In situ hybridization was performed as described [8]. Synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Köping, Sweden) were labelled using 35S-deoxyadenosine-5′-thiotriphosphate (New England Nuclear, Cambridge, MA) with terminal deoxynucleotidyl transferase (Amersham). The oligonucleotide sequences were obtained from GenBank and probes were designed using MacVector software (IBI, Toronto, Canada) (Table 1). The human transforming growth factor-beta 1 (TGF-β1) probe has > 90% homology with rat TGF-β1. A sense probe with the nucleotide sequence for rat IFN-γ exon 4 was used as control of each cytokine studied. After hybridization, emulsion autoradiography and development, slides were stained with cresyl violet and mounted with Entellan (Merck, Darmstadt, Germany). Coded slides were examined by dark field microscopy at × 10 magnification. The results were expressed as numbers of labelled cells per 105 cultured MNC or infiltrates per 100-mm2 tissue section as previously described [8]. Cells were judged as positive when containing > 15 silver grains per cell with a star-like distribution over the cytoplasm. The cellular distribution of the grains was always checked under light microscopy at × 20 and/or × 40 magnification. Variation between duplicates was < 10%. The control probe used in parallel with the cytokine probe revealed no cells with grains exceeding background counts.
Table 1.
Survey of cytokine probes used
Statistical analysis
Differences between pairs of groups were tested by Student's t-test. Differences between the four groups were tested by the Kruskal–Wallis one way analysis of variance (anova). Correlations were analysed using the Spearman rank coefficient. Scheffé's post hoc test was performed for final analyses. All significance tests were two-sided.
RESULTS
Effects of Linomide on clinical EAN
Linomide delayed the appearance of clinical signs of EAN and reduced the severity of the disease. The effects on EAN achieved with Linomide were clearly dose-dependent, and quite similar in the two separate sets of experiments depicted in Fig. 1. Rats treated with Linomide at the three different doses used had milder maximum clinical symptoms of EAN than control EAN rats treated with PBS (Table 2). The groups of rats treated with higher Linomide doses had a later onset of clinical signs (160 versus 16; 160 versus 1.6; and 16 versus 1.6 mg/kg per day; P < 0.001 for all comparisons in the first experiment; 160 versus 16; 160 versus 1.6; and 16 versus 1.6 mg/kg per day; P < 0.05 for all comparisons in the second experiment) and less severe maximum clinical symptoms in both experiments (160 versus 16; and 160 versus 1.6 mg/kg per day; P < 0.01 for both comparisons in both experiments) (Fig. 1a).
Fig. 1.
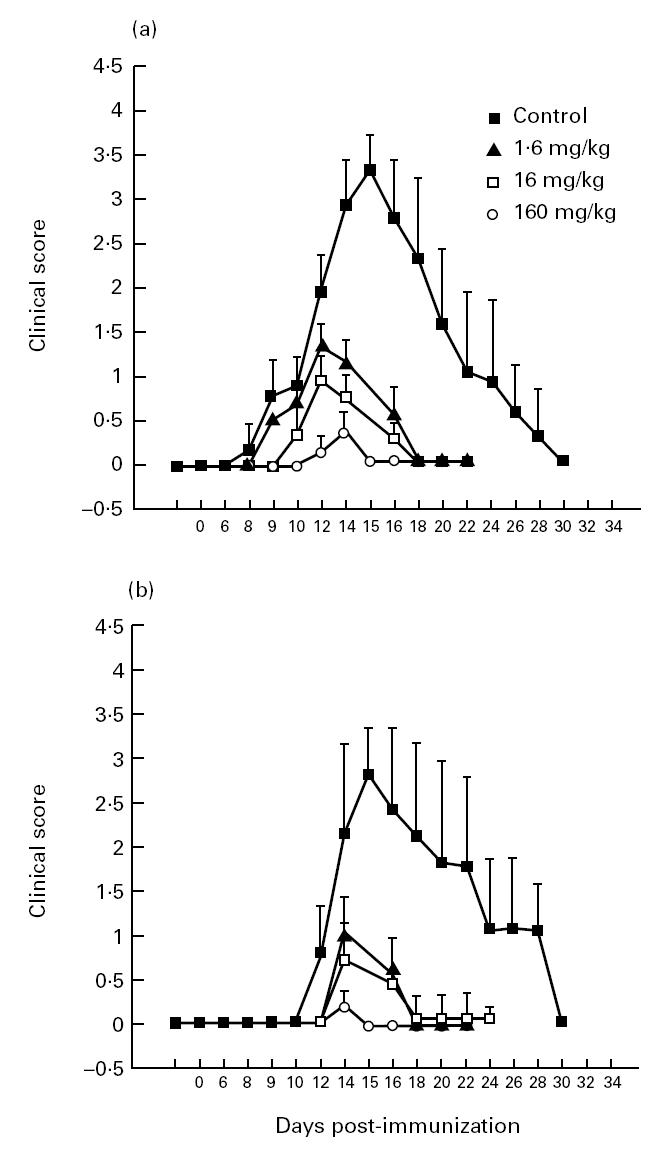
Clinical scores of experimental autoimmune neuritis (EAN) in Lewis rats in two different experiments (a and b) treated with Linomide at 1.6, 16 and 160 mg/kg per day and in control rats receiving PBS. Symbols indicate mean clinical scores for each group on the indicated day.
Table 2.
Clinical course of experimental autoimmune neuritis (EAN) in Lewis rats treated with Linomide at different doses and EAN controls receiving PBS only
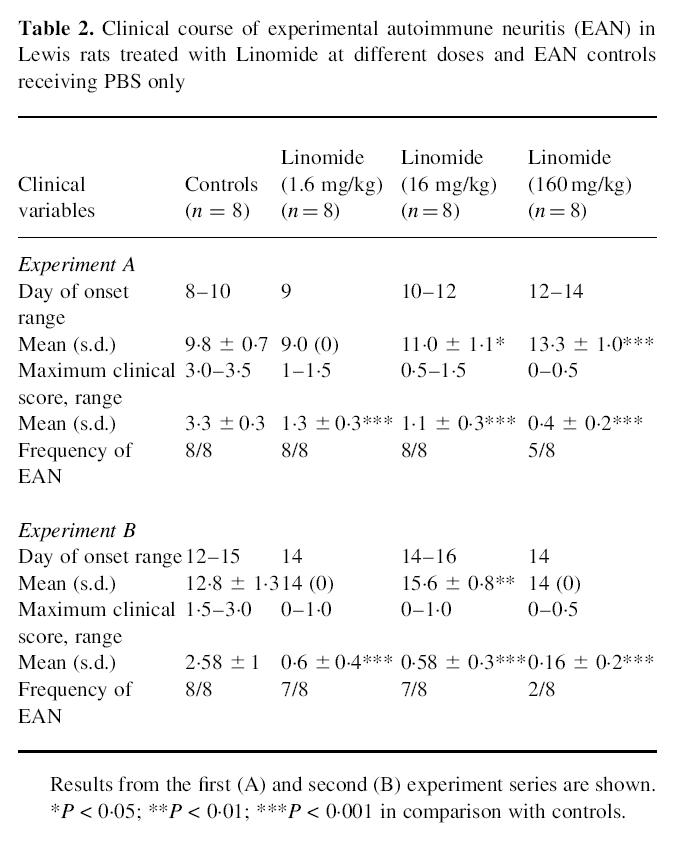
Changes of body weight in the different groups of rats included in two sets of experiments are presented in Fig. 2. In both experiments, PBS-treated control EAN rats exhibited an initial weight loss followed by delayed weight gain during the period of clinical EAN on day 10–21 p.i. In contrast, no initial weight loss was apparent in rats treated with Linomide in the doses of 16 and 1.6 mg/kg per day in both sets of experiments, and the subsequent weight gain was more pronounced in these two groups. The reduction in body weight seen in the group receiving 160 mg/kg per day may indicate that this high dose had reached a limit where non-specific toxic drug effects occur. A negative correlation was observed between the degree of paresis and body weight when considering the whole observation period (day 0–35 p.i.; r = 0.42) as well as the period of maximum clinical EAN (day 9–22 p.i.; r = 0.67) (Fig. 2).
Fig. 2.
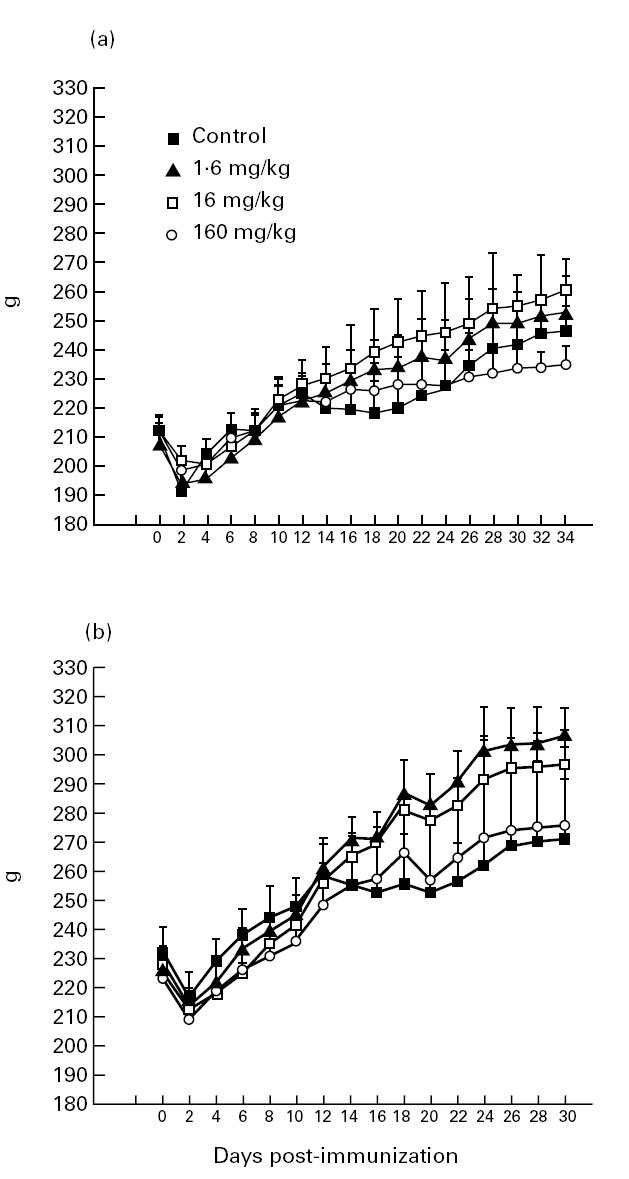
Body weight of experimental autoimmune neuritis (EAN) Lewis rats treated with Linomide at 1.6, 16 and 160 mg/kg per day and of control rats receiving PBS in two different experiments (a and b). Symbols indicate mean body weight of each group on the indicated day.
Effects of Linomide on T cell responses
BPM-induced lymph node MNC proliferation measured on day 27 p.i. was suppressed by the three different Linomide doses. The two higher doses also significantly suppressed PHA-induced MNC proliferation (Fig. 3). The highest Linomide dose had also a suppressive effect on P2 peptide-induced lymphocyte proliferation. In the unstimulated MNC cultures, there were no differences in lymphocyte proliferation between the groups of rats receiving different Linomide doses and control EAN rats treated with PBS.
Fig. 3.
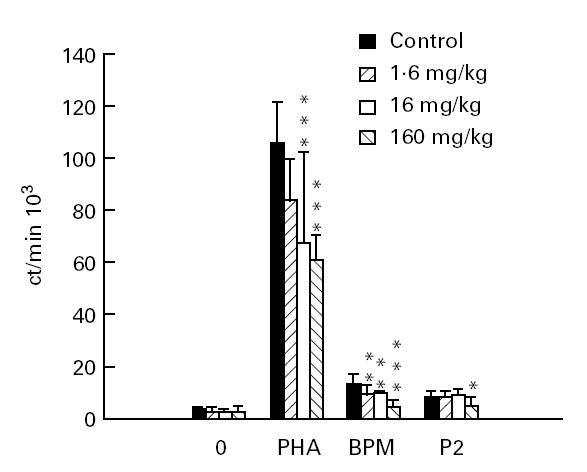
Proliferation of lymph node mononuclear cells (MNC) at day 27 post-immunization from experimental autoimmune neuritis (EAN) rats treated with Linomide (1.6, 16 and 160 mg/kg per day) and control rats treated with PBS, after ex vivo culture without antigen or mitogen, and in the presence of phytohaemagglutinin (PHA), bovine peripheral nerve myelin (BPM) and P2 peptide, respectively. Means and s.d. are indicated. P values refer to comparisons between EAN rats treated with Linomide and PBS-treated EAN control rats. *P < 0.05; **P < 0.01; ***P < 0.001.
When using ELISPOT to enumerate IFN-γ-secreting cells as read-out, all three Linomide doses clearly reduced the levels of BPM- and P2 peptide-reactive IFN-γ-secreting Th1-like lymph node MNC compared with levels registered in the PBS-treated control EAN rats, when evaluated on day 27 p.i. PHA-induced IFN-γ secretion was also significantly suppressed by Linomide treatment (Fig. 4).
Fig. 4.
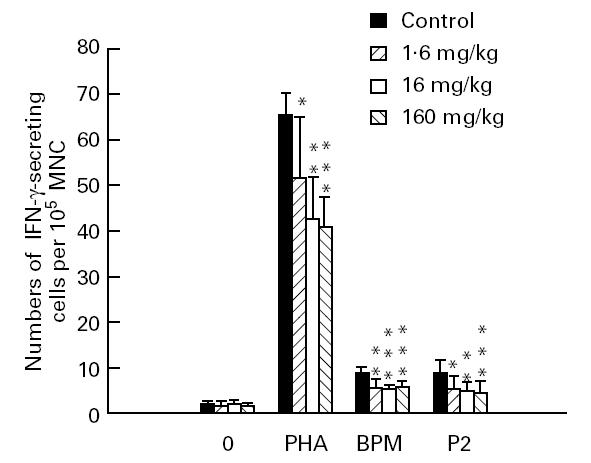
Numbers of IFN-γ-secreting cells per 105 lymph node mononuclear cells (MNC) at day 27 post-immunization from experimental autoimmune neuritis (EAN) rats treated with Linomide (1.6, 16 and 160 mg/kg per day) and controls, after ex vivo culture without antigen or mitogen, and in the presence of phytohaemagglutinin (PHA), bovine peripheral nerve myelin (BPM) and P2 peptide, respectively. Means and s.d. are indicated. P values refer to comparisons between EAN rats treated with Linomide and PBS-treated control EAN rats. *P < 0.05; **P < 0.01; ***P < 0.001.
Effects of Linomide on B cell responses
Linomide also had suppressive effects on B cell responses when evaluated on day 27 p.i.. At the highest dose (160 mg/kg per day), Linomide suppressed the concentrations of IgG antibodies to BPM, P2 and P0 proteins (Fig. 5). The anti-P2 protein antibody concentration was also suppressed by administration of Linomide at a dose of 1.6 mg/kg per day.
Fig. 5.
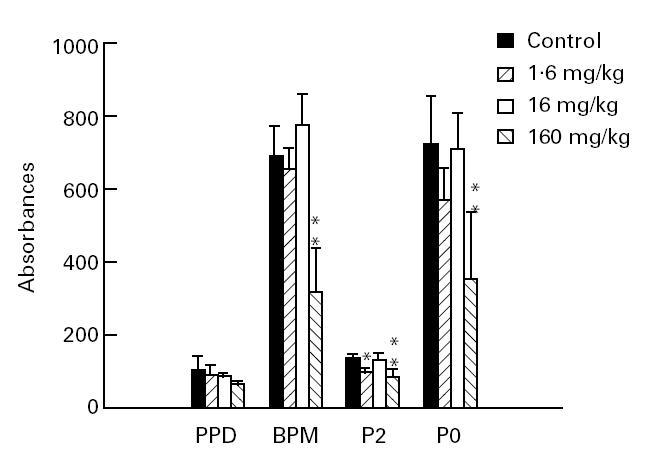
The effect of Linomide on anti-peripheral nervous system (PNS) myelin and anti-purified protein derivative (PPD) IgG antibodies. Serum specimens obtained on day 27 post-immunization from experimental autoimmune neuritis (EAN) rats treated with Linomide (1.6, 16 and 160 mg/kg per day) and from PBS-treated EAN controls. Means and s.d. are indicated. P values refer to comparisons between EAN rats treated with Linomide and PBS-treated control EAN rats. *P < 0.05; **P < 0.01.
Effects of Linomide on cytokine mRNA profiles in lymph node cells and PNS tissue sections
BPM- and P2 peptide-induced mRNA expression of IFN-γ, TNF-α and IL-12 in lymph node MNC was clearly suppressed in rats receiving Linomide compared with PBS-treated control EAN rats when evaluated on day 27 p.i. (Fig. 6a–c). These effects were also dose-dependent, with the exception of BPM-induced IL-12 mRNA expression. In contrast, the effects of Linomide on PNS autoantigen-induced IL-10 and TGF-β mRNA expression in lymph node MNC were minor and inconsistent (Fig. 6d,e).
Fig. 6.
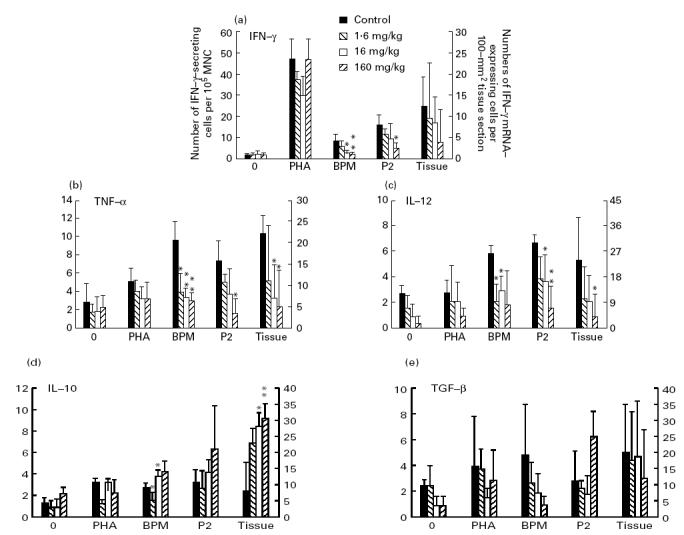
Numbers of cells expressing mRNA for IFN-γ (a), tumour necrosis factor-alpha (TNF-α) (b), IL-12 (c), IL-10 (d) and transforming growth factor-beta (TGF-β) (e) per 105 lymph node mononuclear cells (MNC) from experimental autoimmune neuritis (EAN) rats treated with Linomide (1.6, 16 and 160 mg/kg per day) and PBS-treated control rats, after ex vivo culture without antigen or mitogen, and in the presence of phytohaemagglutinin (PHA), bovine peripheral nerve myelin (BPM) or P2 peptide (left ordinate), and per 100-mm2 tissue section of sciatic nerve (right ordinate), obtained on day 27 post-immunization. mRNA expression was detected by in situ hybridization with 35S-labelled synthetic oligonucleotide probes. Bars indicate 1 s.d. P values refer to comparisons between EAN rats treated with Linomide and PBS-treated EAN control rats. *P < 0.05; **P < 0.01.
Linomide had no significant effects on mRNA expression for any of these cytokines upon culture of lymph node MNC in the presence of PHA, or in the absence of antigen or mitogen (Fig. 6a–e).
In situ hybridization (ISH) performed on PNS tissue sections obtained on day 27 p.i. revealed that the Linomide-treated rats had lower levels of TNF-α and IL-12 mRNA-expressing cells compared with PBS-treated control EAN rats (Fig. 6b,c). Interestingly, IL-10 mRNA expression in PNS was up-modulated in the Linomide-treated rats (Fig. 6d). These effects of Linomide on cytokine mRNA expression were also dose-dependent.
DISCUSSION
Linomide has previously been shown to effectively inhibit manifestations of acute and chronic relapsing EAE [14–16], EAN [13] and EAMG [17,18], lupus-like disease in MRL/lpr mice [23] and collagen-induced arthritis in DBA/1 mice [24]. Various mechanisms have been proposed to be related to therapeutic efficacy, including suppressed antigen-driven T cell proliferation, down-regulated autoantigen-specific B cell responses and interference with T cell sensitization by either reducing the total number of antigen-presenting cells or down-regulating antigen presentation early after immunization [17,25]. In a previous study we observed that Linomide suppressed EAN when using a single dose (160 mg/kg per day), resulting in suppressed macrophage function reflected by decreased IL-1β, TNF-α and IL-6 mRNA expression in response to IFN-γ and LPS at the height of clinical EAN [13]. Here, we show that Linomide dose-dependently suppresses EAN by delaying the interval between immunization and onset of clinical EAN and reducing the severity of EAN symptoms. This suppression of clinical EAN is also associated with down-regulated PNS antigen-induced T and B cell responses and an altered Th1/Th2 balance associated with the suppression of the later phase of EAN.
T cells recognizing antigen and producing cytokines play a pivotal role in the pathogenesis of EAN. Previously, a role for IFN-γ in the evolution of EAN has been suggested. Strigård et al. [26] showed that treatment of rats with antibodies to IFN-γ after the onset of EAN shortened the course of disease, down-regulated MHC class II antigen expression and reduced the numbers of T cells within the nerves. Hartung et al. [27] observed that administration of IFN-γ enhances EAN. Immunocytochemical studies revealed that IFN-γ is produced by T cells and polymorphonuclear cells in the endoneurium during the course of EAN [28]. We described high levels of PNS antigen-induced IFN-γ secretion and IFN-γ mRNA-expressing cells in lymph nodes and increased IFN-γ mRNA expression in sciatic nerve tissue sections in EAN rats [6,10].
TNF-α is present in macrophages in the peripheral nerves of rats with actively or passively induced EAN, and antibodies to TNF-α ameliorate the disease [29]. GBS is also associated with up-regulated IFN-γ secretion in the acute stage [12] and increased serum TNF-α [30]. IL-12 has potent augmenting effects on IFN-γ production and proliferation of T cells as well as natural killer (NK) cells. IL-12 enhances Th1 responses [31]. The present data show that Linomide-induced suppression of clinical EAN is associated with suppression of mRNA expression of the proinflammatory cytokines IFN-γ TNF-α and IL-12 in MNC of peripheral lymphoid organs, and of TNF-α and IL-12 in the PNS.
It is hypothesized that Linomide may influence immunoregulatory circuits through amplification of naturally existing suppressor/regulatory networks. To elucidate this possibility, we evaluated the mRNA expression of the endogenous immunosuppressive cytokines TGF-β and IL-10. TGF-β is related to EAN remission [10,32]. IL-10 has potent suppressive effects on Th1 cells [33]. Its role in EAN is not known. We recently observed increased numbers of PNS autoantigen-reactive IL-10 mRNA-expressing cells among lymph node MNC and in PNS tissue sections during recovery from EAN [8,9]. Further, injection of IL-10 at the day of immunization or at onset of clinical EAN suppressed the severity of EAN and, in parallel, reduced levels of PNS antigen-reactive IFN-γ-secreting lymph node MNC, and of PNS autoantigen-driven IFN-γ and TNF-α mRNA-expressing lymph node cells, and decreased IFN-γ and TNF-α mRNA expression in PNS tissue sections [34]. Thus, both TGF-β and IL-10 seem to favour remission of EAN.
Linomide could promote recovery from EAN, since it does not suppress TGF-β and IL-10 mRNA expression in lymph node MNC, but promotes IL-10 mRNA expression in PNS sections.
The role of B cell responses in the pathogenesis of EAN and GBS is incompletely understood. Only some GBS patients have evidence of circulating antibodies to PNS myelin proteins, and adoptive transfer of antibodies does not usually cause disease in animals [35]. Whether the observed beneficial effects of Linomide on clinical EAN are due in part to a suppression of the B cell responses remains to be settled.
One of the characteristic features of EAN is progressive weight loss during the clinical phase of the disease, which is rapidly reversed when the animals recover. In general, there is a good correlation between disease severity and magnitude of weight reduction. However, rats treated with the highest dose of Linomide showed a more pronounced reduction in body weight than rats treated with lower doses or PBS-treated control rats. This effect of Linomide was unexpected, and is probably explained by toxic effects of Linomide, probably related to relatively high doses of Linomide administered.
In conclusion, evidence is presented that Linomide down-modulates PNS antigen-induced T and B cell responses. Linomide counteracted EAN by suppressing the proinflammatory cytokines IL-12, IFN-γ and TNF-α, without suppressing TGF-β and IL-10 mRNA expression in MNC from peripheral lymphoid organs. Even more important, Linomide suppressed IL-12 and TNF-α, and up-regulated IL-10 in PNS tissues, the target organ in EAN. These effects indicate that Linomide may mediate its effects by regulation of cytokines. The observed effects for Linomide are of interest, since EAN serves as a model also for CD4+ T cell-mediated experimental autoimmune diseases in general.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council, the Swedish MS Society (NHR) and Karolinska Institute.
REFERENCES
- 1.Waksman BH, Adams RD. Allergic neuritis: an experimental disease of rabbits induced by the injection of peripheral nervous tissue and adjuvant. J Exp Med. 1955;102:213–25.. doi: 10.1084/jem.102.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartung HP, Pollard JD, Harvey GK, Toyka KV. Immunopathogenesis and treatment of the Guillain–Barré syndrome. Part I. Muscle and Nerve. 1995;18:137–53.. doi: 10.1002/mus.880180202. [DOI] [PubMed] [Google Scholar]
- 3.McCombe PA. Autoimmune diseases. In: Pender MP, McCombe PA, editors. Autoimmune neurological diseases. Cambridge: Cambridge University Press; 1995. pp. 177–228. [Google Scholar]
- 4.Linington C, Izumo S, Suzuki M, Uyemura K, Meyermann R, Wekerle H. A permanent rat T cell line that mediates experimental allergic neuritis in the Lewis rat in vivo. J Immunol. 1984;133:1946–50. [PubMed] [Google Scholar]
- 5.Linington C, Lassmann H, Ozawa K, Kosin S, Mongan L. Cell adhesion molecules of the immunoglobulin supergene family as tissue-specific autoantigens: induction of experimental allergic neuritis (EAN) by P0 protein-specific T cell lines. Eur J Immunol. 1992;22:1813–7. doi: 10.1002/eji.1830220721. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Link H, Mix E, Olsson T, Huang WX. Th1-like cell responses to peripheral nerve myelin components over the course of experimental allergic neuritis in Lewis rats. Acta Neurol Scand. 1994;90:19–25. doi: 10.1111/j.1600-0404.1994.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Link H, Weerth S, Linington C, Mix E, Qiao J. The B cell repertoire in experimental allergic neuritis involves multiple myelin proteins and GM1. J Neurol Sci. 1994;125:132–7. doi: 10.1016/0022-510x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Bai XF, Mix E, Link H. Experimental allergic neuritis: cytolysin mRNA expression is upregulated in lymph node cells during convalescence. J Neuroimmunol. 1997;78:108–16. doi: 10.1016/s0165-5728(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Bai XF, Mix E, Link H. Cytokine dichotomy in the peripheral nervous tissues influence the outcome of experimental allergic neuritis: dynamics of mRNA expression for IL-1β, IL-6, IL-10, IL-12, tumor necrosis factor-α and TNF-β as well as cytolysin. Clin Immunol Immunopathol. 1997;84:85–94. doi: 10.1006/clin.1997.4356. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Mix E, Link H. Cytokine production and the pathogenesis of experimental allergic neuritis and Guillain–Barré syndrome. J Neuroimmunol. 1998;84:40–52. doi: 10.1016/s0165-5728(97)00238-5. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RA, Atkinson P, Coates P, Hall S, Leibowitz S. Sural nerve biopsies in Guillain–Barré syndrome: axonal degeneration and macrophage-associated demyelination and absence of cytomegalovirus genome. Muscle Nerve. 1992;15:568–75. doi: 10.1002/mus.880150506. [DOI] [PubMed] [Google Scholar]
- 12.Dahle C, Ekerfelt C, Vrethem M, Samuelsson M, Ernerudh J. Th2-like responses to P0 and P2 peptides in the recovery phase of the Guillain–Barré syndrome. Acta Neurol Scand. 1996;94(Suppl. 167):99. (Abstr.) [Google Scholar]
- 13.Bai XF, Shi FD, Zhu J, Xiao BG, Link H. Linomide induced suppression of experimental autoimmune neuritis is associated with down-regulated macrophage functions. J Neuroimmunol. 1997;76:177–84. doi: 10.1016/s0165-5728(97)00051-9. [DOI] [PubMed] [Google Scholar]
- 14.Karussis DM, Lehmann D, Slavin S, et al. Inhibition of acute experimental autoimmune encephalomyelitis by the synthetic immunomodulator Linomide. Ann Neurol. 1993;34:654–60. doi: 10.1002/ana.410340506. [DOI] [PubMed] [Google Scholar]
- 15.Diab A, Levi M, Wahren B, Deng GM, Björk J, Hedlund G, Zhu J. Linomide suppresses acute experimental autoimmune encephalomyelitis in Lewis rats by counter-acting the imbalance of pro-inflammatory versus anti-inflammatory cytokines. J Neuroimmunol. 1998;85:146–54. doi: 10.1016/s0165-5728(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Diab A, Levi M, Wahren B, Björk J, Hedlund G, Mustafa M, Link H. Linomide suppresses chronic-relapsing experimental autoimmune encephalomyelitis in DA rats. J Neurol Sci. doi: 10.1016/s0022-510x(98)00244-5. in press. [DOI] [PubMed] [Google Scholar]
- 17.Karussis DM, Lehmann D, Brenner T, Wirguin I, Mizrachi-Koll R, Sicssic C, Abramsky O. Immunomodulation of experimental autoimmune myasthenia gravis with linomide. J Neuroimmunol. 1994;55:187–93. doi: 10.1016/0165-5728(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang GX, Yu LY, Shi FD, Xiao BG, Bjork J, Hedlund G, Link H. Linomide suppresses both Th1 and Th2 cytokine in experimental autoimmune myasthenia gravis. J Neuroimmunol. 1997;73:175–82. doi: 10.1016/s0165-5728(96)00197-x. [DOI] [PubMed] [Google Scholar]
- 19.Norton WI, Poduslo SE. Myelination in rat nerve: method of myelin isolation. J Neurochem. 1973;21:749–57. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- 20.Kadlubowski M, Hughes RAC, Gregson NA. Experimental allergic neuritis in the Lewis rat: characterization of the activity of peripheral myelin and its major basic protein P2. Brain Res. 1980;184:439–54. doi: 10.1016/0006-8993(80)90811-2. [DOI] [PubMed] [Google Scholar]
- 21.Milner P, Lovelidge CA, Taylor WA, Hughes RAC. P0 myelin protein produces experimental allergic neuritis in Lewis rats. J Neurol Sci. 1987;79:275–85. doi: 10.1016/0022-510x(87)90235-8. [DOI] [PubMed] [Google Scholar]
- 22.Olee T, Powers JM, Brostoff SW. A T cell epitope for experimental allergic neuritis. J. Neuroimmunol. 1988;19:167–73. doi: 10.1016/0165-5728(88)90046-x. [DOI] [PubMed] [Google Scholar]
- 23.Tarkowski A, Gunnarsson K, Nilsson LA, Lindholm L, Stalhandske T. Successful treatment of autoimmunity in MPL/lpr mice with LS-2616, a new immunomodulator. Arthritis Rheum. 1986;29:1405–9. doi: 10.1002/art.1780291115. [DOI] [PubMed] [Google Scholar]
- 24.Bjork J, Kleinau S. Paradoxical effects of LS-2616 (linomide) treatment in the type II collagen arthritis model in mice. Agents Actions. 1989;27:319–21. doi: 10.1007/BF01972810. [DOI] [PubMed] [Google Scholar]
- 25.Karussis DM, Lehmann D, Slavin S, Kalland T, Vourka-Karussis U, Mizrachi-Koll R, Ovadia H, Abramsky O. Immunomodulation of autoimmunity by linomide. Isr J Med Sci. 1995;31:38–41. [PubMed] [Google Scholar]
- 26.Strigård K, Holmdahl R, van der Meide P, Klareskog L, Olsson T. In vivo treatment of rats with monoclonal antibodies against gamma interferon: effects on experimental allergic neuritis. Acta Neurol Scand. 1989;80:201–7. doi: 10.1111/j.1600-0404.1989.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 27.Hartung HP, Schäfer B, van der Meide PH, Fierz W, Heininger K, Toyka KV. The role of interferon-gamma in the pathogenesis of experimental autoimmune disease of the peripheral nervous system. Ann Neurol. 1990;27:247–57. doi: 10.1002/ana.410270306. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt B, Stoll G, van der Meide P, Jung S, Hartung HP. Transient cellular expression of gamma-interferon in myelin-induced and T-cell line-mediated experimental autoimmune neuritis. Brain. 1992;115:1633–46. doi: 10.1093/brain/115.6.1633. [DOI] [PubMed] [Google Scholar]
- 29.Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-α in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993;45:175–82. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- 30.Sharief MK, McLean B, Thompson EJ. Elevated serum levels of tumor necrosis factor-a in Guillain–Barré syndrome. Ann Neurol. 1993;33:591–6. doi: 10.1002/ana.410330606. [DOI] [PubMed] [Google Scholar]
- 31.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz RH. Advances in immunoregulation and immunotherapy. The Immunologist. 1995;3:5–6. [Google Scholar]
- 33.Kiefer R, Funa K, Schweitzer T, Jung S, Bourde O, Toyka KV, Hartung HP. Transforming growth factor-β1 in experimental autoimmune neuritis. Cellular localization and time course. Am J Pathol. 1996;148:211–23. [PMC free article] [PubMed] [Google Scholar]
- 34.Howard M, O'Garra A. Biological properties of interleukin-10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 35.Bai XF, Zhu J, Zhang GX, Kaponides G, Höjeberg B, van der Meide PH, Link H. IL-10 suppresses experimental allergic neuritis, and down-regulates cytokine mRNA expression of Th1 and macrophage source. Clin Immunol Immunopathol. 1997;2:117–26. doi: 10.1006/clin.1997.4331. [DOI] [PubMed] [Google Scholar]
- 36.Harvey GK, Pollard JD. Peripheral nervous system demyelination from systemic transfer of experimental allergic neuritis serum. J Neuroimmunol. 1992;41:159–66. doi: 10.1016/0165-5728(92)90066-t. [DOI] [PubMed] [Google Scholar]


