Abstract
Exposure to swine dust causes airway inflammation with increased levels of proinflammatory cytokines, and inflammatory cells in nasal and bronchoalveolar lavage fluid (BALF) in healthy subjects. Earlier studies have suggested that lipopolysaccharides (LPS) might be an important proinflammatory factor in swine dust. Since respiratory epithelial cells and alveolar macrophages are target cells for the inhaled dust, we therefore compared the release of proinflammatory cytokines from normal human bronchial epithelial cells (NHBE), an epithelial cell line (A549) and from human alveolar macrophages obtained from BALF from healthy subjects in vitro after incubation with dust collected in swine houses or LPS. Swine dust or LPS was added to the wells with A549 cells or macrophages and incubated for 8 h at concentrations of 12.5, 25, 50 and 100 μg/ml. NHBE cells were incubated with swine dust at a concentration of 25, 50 or 100 μg/ml or with LPS at a concentration of 50 or 100 μg/ml and incubated for 24 h. The supernatants were collected, centrifuged, and IL-6, IL-1β and tumour necrosis factor-alpha (TNF-α) production was measured using an ELISA method and expressed per 106 cells. Swine dust and LPS caused a dose-dependant increase of IL-6 production in NHBE cells, swine dust being more potent than LPS. In A549 cells, only swine dust, but not LPS caused an increase of IL-6 production. Neither swine dust nor LPS induced IL-1β or TNF-α release from A549 cells. Both swine dust and LPS caused a dose-dependent increase of IL-1β, IL-6 and TNF-α in alveolar macrophages. Swine dust which contained 2.2 (0.2) ng endotoxin/100 μg swine dust (0.02‰) was almost as potent as LPS in inducing cytokine release from alveolar macrophages in vitro. We conclude that both epithelial cells and alveolar macrophages have the capability to contribute to the release of proinflammatory cytokines following exposure to swine dust. Some agent(s) other than LPS in the dust contribute to the marked airway inflammatory reaction.
Keywords: epithelial cells, alveolar macrophages, IL-6, tumour necrosis factor-alpha, organic dust (swine dust), lipopolysaccharide
INTRODUCTION
Inhalation of dust from swine confinement buildings (swine dust) induces airways inflammation and systemic effects such as fever, chills and fatigue [1,2]. The proinflammatory cytokines IL-1β, IL-6, and tumour necrosis factor-alpha (TNF-α) increase in nasal and bronchoalveolar lavage fluid (BALF) and the latter two increase in serum in healthy human volunteers exposed for 3 h to dust in a swine confinement building [3]. These cytokines are likely to contribute to the local and systemic reactions [4,5]. Little is known about which agents in the swine dust cause the production of cytokines and the relative importance of epithelial cells and alveolar macrophages as a source of these mediators. Although inhalation of lipopolysaccharide (LPS) may mimic many of the changes observed following inhalation of swine dust, several observations suggest that agents other than endotoxin may also contribute to the reaction following swine dust exposure [5,6].
IL-1, IL-6 and TNF-α are cytokines with proinflammatory properties, with a potential capability to participate in the systemic and local effects. Probable sources for these cytokines in the airways are epithelial cells and alveolar macrophages. In the present study, the secretion of IL-6 from normal human bronchial epithelial cells (NHBE) and the secretion of IL-6, IL-1β and TNF-α from an epithelial carcinoma cell line (A549) and human alveolar macrophages obtained by BAL was studied following in vitro exposure to swine dust and LPS.
MATERIALS AND METHODS
Preparation of swine dust
Settled dust was collected approx. 1.2 m above the floor from a swine confinement building with approx. 700–900 swine weighing around 100 kg each. The dust (10 mg) was dissolved in Ham's F-12 or RPMI 1640 (Seromed Biochrom KG, Berlin, Germany) medium to a final concentration of 1 mg/ml and was mixed for 15 min using a rotator and subsequently sonicated (Eurosonic, Medelco AB, Stockholm, Sweden) for 15 min. The endotoxin content of swine dust was analysed with chromogen version of Limulus amebocyte lysate assay (QCL-1000, Endotoxin; BioWhittaker, Walkersville, MD, with Escherichia coli 0111:B4 as standard).
NHBE
NHBE in primary culture (Clonetics Corp., San Diego, CA) were cultured in serum-free Bronchial Epithelial Cell Growth Medium (BEGM; Clonetics Corp.). A total of 3.1 × 105 cells were seeded into 80-cm2 plastic flasks and grown in BEGM medium. The cultures were kept at 37°C in a humidified atmosphere of 5% CO2 in air and medium was changed every second day. At confluence, the cells were detached by exposure to trypsin/EDTA solution (0.025/0.01% in calcium- and magnesium-free PBS; Clonetics Corp.) and reseeded in 24-well plates at a concentration of 4–7 × 104 cells/well and grown to subconfluence.
At subconfluence, 25, 50 and 100 μg/ml swine dust or 50 and 100 μg/ml LPS (lipopolysaccharide B E. coli 0111:B4; Difco, Detroit, MI) in BEGM medium, 1 ml of each, were added to the wells with NHBE cells in triplicate and incubated for 24 h.
All culture supernatant samples were centrifuged at 1000 g for 10 min to remove cell debris and particulate material and stored at −70°C until analysis. The cells were detached by trypsinization and counted in a haemocytometer. Cell viability was determined by the exclusion of trypan blue (0.4% in saline; Sera-Lab, Crawley Down, UK).
Pulmonary epithelial cell line (A549)
Cells from the human lung epithelial carcinoma cell line A549 (American Type Culture Collection, Rockville, MD; CCL 185) were cultured in Ham's F-12 supplemented with penicillin/streptomycin (100 μg/ml), and heat-inactivated (56°C, 1 h in oven) fetal calf serum (FCS) (10% Seromed, Biochrom KG). Frozen cells were taken from liquid nitrogen and were seeded onto 80-cm2 plastic flasks (Nunc, Roskilde, Denmark) and grown to confluence in humidified a 5% CO2 atmosphere at 37°C for 5–7 days. The cells were removed from the flask by trypsin/EDTA treatment (0.05%/0.02% in calcium- and magnesium-free PBS; Seromed Biochrom KG). The cells were seeded onto six-well plates to a final concentration of 3 × 105 cells/well. At subconfluence the cells were stimulated with LPS or swine dust to a final concentration of 12.5, 25, 50 or 100 μg/ml in triplicate or quadruplicate and incubated for 8 h in medium without serum. Control media were prepared from cell-free dishes in the same manner. The supernatants were collected and centrifuged (1000 g, 10 min at 20°C) and stored at −70°C until analysis. The cells were detached by trypsinization and counted in a haemocytometer. Cell viability was determined by exclusion of trypan blue (0.4% in saline; Sera-Lab).
Alveolar macrophages
Alveolar macrophages were obtained by BAL from six healthy volunteers. Bronchoscopy was performed through the nose with a flexible fibreoptic bronchoscope (Olympus Type 4B2) under local anaesthesia with 2% lidocaine (Xylocaine; Astra, Södertälje, Sweden) after premedication with morphine-scopolamine. The bronchoscope was wedged in a middle lobe bronchus and 250 ml of sterile saline solution at 37°C were instilled in five aliquots of 50 ml. After each instillation, the fluid was gently aspirated and collected in a siliconized plastic bottle kept on ice.
The lavage fluid was immediately centrifuged at 200 g for 10 min at 4°C. The cells were then resuspended in RPMI medium supplemented with 5% FCS (heat-inactivated), 100 μg/ml penicillin/streptomycin + 50 μg/ml gentamycin. The total number of cells was determined by counting in a haemocytometer and cell viability was determined by the exclusion of trypan blue. Total cell count obtained per lavage was 17–26 × 106 and viability was > 80%. A total of 106 cells/well were seeded in six-well plates for dose response studies or 0.3 × 106 cells/well in 24-well plates for TNF-α and IL-6 production over time study and incubated for 2 h at 37°C in 5% CO2. Non-adherent cells were removed after 2 h by washing with RPMI and the adherent cells were incubated for 18 h in serum-free medium. After about 18 h of incubation, the cells were washed with RPMI medium and stimulated with LPS or swine dust at concentrations of 12.5, 25, 50 and 100 μg/ml in triplicate or quadruplicate and incubated for 8 h in serum-free RPMI medium. To study TNF-α and IL-6 production over time, swine dust at a concentration of 100 μg/ml, or medium only as a control, was added to the wells in quadruplicate for each time point and incubated for 2, 4, 6, 8, 10 and 24 h. The supernatants were collected and centrifuged (1000 g, 10 min for 20°C) and stored at −70°C until assay. Control media were prepared from cell-free dishes in the same manner. The viability of adherent alveolar macrophages was assessed using trypan blue exclusion.
Cytokine interactions and decay
To study the effect of TNF-α on IL-6 production, 2 ml of purified recombinant human TNF-α (rTNF-α) (210-TA-010; R&D Systems Europe Ltd, Abingdon, UK) at concentrations of 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0 or 7.5 ng/ml in Ham's F-12 were added at subconfluence to the wells with A549 cells in quadruplicate and incubated for 24 h, after which IL-6 was analysed in the supernatant.
To study IL-6 production over time, rTNF-α at a concentration of 1 ng/ml or medium only as a control were added to the wells in triplicate and incubated for 1, 2, 4, 6, and 24 h.
In order to study interactions between IL-6 and TNF-α, and possible cytokine degradation during incubation, IL-6 production induced by swine dust alone and by swine dust in combination with TNF-α from two different sources were compared. The A549 epithelial cells were incubated with r-TNF-α or TNF-α standard 30 pg/ml (using kit standard, R&D Systems Europe Ltd) with and without swine dust 100 μg/ml. The supernatants were harvested after 24 h incubation, centrifuged, stored and assayed by ELISA technique. In addition the decay of TNF-α immunoreactivity during culture was studied.
Cytokine assays
The IL-6, IL-1β and TNF-α in alveolar macrophage and epithelial cell (A549) supernatants were measured in duplicate by ELISA using IL-6, IL-1β and TNF-α commercial kits (R&D Systems) with a detection limit of 3.1, 3.9 and 15.7 ng/l, respectively. The intra- and interassay coefficient of variation was < 10%.
In the analysis of IL-6 production from NHBE cells and the interaction between IL-6 and rTNF-α with and without swine dust, an IL-6 ELISA method developed in our laboratory was used. A human MoAb against IL-6 (R&D Systems; MAB206) was used as a capture antibody, at a concentration of 4 μg/ml (in PBS), which was bound to 96-well microtitre plates (Maxisorp; Nunc) overnight at room temperature. After washing, the wells were incubated with a blocking buffer (PBS with 1% bovine serum albumin (BSA), 5% sucrose and 0.15% Kathon) for 1 h at room temperature. After washing, standard dilutions (3–375 pg/ml) of recombinant human IL-6 (R&D Systems; 206-IL-010) or sample diluted in PBS/0.1% Tween (dilution buffer) were added to the wells in duplicate, mixed on a shaker and incubated for 2 h at room temperature. After repeated washing, the detection biotinylated antibody (1:250; R&D Systems, BAF206) in dilution buffer was added and incubated for 2 h at room temperature. After repeated washing, the conjugate streptavidin–horseradish peroxidase (HRP) (PQ35580; in house) was added for 1 h incubation. Following washing, the substrate K-blue was added and the reaction terminated after 10 min with 1 m H2SO4. Absorbance was read at 450 nm using a Thermomax 250 reader (Molecular Devices, Sunnyvale, CA), and the results were analysed with Softmax software (Molecular Devices). Quantikine serum controls at three different levels were used as calibrators. The lower detection limit of the assay is 3 ng/l. The cytokine production was expressed as ng/106 cells. For duplicated samples an intra-assay coefficient of variation (CV) of < 10% and an interassay CV of < 20% was accepted. There was a high correlation (r = 0.93) between results obtained using the commercial kit and our method.
Statistical analysis
Data are expressed as mean (s.e.m.). Comparisons are made using analysis of variance (anova; Statview program, version 4.5; Abacus Concepts, Inc., Berkeley, CA) and a Fisher's PLSD test when appropriate. P < 0.05 was considered significant.
RESULTS
The endotoxin content of the swine dust solution was 2.2 ng/100 μg swine dust. Both swine dust and LPS stimulated NHBE cells to produce IL-6 at 50 and 100 μg/ml (Fig. 1a). Baseline IL-6 production in NHBE cells was 0.34 (0.04) ng/million cells. At the highest concentration swine dust increased IL-6 production 14-fold and LPS four-fold compared with the control. Neither swine dust nor LPS stimulated NHBE cells to secrete measurable TNF-α (below detection limit). None of the tested agents altered cell viability at any concentration as assessed by trypan blue exclusion (viability > 90%).
Fig. 1.
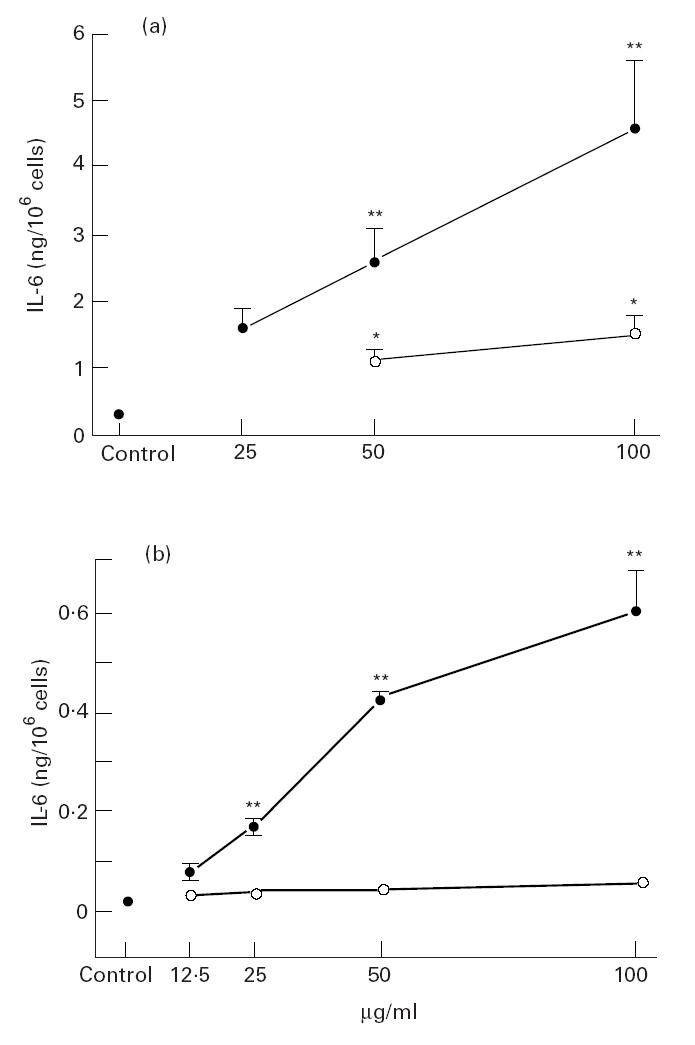
IL-6 release from normal human bronchial epithelial cells (NHBE) (a) and A549 epithelial cells (b) exposed to swine dust (•) or lipopolysaccharide (LPS; ○). Cells were cultured to subconfluence, then LPS or swine dust was added and the culture supernatants were harvested after 24 h (NHBE) or 8 h (A549) of incubation. The amount of IL-6 released per 106 cells was calculated and expressed as mean (s.e.m.). Each data point is based on three experiments performed in triplicate (a) and two experiments performed in triplicate or quadruplicate (b). *P < 0.05; **P < 0.01 compared with baseline (supernatant from cell culture without LPS or swine dust).
Baseline IL-6 production in A549 cells was 0.01 (0.03) ng/million cells. Swine dust, but not LPS induced a dose-dependent release of IL-6 from A549 cells (Fig. 1b). TNF-α and IL-1β were not detected in the supernatants from epithelial cell cultures after stimulation with swine dust or LPS. Viability (trypan blue exclusion) remained unchanged (> 90%) after incubation with both agents at all concentrations.
Baseline IL-6, IL-1β and TNF-α in unstimulated macrophages was 0.01 (0.01), 0.01 (0.01) and 0.29 (0.29) ng/million cells, respectively. IL-6 and TNF-α production increased over time after incubation with swine dust, but under control conditions the IL-6 and TNF-α release was below detection limit at all tested time points (Fig. 2). Swine dust and LPS caused a dose-related increase in IL-6, IL-1β and TNF-α in alveolar macrophages (Fig. 3). Both LPS and swine dust induced significant increases in TNF-α at all concentrations, LPS being more potent than swine dust. LPS at the two highest concentrations was also a more potent stimulus for IL-6 release than was swine dust. There were no significant differences in the potency of LPS and swine dust in causing release of IL-1β by alveolar macrophages.
Fig. 2.
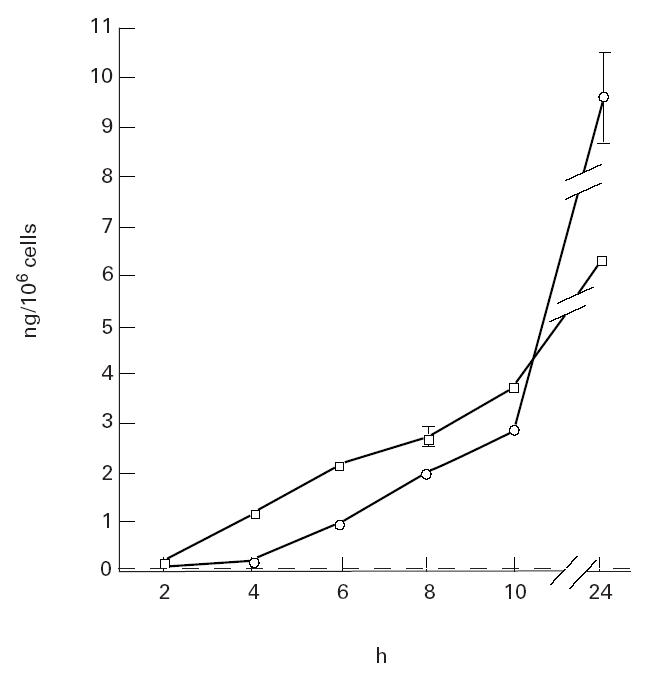
Release of tumour necrosis factor-alpha (TNF-α; □) and IL-6 (○) from alveolar macrophages over time in unstimulated control situation and after stimulation with swine dust. Alveolar macrophages were incubated with medium only or with swine dust (100 μg/ml) for indicated time points (n = 4, with exception of 8 and 24 h where n = 2). The amount of TNF-α and IL-6 release (ng) per 106 cells was calculated and expressed as mean (s.e.m.). In the unstimulated control situation the TNF-α and IL-6 release was below detection limit at all tested time points.
Fig. 3.
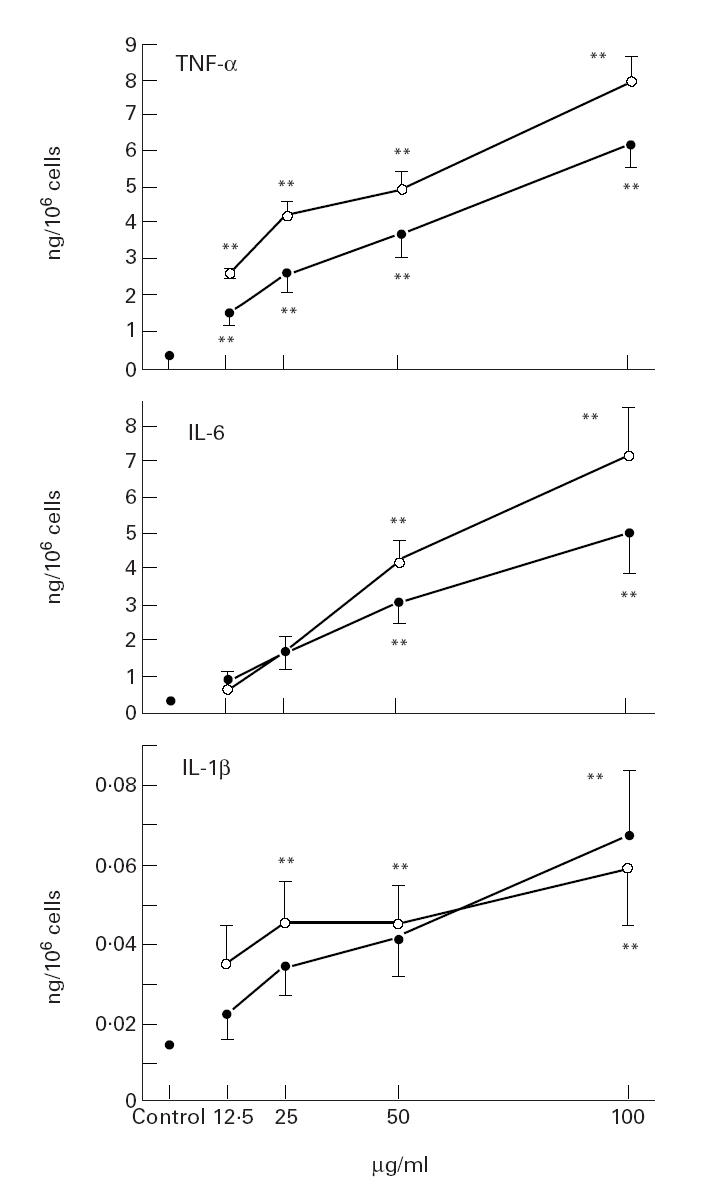
Release of tumour necrosis factor-alpha (TNF-α), IL-6 and IL-1β from alveolar macrophages incubated with swine dust (•) or lipopolysaccharide (LPS; ○). Alveolar macrophages were obtained from six healthy subjects and cultured overnight. LPS or swine dust was then added at indicated concentrations, and incubated for 8 h. The amount of TNF-α, IL-6 and IL-1β released per 106 cells was calculated and expressed as mean (s.e.m.). Each data point is based on two experiments performed in triplicates or quadruplicate. **P < 0.01 compared with baseline (supernatant from cell culture without LPS or swine dust).
In control experiments where swine dust or LPS were incubated in cell-free media no IL-1β, IL-6 or TNF-α immunoreactivity was found.
IL-6 production increased in a dose-dependent manner after stimulation of A549 cells with rTNF-α (Fig. 4a).
Fig. 4.
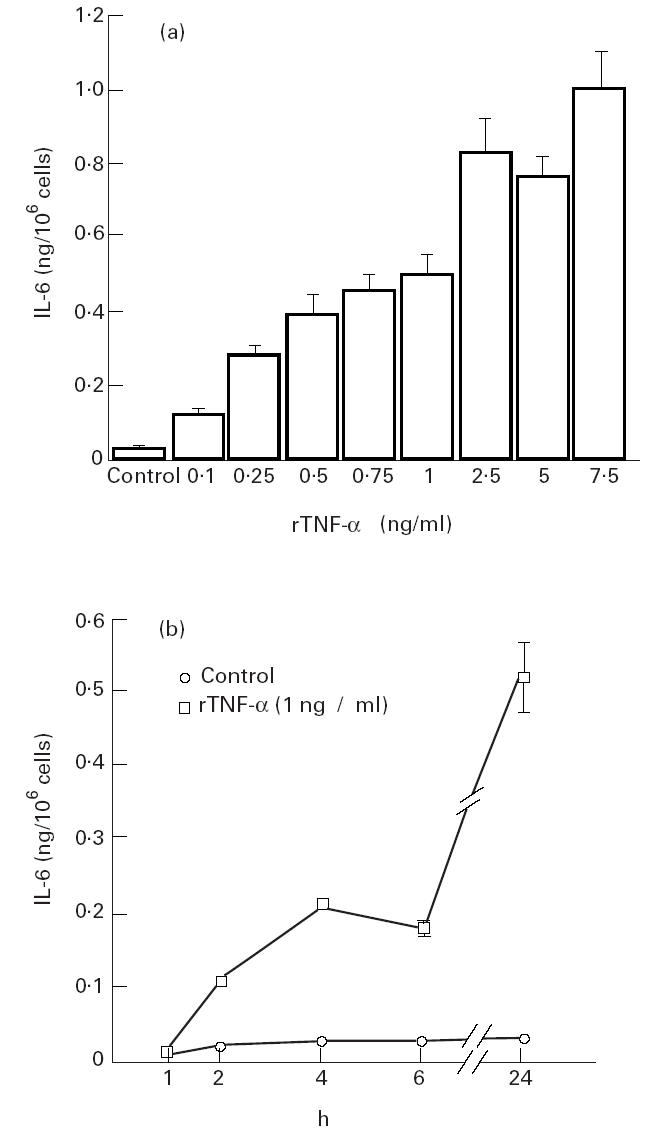
(a) IL-6 release from A549 epithelial cells exposed to recombinant human tumour necrosis factor-alpha (rTNF-α). Cells were cultured to subconfluence, then rTNF-α was added at different concentrations and the culture supernatant was harvested after 24 h. The amount of IL-6 released per 106 cells was calculated and expressed as mean (s.e.m.). Each data point is based on one experiment performed in quadruplicate. (b) IL-6 release over time from A549 epithelial cells exposed to rTNF-α. Cells were cultured to subconfluence, then rTNF-α or only medium as a control was added and the culture supernatant was harvested after the indicated time points. The amount of IL-6 released per 106 cells was calculated and expressed as mean (s.e.m.). Each data point is based on one experiment performed in triplicate.
IL-6 production increased over time in A549 cells, when incubated with medium only or after stimulation with rTNF-α (1 ng/ml). rTNF-α stimulation resulted in a significantly higher IL-6 production at all tested time points (Fig. 4b).
Swine dust (100 μg/ml) induced a 70-fold increase, rTNF-α (30 pg/ml) a 4.5-fold increase and the combination of rTNF-α (30 pg/ml) and swine dust (100 μg/ml) a 80-fold increase in IL-6 production in A549 cells (Fig. 5a). A synergistic effect of swine dust and TNF-α was found when TNF-α standard was used (Fig. 5b). After 24 h incubation with TNF-α standard or TNF-α standard combination with swine dust TNF-α recovery was 64% (n = 8) and 125%, respectively (n = 10).
Fig. 5.
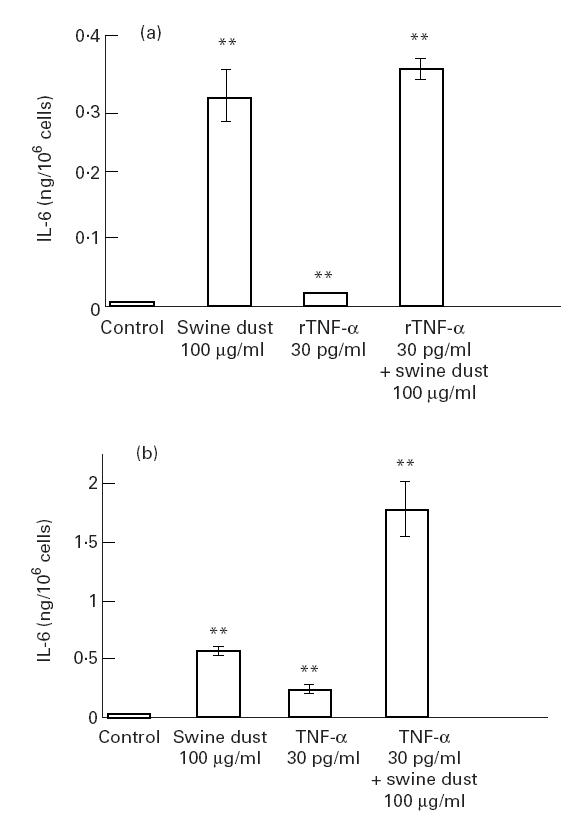
(a) IL-6 release from A549 epithelial cells exposed to swine dust, recombinant human tumour necrosis factor-alpha (rTNF-α) or a combination of swine dust and rTNF-α. Cells were cultured to subconfluence, then swine dust, rTNF-α or a combination of swine dust and rTNF-α was added and the culture supernatant was harvested after 24 h. The amount of IL-6 released per 106 cells was calculated and expressed as mean (s.e.m.). Each data point is based on three experiments performed in triplicate or quadruplicate. (b) IL-6 release from A549 epithelial cells exposed to swine dust, TNF-α standard supplied with commercial kits or a combination of swine dust and TNF-α standard. Cells were cultured to subconfluence, then swine dust, TNF-α standard or a combination of swine dust and TNF-α standard was added and the culture supernatant was harvested after 24 h. The amount of IL-6 released per 106 cells was calculated and expressed as mean (s.e.m.). Each data point is based on two experiments performed in quadruplicate.
DISCUSSION
In this study, we have demonstrated that swine dust and pure LPS trigger IL-1, IL-6 and TNF-α release in human alveolar macrophages in a dose-dependent manner. Swine dust and LPS were almost equipotent based on weight. Since swine dust contains approximately 0.002% LPS (by weight), we suggest that other components than LPS in swine house dust contribute to the cytokine production in human alveolar macrophages in vitro. This suggestion is strengthened by the in vitro findings in epithelial cell cultures. Thus in primary culture of human epithelial cells (NHBE), swine dust induced a much higher production of IL-6 than did LPS in equal amounts by weight. Furthermore, in the carcinoma epithelial A549 cells swine dust stimulated IL-6 release while LPS had no effect in this respect. Since the cytokine concentrations in the cell culture supernatant increased in a dose-dependent manner, the findings cannot be explained by inappropriate dosage of the agents. The IL-6 release from NHBE cells was about 10 times higher than from A549 cells. However, since the time between exposure and collection of supernatants was longer in the NHBE experiments, no definite conclusions regarding the relative potency of the cells to produce and release IL-6 can be drawn from this study. In a previous study swine dust induced a 10-fold greater IL-8 production in NHBE in primary culture than in A549 cell line measured at the same time after exposure [7]. Thus the A549 epithelial cell line may not be representative of human epithelial cells in all respects.
In a previous study we showed that alveolar macrophages and epithelial cells release IL-8 after exposure to swine dust and LPS and that glucan and grain dust are much weaker stimuli in this respect [7]. We have also found that both Gram-positive and Gram-negative bacteria, which are known to be present in swine dust, induced IL-8 [9] and IL-6 secretion in epithelial cells (unpublished data). Swine dust contains different bacteria, both dead and cultivable. The cultivable flora is dominated by Gram-positive bacteria [10]. In a human exposure study, bacterial markers both Gram-positive (muramic acid) and Gram-negative (3-hydroxyfatty acids) bacteria showed better correlation with IL-6 changes in serum than did the inhalable dust concentration [5]. The systemic inflammatory response syndrome (SIRS) ‘sepsis’ may be mimicked by i.v. infusion of LPS, but a large proportion of the cases of SIRS are caused by Gram-positive bacteria which have no LPS, suggesting that profound inflammation may be induced by other agents than LPS emanating from Gram-positive bacteria.
LPS is an abundant component of the outer membrane of Gram-negative bacteria [11]. Systemic administration of LPS elicits a marked inflammatory response, and it is a powerful stimulant for monocytes/macrophages and other cells to release cytokines in vitro [12]. Thus LPS triggers the production of TNF-α, IL-1β, IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) in monocytes, endothelial cells [13–16] and alveolar macrophages [17]. In the present study, LPS was used as reference agent and stimulated alveolar macrophages and NHBE cells to release cytokines in a dose-dependent fashion as expected.
LPS interacts with several receptors. So far only interaction with CD14 has been demonstrated to activate monocytes and macrophages, as well as endothelial cells and neutrophils [18]. CD14 exist in a membrane-bound form (on monocytic cell membranes) and as a circulating soluble protein (sCD14). Interaction between LPS and CD14 is greatly facilitated by a circulating lipid-binding protein (LBP). A complex of sCD14–LPS formed in the presence of LBP may interact, e.g. endothelial cells, and activate them to induce an inflammatory response. LPS did not induce IL-6 release from the A549 epithelial cell line and induced only a weak response in NHBE epithelial cells, which is probably a consequence of the absence of CD14 on epithelial cells. It is not known if human airway epithelial cells in vivo in normal conditions interact with LPS via sCD14 and LBP in airway lining fluid. It has, however, been speculated that in airway inflammatory conditions, sCD14 and LBP may become available through extravasation of serum proteins. It is thus unlikely that the CD14 receptor is involved in the responses of epithelial cell cultures to swine dust or LPS in vitro. It is therefore of interest to note that swine dust, but not LPS, induced marked IL-6 release under such conditions, suggesting that other mechanisms and agents, possibly present in bacteria, may be involved.
The present study confirms previous findings that carcinoma epithelial cell lines do not produce TNF-α [19]. This is in contrast to findings in cultured normal human epithelial cells [20,21]. We wanted to ascertain that the absence of TNF-α response was not due to concomitant breakdown of immunoreactivity of this cytokine. Our data speak in favour of such a breakdown of TNF-α by epithelial cells, since we found only 64% recovery after incubation of the cells with TNF-α. It can thus not be excluded, from the present study, that the A549 epithelial cells produce TNF-α in small amounts, since incubation with swine dust and TNF-α gave a recovery of 125%. Our findings do, however, suggest that epithelial cells have a more restricted cytokine response than alveolar macrophages in the response to swine dust.
It is known that airway epithelial cells possess TNF binding sites [22]. In the present study both swine dust and TNF-α induced IL-6 release from epithelial cells. An additive response was observed when swine dust and rTNF-α were used in combination. However, when we used TNF-α supplied as the standard of commercial kits (R&D Systems) a synergistic effect between TNF-α and swine dust was shown. The TNF-α standard probably contains a component, apart from TNF-α, which is a very potent inducer of cytokine release. This component was probably toxic to the cells, since higher concentrations than 30 pg/ml influenced cell viability. Higher concentrations of rTNF-α used in the dose–response curve did not influence the viability of the cells. Our results imply that TNF-α used as the standard of commercial kits (R&D Systems) can not be used as a TNF-α stimulus in experiments such as this.
In conclusion, the present study suggests that both alveolar macrophages and epithelial cells may participate in the release of inflammatory cytokines following exposure to dust from swine confinement buildings. Some agent(s) apart from LPS in the dust are at least equally potent compared with LPS in this in vitro situation.
Acknowledgments
We thank Siw Siljerud, Britt-Marie Larsson and Per Larsson for skilful technical assistance. This study was supported by the Swedish Council for Work Life Research (formerly National Work Environmental Fund) grant numbers 94-1383 and 94-0318 and by Swedish Heart & Lung Foundation.
REFERENCES
- 1.Larsson K, Eklund A, Hansson L-O, Isaksson B-M, Malmberg P. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med. 1994;150:973–7. doi: 10.1164/ajrccm.150.4.7921472. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Suur C, Larsson K, Malmberg P, Larsson PH. Increased number of activated lymphocytes in human lung following swine dust inhalation. Eur Respir J. 1997;10:376–80. doi: 10.1183/09031936.97.10020376. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J. 1997;10:381–7. doi: 10.1183/09031936.97.10020381. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Malmberg P, Larsson P, Larsson B-M, Larsson K. Time course of interleukin-6 and tumor necrosis factor-α increase in serum following inhalation of swine dust. Am J Respir Crit Care Med. 1996;153:147–52. doi: 10.1164/ajrccm.153.1.8542108. [DOI] [PubMed] [Google Scholar]
- 5.Zhiping W, Malmberg P, Larsson B-M, Larsson K, Larsson L, Saraf A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med. 1996;154:1261–6. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]
- 6.Sandström T, Bjermer L, Rylander R. Lipopolysaccharide (LPS) inhalation in healthy subjects increases neutrophils, lymphocytes and fibronectin levels in bronchoalveolar lavage fluid. Eur Respir J. 1992;5:992–6. [PubMed] [Google Scholar]
- 7.Palmberg L, Larsson B-M, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax. 1998;53:260–4. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huag ZB, Eden E. Effect of bronchoalveolar lavage fluid from patients with sarcoidosis or AIDS on interleukin 1β release from alveolar macrophages. Chest. 1990;98:576–80. doi: 10.1378/chest.98.3.576. [DOI] [PubMed] [Google Scholar]
- 9.Larsson B-M, Palmberg L, Larsson K, Malmberg P. Gram positive bacteria induce IL-8 production in epithelial cell line A549. Eur Respir J. 1995;8:225s. doi: 10.1023/a:1020269802315. [DOI] [PubMed] [Google Scholar]
- 10.Crook B, Robertson JF, Travers-Glass SA, Botheroyd E, Lacey J, Topping MD. Airborne dust, ammonia, microorganisms and antigens in pig confinement houses and respiratory health of exposed farm workers. Am Ind Hyg Assoc J. 1991;52:271–9. doi: 10.1080/15298669191364721. [DOI] [PubMed] [Google Scholar]
- 11.Burrel R. Immunomodulation by bacterial endotoxin. Crit Rev Microbiol. 1990;17:189–208. doi: 10.3109/10408419009105725. [DOI] [PubMed] [Google Scholar]
- 12.Andersson J, Nagy S, Björk L, Abrams J, Holm S, Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 13.Hedges S, Svensson M, Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992;60:1295–301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loppnow H, Libby P, Freudenberg M, Krauss JH, Weckesser J, Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990;58:3743–50. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jirik FR, Podor TJ, Hirano T, Kishimoto T, Loskutoff DJ, Carson DA, Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989;142:144–7. [PubMed] [Google Scholar]
- 16.de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Svanborg Edén C. Interleukin-6 induced at mucosal surfaces by Gram-negative bacterial infection. Infect Immun. 1989;57:3383–8. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing Z, Jordana M, Kirpaleni H, Dirscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994;10:148–53. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]
- 18.Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1990;176:1665–71. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spriggs DR, Imamura K, Rodriguez C, Sariban E, Kufe DW. Tumor necrosis factor expression in human epithelial tumor cell lines. J Clin Invest. 1988;81:455–69. doi: 10.1172/JCI113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khair OA, Devalia JL, Abdelaziz MM, Sapsford RJ, Tarraf H, Davies RJ. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-α and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur Respir J. 1994;7:2109–16. doi: 10.1183/09031936.94.07122109. [DOI] [PubMed] [Google Scholar]
- 21.Devalia JL, Campbell AM, Sapsford RJ, Rusznak C, Quint D, Godard P, Bousquet J, Davies RJ. Effect of nitrogen dioxide on synthesis of inflammatory cytokines expressed by human bronchial epithelial cell in vitro. Am J Respir Cell Mol Biol. 1993;9:271–8. doi: 10.1165/ajrcmb/9.3.271. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ. Airway epithelial receptors. Eur Respir Rev. 1994;4:371–9. [Google Scholar]


