Abstract
CD8 deficiency is a rare primary immunodeficiency caused by the defect of a tyrosine kinase, ZAP-70, which transduces signals from the T cell receptor. We report here a case of CD8 deficiency, having CD4+ T cells with a unique phenotype. The patient's T cells did not respond to anti-CD3 stimulation in vitro, suggesting that they were naive. However, many CD4+ T cells with activated and memory phenotypes, which expressed CD45RO+, HLA-DR+ and CD25+, were present in the peripheral blood, and these cells accumulated in the perivascular area of his infiltrative erythematous skin lesions. The patient's T cells could be activated by a high concentration of phytohaemagglutinin (PHA), indicating the presence of an alternate signalling pathway which bypasses ZAP-70 and activates CD4+ T cells in vivo. The origin and role of activated CD4+ T cells in the pathogenesis involved in the skin lesions are discussed.
Keywords: CD8 deficiency, ZAP-70, activated and memory CD4+ T cells, skin infiltration
INTRODUCTION
CD8 deficiency is a rare primary immunodeficiency which was first reported by Roifman et al. in 1988 [1]. A tyrosine kinase, ZAP-70, which had been shown to transduce the signals from T cell receptors (TCR) [2], was identified as the responsive gene product of this disease [3–5]. In contrast to other types of severe combined immunodeficiency (SCID), CD8-deficient patients lacked peripheral CD8+ T cells but had a normal number of circulating CD4+ T cells [1,6]. This is in marked contrast to the result of gene targeting of ZAP-70 in mice that lack both CD4+ and CD8+ T cells from peripheral blood [7]. Although we can study the role or functions of ZAP-70-deficient CD4+ T cells only in these patients, there have been no studies concerning the surface phenotypes of ZAP-70-deficient CD4+ T cells in these patients, especially whether these cells are naive or memory cells.
Patients develop many symptoms suggesting T cell deficiency, such as frequent upper respiratory infection, oral ulceration, eczematous lesions, chronic diarrhoea and opportunistic infections [1,6]. The patient presented here had infiltrative erythematous skin lesions on his face and extremities. As an immunohistochemical examination revealed infiltration of activated and memory phenotypes of CD4+ T cells in skin lesions, we performed studies to characterize CD4+ T cells in this patient to better understand the role of ZAP-70 in the human immune system.
PATIENT AND METHODS
Patient and his skin lesions
A 2-month-old boy who was the third-born child to unrelated Japanese parents was referred to our hospital for immunological examination because he had infiltrative erythematous skin lesions on his face and extremities, and similar skin lesions had been present on his deceased sister who died of pneumonia caused by cytomegalovirus at 6 months. These skin lesions, with slight exudate and excoriation but no lichenification, were present from birth in both this patient and his sister. There was no family history of allergic diseases or immunodeficiency among first and second degree relatives except for his sister. The patient was healthy and afebrile, and had no abnormal physical findings except the skin lesions. He was kept in protective isolation after diagnosis, and has remained well until 17 months of age. There was no evidence of opportunistic infection, diarrhoea, chest infections or lymphadenopathy. His elder brother was healthy and had no skin lesions.
Analysis of patient's lymphocytes by flow cytometry
Cell surface markers were examined by three-colour flow cytometry using a FACScan. After erythrocyte lysis of heparinized whole blood, leucocytes were stained by the following labelled MoAbs. PE- or FITC-labelled anti-CD3, CD4, CD8, CD56, CD20, CD45RA, CD45RO, CD25, CD62L and CD71 MoAbs were obtained from Becton Dickinson Immunocytometry System (San Jose, CA). FITC-labelled anti-CD11a MoAb was obtained from Dakopatts (Glostrup, Denmark). PE-Cy5-labelled anti-CD4 MoAb was obtained from Immunotech (Marseille, France). Data were analysed using LYSYS II software (Becton Dickinson). CD4+ T cells were electronically gated based on the staining pattern with PE-Cy5-labelled anti-CD4 MoAb and further analysed for other surface markers by staining with the indicated labelled antibodies.
Analysis of expression of ZAP-70 protein
Expression of ZAP-70 protein was analysed by Western blotting as previously described [8]. Peripheral blood mononuclear cells (PBMC) from the patient and a healthy control were stimulated with 10 ng/ml phorbol myristate acetate (PMA; Sigma Co., St Louis, MO) and 1 μg/ml ionomycin (Calbiochem Co., La Jolla, CA) and expanded in medium containing 50 U/ml of rIL-2 (Shionogi Pharmaceutical Co., Osaka Japan) for 6 days. These expanded cells (5 × 106) were solubilized in 250 μl lysis buffer solution as described [8]. After incubation on ice for 30 min, samples were centrifuged at 14 000 g for 10 min, and the concentration of protein in the supernatant was measured with a protein assay kit (BioRad, Richmond, CA). Aliquots of 20 μg protein were boiled with Laemmli's sample buffer solution. The samples were then applied to 10% SDS–polyacrylamide gels, electrophoresed and then transferred on PVDF membranes (BioRad). The membranes were blocked with 1% bovine serum albumin (BSA) in PBS and 0.1% Tween 20, then incubated with rabbit antibody against ZAP-70 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:1000 dilution, followed by incubation with peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz) at 1:1000 dilution. The immunoreactive bands were visualized by enhanced chemiluminescence using the ECL reagent (Amersham, Aylesbury, UK).
Immunohistochemical examination of the patient's skin lesion
A small skin specimen was obtained by a punch biopsy from a skin lesion on his right forearm. The specimen was fixed in 10% buffered formalin, routinely processed, sectioned at 4 μm and stained with haematoxylin and eosin. These sections were stained with polyclonal anti-CD3 (Dakopatts) and MoAbs against CD4 (1F6; Novocastra, Newcastle upon Tyne, UK), CD8 (C8/144B; Dakopatts), CD45RO (UCHL1; Dakopatts) and HLA-DR (LN3; Techniclone, Tustin, CA) by the avidin-biotin-peroxidase method described by Hsu et al. [9]. Sections were treated by autoclave before CD4 and CD8 staining.
Analysis of cell proliferation
PBMC were isolated by density gradient centrifugation, washed twice with RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), penicillin G 100 U/ml, streptomycin 100 μg/ml, 50 μm 2-mercaptoethanol (2-ME) and l-glutamine 2 mm. The cells were stimulated with phytohaemagglutinin (PHA; Murex Diagnostics Ltd, Dartford, UK) in 96-well plates (200 μl/well) or anti-CD3 MoAb (UCHT1, 100 ng/ml; Pharmingen, San Diego, CA) in 96-well plates coated with goat anti-mouse IgG at a cell density of 106/ml for 72 h and pulsed with 0.5 μCi/well of methyl-3H-thymidine (Moravex Biochemicals, Brea, CA) for the last 8 h of culture. After harvesting the cells, methyl-3H-thymidine uptake was measured on a liquid scintillation counter. All the experiments were carried out after informed consent was obtained from the patient's parents.
RESULTS
Lack of ZAP-70 protein in a CD8-deficient patient
Laboratory findings showed 25 000 leucocytes/mm3 consisting of 39% lymphocytes, 8% monocytes, 13% neutrophils, 38% eosinophils, 1% basophils, and 1% atypical lymphocytes. Serum immunoglobulin concentrations were IgG 6.1 g/l (age-matched reference range 3.0–7.0 g/l), IgA 0.18 g/l (0.05–0.5 g/l), IgM 0.67 g/l (0.2–0.7 g/l), IgE 57 U/ml (< 10 U/ml). C-reactive protein (CRP) was negative. Antigen-specific IgE examined by radioallergosorbent test (RAST) was negative for mites, egg white, cows' milk, soy bean, rice and wheat. The patient lacked peripheral CD8+ T cells (< 1%) (Fig. 1) and had CD4+ T cells which did not proliferate upon stimulation with 1 μg/ml PHA or anti-CD3 stimulation (Table 1) as reported previously for CD8-deficient patients [6]. Since similar CD8-deficient patients were reported to lack functional ZAP-70 protein in the remaining T cells [3–5], we analysed ZAP-70 expression in the patient's CD4+ T cells and confirmed the lack of ZAP-70 by Western blotting (Fig. 2). Detailed analysis of the ZAP-70 gene of this patient will be described elsewhere. His PBMC were 100% male phenotype by fluorescence in situ hybridization method (FISH) using human chromosome Y-specific probe (Vysis, Inc., Downer's Grove, IL).
Fig. 1.
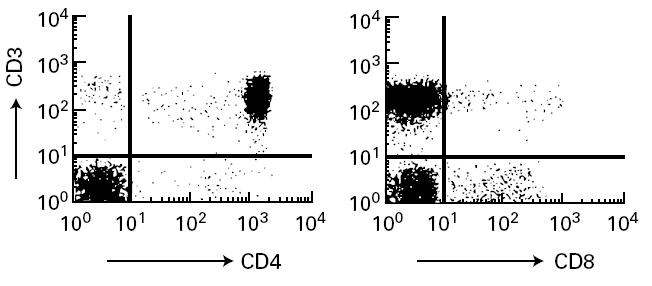
Flow cytometric analysis of patient's peripheral T cells. Patient's peripheral blood mononuclear cells (PBMC) were stained with FITC-labelled anti-CD3 and PE-labelled anti-CD4 or anti-CD8. Stained cells were analysed as described in Patient and Methods.
Table 1.
Proliferative response of T cells to various mitogens
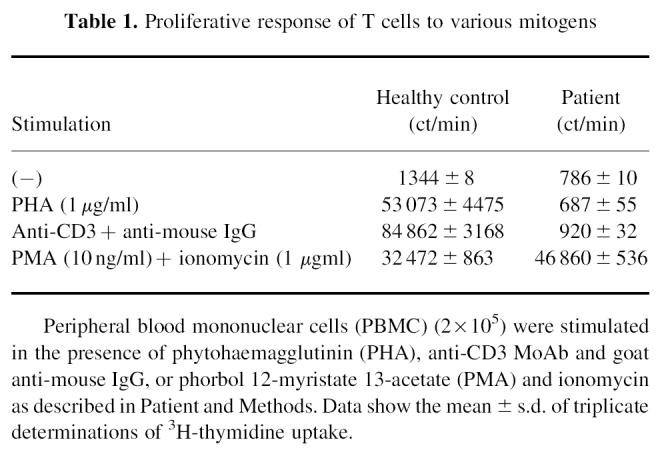
Fig. 2.
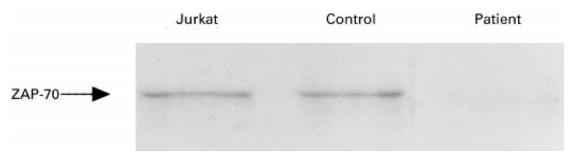
Western blotting of ZAP-70 protein. Patient's T cells, normal control's T cells and Jurkat cells were lysed and ZAP-70 protein was analysed with Western blotting as described in Patient and Methods.
Accumulation of activated and memory CD4+ T cells in skin lesions
To investigate the immunopathology of infiltrative erythematous skin lesions on his face and extremities (Fig. 3), we examined the skin biopsy specimen by immunohistological methods. As shown in Fig. 4a, these skin lesions were characterized by spongiotic and slightly thickened epidermis. In the dermis, increased numbers of infiltrating cells consisting predominantly of mononuclear cells and eosinophils were detected in the perivascular area, and partially in peri-appendageal and adipose tissue areas. To characterize mononuclear cells infiltrating the perivascular area, these sections were stained by the immunoperoxidase method using antibodies against CD3, CD4, CD8, CD20, CD14, CD45RO and HLA-DR. The infiltrated cells were almost all positive for CD3 and CD4, and negative for CD8, CD20 and CD14. Furthermore, these CD4+ T cells also expressed CD45RO and HLA-DR, which are the markers of memory and activated T cells, respectively (Fig. 4b).
Fig. 3.
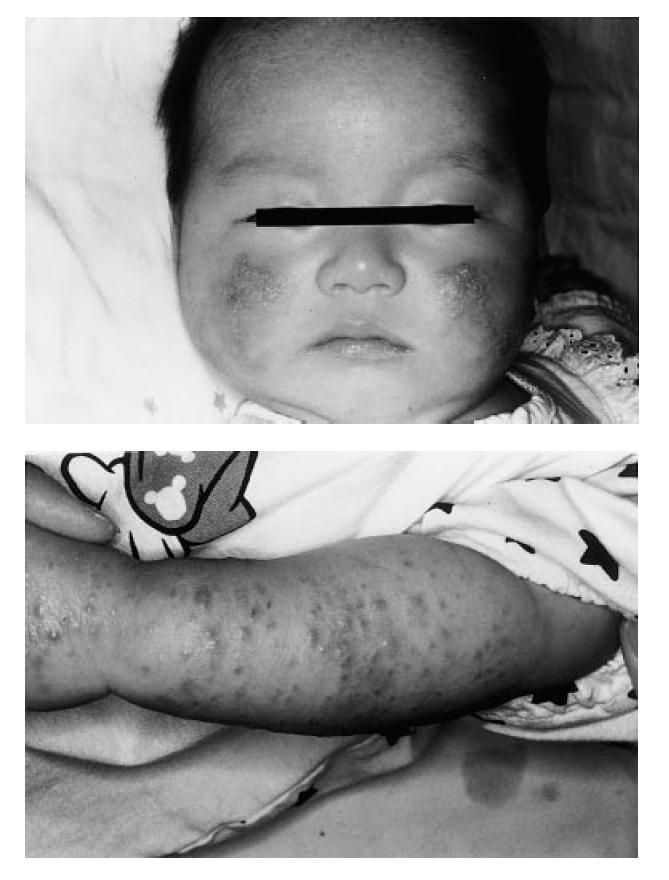
The patient's skin lesions on his face and extremities.
Fig. 4.
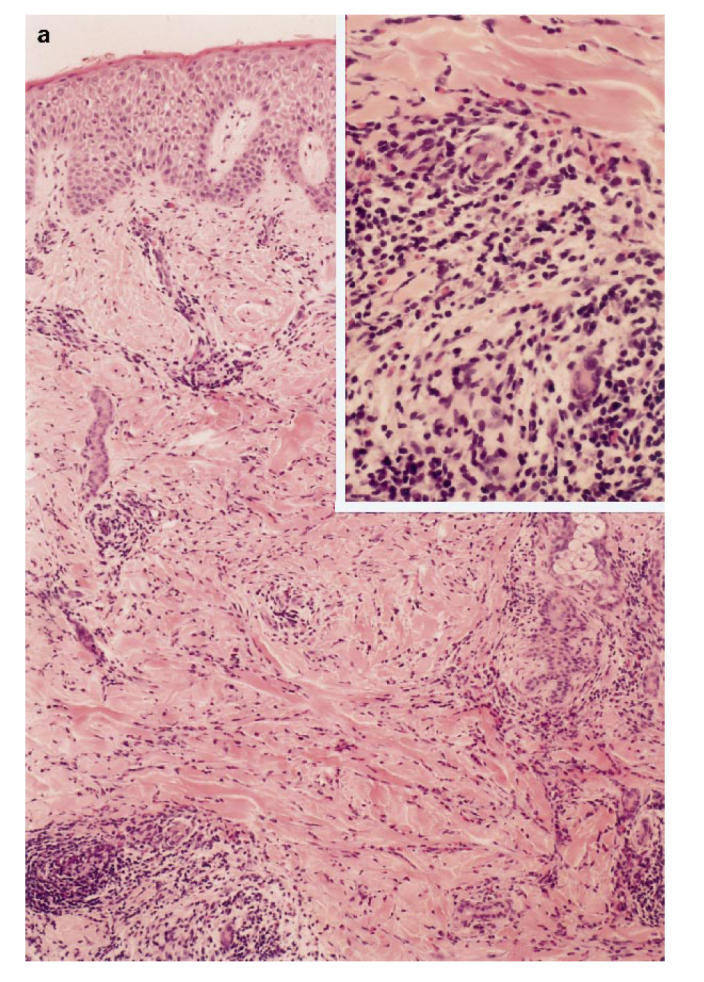

(See next page.) Immunohistochemical analysis of patient's skin lesion. (a) Haematoxylin–eosin stain of a skin lesion. The perivascular area is magnified in right upper portion. (b) Immunohistochemical analysis of infiltrated cells. Cells were stained with indicated antibodies. Upper left: anti-CD3; upper middle, anti-CD4; upper right, anti-CD8; lower left, anti-HLA-DR; lower middle, anti-CD45RO.
Presence of activated memory CD4+ T cells in peripheral blood
As shown in Table 1, peripheral CD4+ T cells in this patient were not activated by signals through TCR such as anti-CD3 MoAb. Since they did not respond to TCR stimulation alone, these cells were initially regarded as being naive. However, the immunohistological examination of the skin lesion demonstrated the presence of activated and memory types of CD4+ T cells. To characterize further the CD4+ T cells from this patient, we performed flow cytometric analysis of the patient's peripheral CD4+ T cells. As shown in Fig. 5, about half of the patient's peripheral CD4+ T cells were CD45RO+CD45RA− and contained CD11ahigh and CD62Llow cells, which are characteristics of memory T cells. Furthermore, most of these CD4+CD45RO+ T cells also expressed HLA-DR, CD25 and CD71: markers of activated T cells, which are considered to be induced by stimulation via TCR under physiological conditions. Although these memory T cells did not express other activation markers such as CD69 and CD154 (data not shown), a considerable fraction of CD4+ T cells in this patient expressed memory phenotype and activation markers in the absence of ZAP-70 protein kinase, which is considered to be essential in TCR signal transduction.
Fig. 5.
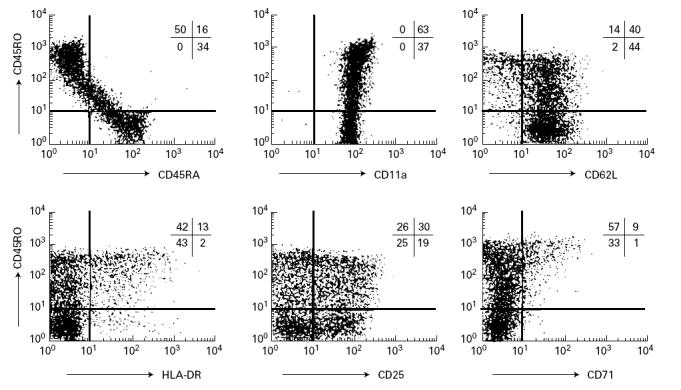
Three-colour analysis of peripheral CD4+ T cells from the ZAP-70-deficient patient. CD4+ T cells were gated by staining with PE-Cy5-labelled anti-CD4, and analysed with FITC-conjugated and PE-conjugated antibodies. Vertical and horizontal axes represent logarithm of fluorescence intensity, and the percentages of cells in each region are indicated.
In vitro activation of the patient's CD4+ T cells with high concentrations of PHA
The presence of memory and activated CD4+ T cells in this ZAP-70-deficient patient suggested the presence of an alternate signalling pathway that bypassed ZAP-70 in vivo and activated these ZAP-70-deficient CD4+ T cells. We stimulated the patient's PBMC with higher concentrations of PHA as shown in Fig. 6. Normal PBMC proliferated well between 1 μg/ml and 10 μg/ml. However, although the patient's PBMC could not respond to 1 μg/ml PHA, as reported previously, they showed considerable proliferation upon stimulation with 10 μg/ml PHA. These results indicate that some signalling pathway(s) compensates for the lack of ZAP-70 in this patient's CD4+ T cells.
Fig. 6.
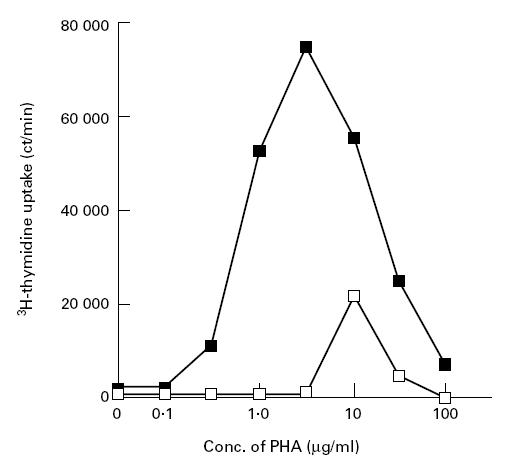
Proliferative responses of control's (▪) and patient's (□) peripheral blood mononuclear cells (PBMC) to various concentrations of phytohaemagglutinin (PHA). Data represent the mean of triplicate determinations. S.d. values are within 10% of each data point.
DISCUSSION
In the present study we have demonstrated the presence of activated and memory CD4+ T cells in the peripheral blood of a CD8-deficient SCID patient. Immunohistological examination of a skin lesion revealed dense infiltration of CD4+ T cells, which had characteristics of memory and activated CD4+ T cells. Furthermore, three-colour flow cytometric analysis of this patient's peripheral lymphocytes showed many CD45RO+CD4+ T cells expressing activation markers, such as HLA-DR and CD25. Although similar characteristics of peripheral CD4+ T cells are reported in CD4+ T cells of X-linked combined immunodeficiency (XCID) patients who have defects in the signalling pathway through common cytokine receptor γ-chain (γc) to Jak3 [10,11], their CD4+ T cells had a signalling pathway through TCR, which was considered to be normal. However, a similar phenotype of CD4+ T cells was observed in the CD8-deficient patient lacking ZAP-70, which is currently considered to be essential in signal transduction via TCR.
These activated and memory CD4+ T cells selectively infiltrated the skin lesions of this patient. The patient's deceased sister, who also lacked peripheral CD8+ T cells and had CD4+ T cells unresponsive to mitogen (data not shown), presented with similar skin lesions. These skin lesions were present from birth and refractory to corticosteroid ointment or antibiotics, and did not increase in size or extend for > 1 year, while the patient remained in good condition. We could not detect any significant pathogenic organisms including bacteria, herpesvirus or Epstein–Barr virus (EBV) by culture or polymerase chain reaction (PCR) methods (data not shown). His serum IgE level was slightly elevated for his age and there was severe eosinophilia. However, the clinical course and the histological findings differed from that of atopic dermatitis or eosinophilic cellulitis. Furthermore, although about half of the patient's peripheral CD4+ T cells were CD45RO+CD45RA− (memory type), we could not detect the female phenotype PBMC by FISH analysis. These results exclude graft-versus-host disease by transplanted maternal T cells, which has been reported in SCID patients [12,13]. The skin lesions of CD8-deficient patients have not been described precisely [6], but these findings, taken collectively, suggest that these skin lesions in this patient might be one characteristic of CD8 deficiency and that these activated and memory CD4+ T cells might have a role in the pathogenesis of such skin lesions. Similar infiltration of activated and memory CD4+ T cells with a functional defect was also reported in the inflammatory colon of IL-2 receptor α-chain targeted mice [14], but our patient did not present any signs or symptoms suggesting the infiltration of CD4+ T cells into other organs.
Although not shown, we examined the patient's peripheral CD4+ T cells for the expression of a variant type of sialyl Lewis X antigen defined by 2F3 MoAb, which was strongly expressed on skin-infiltrating leukaemia cells and is suggested to be a skin-specific adhesion molecule [15]. However, the patient's CD4+ or CD4+CD45RO+ T cells expressed a comparable amount of the antigen to those of a normal control's CD4+ T cells, suggesting that the memory and activated CD4+ T cells utilize adhesion molecules other than 2F3-defined antigen for the skin infiltration.
Recent studies using gene targeting in mice have also revealed that many activated and memory CD4+ T cells accumulated in the spleen of Jak3 or γc targeted mice [16–18]. These results, including the reports of XCID patients [10,11], demonstrated that Jak3 and γc are constitutively required to regulate the fate of activated CD4+ T cells and control lymphoid homeostasis. The accumulation of activated memory CD4+ T cells in the periphery might be a characteristic of CD4+ T cells incapable of passing through a normal cell cycle after activation because of functional deficiencies. Once T cells are activated, it may be necessary to pass through the entire cell cycle including cell division to return to the resting phenotype. Furthermore, previous studies showed that many self-reactive cells are found among activated memory CD4+ T cells in the spleen of these targeted mice, and that the signalling function of the γc and Jak3 system is essential for the negative selection of self-reactive CD4+ T cells [17,18].
Many studies have demonstrated that signals via the TCR are necessary for the differentiation of CD4+ T cells in the thymus. However, it remains to be clarified how CD4+ T cells of ZAP-70-deficient patients can differentiate in the absence of a normal signalling pathway via TCR, which normally involves ZAP-70. Recently, Gelfand et al. reported that the TCRs on thymocytes, but not peripheral T cells, from a ZAP-70-deficient patient were able to transduce signals induced by anti-CD3 antibody [19]. They also found that the Syk kinase homologous to ZAP-70 was present at high levels and was phosphorylated after TCR stimulation in a thymocyte line derived from the patient, suggesting that Syk compensates for the loss of ZAP-70 and accounts for the partial thymic selection of CD4+ T cells. Indeed, Syk can reconstitute the development of CD4 and CD8 T cells in the periphery of ZAP-70−/− mice, which expressed Syk as a transgene [20]. However, even though such alternate signal transduction via TCR in this patient's thymocytes barely reached a threshold leading to the CD4 but not the CD8 lineage selection, it was apparently insufficient to eliminate self-reactive T cells. Thus, self-reactive CD4+ T cells, which escape from negative selection, may be released from the thymus and activated in peripheral organs as observed in γc or Jak3 targeted mice [15,16]. Although the mechanism which activates these ZAP-70-deficient CD4+ T cells in the periphery is still unknown, we found that a high concentration of PHA could activate these CD4+ T cells (Fig. 6), suggesting that an alternate pathway, which bypasses ZAP-70, activates ZAP-70-deficient CD4+ T cells in vivo. Cells migrating from the thymus may initially express a considerable level of Syk which enables them to respond to strong cross-linking of surface TCR molecules by self-antigens in the periphery. A high density of self-antigens and an array of costimulatory molecules on antigen-presenting cells (APC) in the periphery may account for the stimulation of self-reactive cells among the ZAP-70-deficient CD4+ T cells. After acquiring the memory phenotype, these ZAP-70-deficient CD4+ T cells might be activated more easily, because TCR-mediated signalling in memory CD4+ T cells is less dependent on ZAP-70 than in naive CD4+ T cells [21].
We report here that there are many activated and memory CD4+ T cells in the periphery of a ZAP-70-deficient SCID patient and that they are selectively infiltrated in his skin lesions. These results suggest that there is an alternate pathway, which bypasses ZAP-70 and activates T cells, and these activated and memory CD4+ T cells might play some role in the pathogenesis of this patient's unique skin lesions, and they might be self-reactive. Further analysis will elucidate the molecular mechanism of activation of peripheral CD4+ T cells in ZAP-70-deficient patients and their roles in the pathogenesis of the skin lesions.
REFERENCES
- 1.Roifman CM, Hummel D, Martinez-Valdez H, Thorner P, Doherty PJ, Pan S, Cohen F, Cohen A. Depletion of CD8+ cells in human thymic medulla results in selective immune deficiency. J Exp Med. 1989;170:2177–82. doi: 10.1084/jem.170.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase associates with the TCR ζ chain. Cell. 1992;71:649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 3.Elder ME, Dong L, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–99. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 4.Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo W-L, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 5.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–58. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 6.Monafo WJ, Polmar SH, Neudorf S, Mather A, Filipovich AH. A hereditary immunodeficiency characterized by CD8+ T lymphocyte deficiency and impaired lymphocyte activation. Clin Exp Immunol. 1992;90:390–3. doi: 10.1111/j.1365-2249.1992.tb05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negishi I, Motoyama N, Nakayama K-I, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–8. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 8.Tai G, Jeung E-Y, Horiguchi Y, Kawai M, Heike T, Katamura K, Furusho K, Mayumi M. Different effects of cyclic AMP and butyrate on eosinophilic differentiation, apoptosis and bcl-2 expression of a human eosinophilic leukemia cell line, EoL-1. Hematol Oncol. 1996;14:181–92. doi: 10.1002/(SICI)1099-1069(199612)14:4<181::AID-HON589>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Hsu S-M, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and anlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 10.Brooks EG, Schmalstieg FC, Wirt DP, et al. A novel X-linked combined immunodeficiency disease. J Clin Invest. 1990;86:1623–31. doi: 10.1172/JCI114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell SM, Johnston JA, Noguchi M, et al. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–45. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 12.Flomenberg N, Dupont B, O'Reilly RJ, Hayward A, Pollack MS. The use of T cell culture techniques to establish the presence of an intrauterine-derived maternal T cell graft in a patient with severe combined immunodeficiency (SCID) Transplantation. 1983;36:733–5. [PubMed] [Google Scholar]
- 13.Geha RS, Rheinherz E. Identification of circulating maternal T and B lymphocytes in uncomplicated severe combined immunodeficiency by HLA-typing of subpopulations of T cells separated by the fluorescence-activated cell sorter and of Epstein–Barr virus-derived B cell lines. J Immunol. 1983;130:2493–5. [PubMed] [Google Scholar]
- 14.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa Y, Tara M, Ohmori K, Kannagi R. Variant type of sialyl Lewis X antigen expressed on adult T cell leukemia cells is associated with skin involvement. Cancer Res. 1994;54:6533–8. [PubMed] [Google Scholar]
- 16.Thomis D, Berg LJ. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J Exp Med. 1997;185:197–206. doi: 10.1084/jem.185.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor γ chain plays an essential role in regulating lymphoid homeostasis. J Exp Med. 1997;185:189–95. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saijo K, Park SY, Ishida Y, Arase H, Saito T. Crucial role of Jak3 in negative selection of self-reactive T cells. J Exp Med. 1997;185:351–6. doi: 10.1084/jem.185.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfand EW, Weinberg K, Mazer BD, Kadlecek TA, Weiss A. Absence of ZAP-70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J Exp Med. 1995;182:1057–66. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Q, White L, Johnson R, White M, Negishi I, Thomas M, Chan AC. Restoration of thymocyte development and function in zap-70 −/− mice by the Syk protein tyrosine kinase. Immunity. 1997;7:369–77. doi: 10.1016/s1074-7613(00)80358-1. [DOI] [PubMed] [Google Scholar]
- 21.Farber DL, Acuto O, Bottomly K. Differential T cell receptor-mediated signaling in naive and memory CD4 T cells. Eur J Immunol. 1997;27:2094–101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]


