Abstract
Ws/Ws rats have a small deletion of the c-kit gene, and are deficient in both mucosal and connective tissue-type mast cells. In this study, the role of mucosal type mast cells (MMC) in the development of intestinal ischaemia-reperfusion injury was investigated in Ws/Ws rats. Autoperfused segments of the jejunum were exposed to 60 min of ischaemia, followed by reperfusion for various time periods. The epithelial permeability was then assessed by the 51Cr-EDTA clearance rate. In the control (+/+) rats, the maximal increase in mucosal permeability was achieved at 45 min of reperfusion. In contrast, this increase was significantly and potently attenuated in the Ws/Ws rats. Mucosal alkaline phosphatase activity decreased in the control (+/+) rats, but was not altered in the Ws/Ws rats. There were no differences in mucosal myeloperoxidase activity, indicating that granulocytes did not contribute to tissue injury. These results provide direct evidence for the role of mast cells in the pathogenesis of intestinal ischaemia-reperfusion injury.
Keywords: mucosal mast cell, RMCP-II, permeability
INTRODUCTION
Ischaemia-reperfusion (I/R) of the small bowel is associated with both increased microvascular permeability and mucosal barrier dysfunction, and results in systemic shock under clinical and experimental conditions. Recent studies have indicated that the mucosal and vascular dysfunction induced by hypoxia-reoxygenation damage is associated with the release of toxic factors into the systemic circulation, thus leading to acute circulatory collapse [1–4]. However, the mechanisms mediating this mucosal barrier dysfunction after reperfusion are not fully understood.
There are at least two types of mast cells; the mucosal type of mast cells (MMC) are found primarily in the mucosa, whereas the connective tissue-type of mast cells are found in the submucosa and muscularis [5]. The small intestine contains MMC at a high density and at close proximity to the microvasculature of the villus [6]. These MMC are the local source of large amounts of vasoactive mediators postulated to participate in the pathogenesis of I/R-induced tissue injury [1–4]. The purpose of the present study was to elucidate the role of MMC activation in the induction of rapid mucosal barrier dysfunction after I/R treatment. This was mainly evaluated by experiments using genetically mast cell-deficient Ws/Ws (white spotting in the skin) rats [7–9]. Ws/Ws rats have a 12-base deletion in the tyrosine kinase domain of the c-kit cDNA and are deficient in both connective tissue type and mucosal type mast cells. These rats are useful tools for studying the role of mast cells in the pathogenesis of intestinal I/R injury.
MATERIALS AND METHODS
Surgical preparation
Ws/Ws and control (+/+) rats were purchased from Japan SLC, Inc. (Shizuoka, Japan), and these animals were checked as free of parasites. The origin of Ws/Ws rats has been described in detail [7, 8]. Experiments were performed on male Ws/Ws or control (+/+) rats (200–250 g) anaesthetized with sodium pentobarbital (Nakalai, Kyoto, Japan). The superior mesenteric artery was isolated and a 12–15-cm loop of jejunum was externalized; the blood vessels remained intact. The jejunal loop was fitted with inflow and outflow tubes to allow the perfusion of warm tyroid solution at a rate of 0.4 ml/min. Jejunal ischaemia was subsequently induced by clamping the superior mesenteric artery for 60 min, after which the clamp was removed and then reperfusion was monitored for up to 120 min. Sham-operated rats were treated in an identical fashion with the omission of vascular occlusion.
Measurement of epithelial permeability
51Cr-EDTA was injected via the jugular vein and allowed to equilibrate. The luminal perfusate was collected during both ischaemia and reperfusion. Plasma samples were also collected. The plasma-to-lumen clearance of 51Cr-EDTA was calculated as described by Kanwar & Kubes [10]:
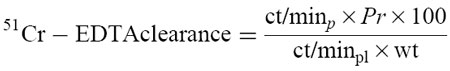 |
where the clearance of 51Cr-EDTA is expressed as ml/min per 100 g, ct/minp is the ct/min per ml of the luminal perfusate, Pr is the perfusate rate (ml/min), ct/minpl is the counts per ml of plasma, and wt is the weight of the intestinal segment (g). This 51Cr-EDTA clearance was calculated every 15 min.
Alkaline phosphatase and myeloperoxidase activity
Mucosal scrapings were homogenized and used as samples. The degree of mucosal damage was estimated by the decrease in mucosal alkaline phosphatase (ALP) activity determined by Kind & King's method [11–13]. Intestinal mucosal myeloperoxidase (MPO) activity, a biochemical marker of MPO-positive granulocytes, was measured using a standard assay as previously described [14, 15].
Histological techniques
The specimens were immediately fixed in 10% formaldehyde-saline solution, followed by sectioning and haematoxylin–eosin staining. For the immunohistological detection of MMC, samples were reacted with a polyclonal rabbit anti-rat mast cell protease II (RMCP II; Moredum Animal Health, Edinburgh, UK) antibody, followed by reaction with immunoperoxidase-streptavidin-biotin complex system (Dako, Glostrup, Denmark). RMCP II is regarded as a specific marker of mucosal mast cells [10, 16, 17]. The number of MMC per 100 villi per animal was counted by microscopic examination for the evaluation of the degranulation of MMC.
Statistical analysis
Data are presented as means ± s.d. Statistical analysis was performed by the unpaired Student's t-test. Statistical significance was accepted at P < 0.05.
RESULTS
The mucosal permeability, as determined by 51Cr-EDTA clearance rate, was assessed for 120 min (Fig. 1). Sixty minutes of ischaemia alone did not induce any significant changes. In the control (+/+) rats there was a rapid and significant increase in mucosal permeability within 15 min of reperfusion. This change reached a maximum as early as 45 min after the start of reperfusion, and was sustained for approx. 120 min. However, these responses were potently and significantly attenuated in Ws/Ws rats (P < 0.01). This indicates that mast cell activation is closely associated with the development of the rapid mucosal injury induced by I/R treatment.
Fig 1.
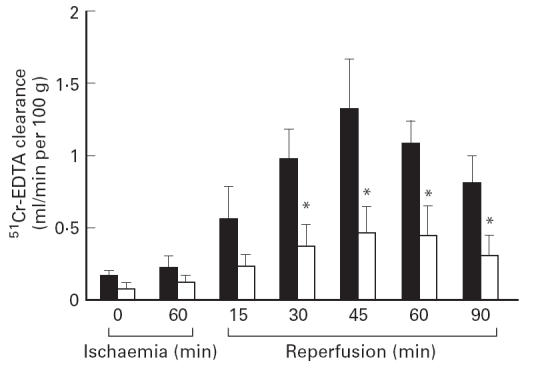
Changes in epithelial permeability induced by ischaemia-reperfusion (I/R) treatment in control (+/+) rats (▪) and Ws/Ws rats (□). Data are expressed as mean ± s.d. (n = 5 per group). *P < 0.01 versus value of respective control (+/+) rats.
The mucosal damage induced by 45 min of reperfusion was assessed histologically by light microscopy. As demonstrate in Fig. 2, in the control (+/+) rats, the I/R treatment induced severe mucosal destruction, as characterized by a disruption of the villus tip, a loss of villus height, dilated capillaries with haemorrhage and ulceration. On the other hand, these changes were completely abolished in the Ws/Ws rats; there was a residual subepithelial space at the villus tip, with mild lifting of the epithelium.
Fig 2.
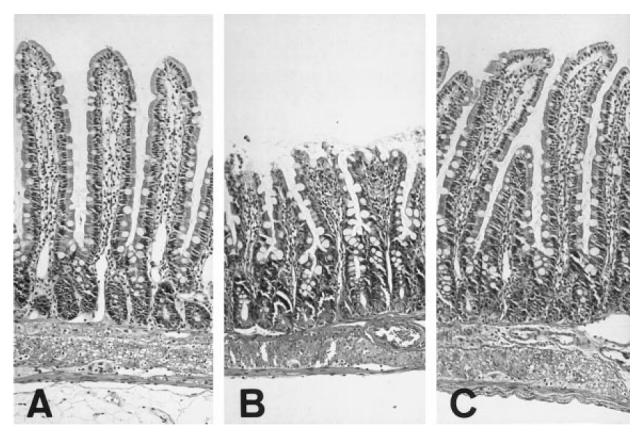
Light micrographs of H–E-stained section of rat intestinal mucosa after ischaemia-reperfusion (I/R) treatment. (A) Sham operation. (B) Control (+/+) rats. (C) Ws/Ws rats. (Mag. × 100.)
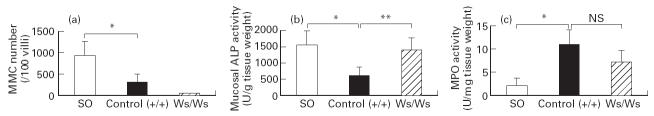
As shown in Fig. 3a, the I/R treatment induced a significant decrease in the number of immunoreactive MMC in the control (+/+) rats. This indicates that the degranulation of the MMC was closely associated with the development of intestinal I/R injury. In the Ws/Ws rats there were no MMC detected in the mucosa. Next, we evaluated mucosal ALP activity as an indicator of the mass of the intact epithelium (Fig. 3b). Mucosal ALP is a brush border membrane enzyme and so represents the total integrity of epithelial cells. The mucosal ALP activity at 45 min of reperfusion was significantly decreased in the control (+/+) rats, but remained unaffected in the Ws/Ws rats. This indicates that the mucosal damage was more severe in the control (+/+) rats compared with Ws/Ws rats. To evaluate the effects of infiltrating granulocytes, we assessed mucosal MPO activity (Fig. 3c). In contrast to the above results, no significant differences in MPO activity could be detected, thus indicating that granulocyte infiltration has little or no effect on the development of rapid I/R-induced mucosal injury.
Fig 3.
Changes in the number of mucosal mast cells (a), mucosal alkaline phosphatase (ALP) activity (b), and myeloperoxidase (MPO) activity (c). Data are expressed as mean ± s.d. (n = 5 per group). *P < 0.01; **P < 0.05. NS, Not significant; SO, sham operation.
DISCUSSION
An intact mucosal barrier is important in allowing for nutrient absorption while preventing bacterial translocation and other toxic antigens into the systemic circulation. It is well recognized that a disruption of the mucosal barrier causes sepsis, thus leading to multiple organ failure. Ischaemia and reperfusion of the small bowel has been previously shown to induce both microvascular and mucosal dysfunction [18, 19]. The prevention of intestinal I/R-induced mucosal injury would be a great advance in the prognosis of small bowel transplantation in the future. Thus, it is important to understand the mechanisms mediating the development of intestinal I/R-induced mucosal injury. The objective of this study was to investigate the potential role of mast cell activation in the pathogenesis of rapid (30–45 min of reperfusion) intestinal I/R injury. Our approach was to compare the response of genetically mast cell-deficient Ws/Ws rats with control (+/+) rats.
This increase in I/R-induced epithelial permeability has been extensively studied; it is now believed that mast cell activation leads to the release of large quantities of chemical mediators, including histamine [1, 2], leukotrienes [4], and platelet-activating factor (PAF) [3], which have been postulated to play important functions in acute and chronic tissue injury. The mast cells, in turn, seem to be one of the more important mediators of I/R-induced microvascular and epithelial dysfunction in the small bowel. In a previous report by Kanwar et al. [10], the administration of a mast cell stabilizer, which blocked mast cell degranulation and inhibited chemical mediator release, prevented I/R-induced mucosal damage of the small bowel. However, it remains unclear whether the mast cell stabilizer actually modulated mast cell function alone, or exerted other effects. Thus, there has been some evidence implicating mast cell activation in I/R-induced mucosal injury [10, 16], but this has not been resolved completely. Furthermore, whether mast cell activation and degranulation represents the primary event remains controversial.
The kinetics of epithelial permeability changes in the control (+/+) rats was similar to previous reports [10, 16], where the maximum increase was observed at 30–45 min after the start of the reperfusion. In the Ws/Ws rats, this increase in mucosal permeability was markedly and significantly attenuated during the entire reperfusion period, compared with control (+/+) rats, thus indicating that mast cells play a critical role in the development of intestinal I/R-induced mucosal injury. These responses in terms of epithelial permeability were also evaluated histologically. In the control (+/+) rats, the I/R treatment induced severe histological damage in the mucosa, where the number of RMCP II-positive MMC had decreased significantly. This indicates that the MMC degranulation was closely associated with the development of I/R-induced mucosal injury in the control (+/+) rats. On the other hand, this histological damage was completely abolished in the Ws/Ws rats. In addition, tissue ALP activity was maintained in the Ws/Ws rats, but was significantly decreased in the control (+/+) rats. Since tissue ALP activity represents the mass of the intact epithelium [12, 13], this finding indicates that mucosal damage was greater in the control (+/+) rats than in the Ws/Ws rats. In previous reports [10, 13, 20], it has been demonstrated that infiltrating granulocytes participate in the pathogenesis of intestinal I/R-induced mucosal injury. However, we could not detect such evidence in our model; there was no difference in mucosal MPO activity, a biochemical marker of MPO-positive granulocytes, between the control (+/+) and Ws/Ws rats. Our results are consistent with other reports on hepatic I/R injury [19, 20]. They demonstrate that early granulocyte infiltration has no effect on hepatic I/R injury, because the cells were not yet fully activated. However, after 5–6 h of reperfusion, the granulocytes can contribute to the post-oxidant stress and tissue injury. In our model, it is likely that the 30–45 min of reperfusion was too short to induce full infiltration and activation of the granulocytes [21, 22]. These findings support a major role for mast cells, but not granulocytes, in the early development of intestinal I/R mucosal injury.
In this study, we clearly demonstrate that MMC are important contributors to the pathogenesis of rapid I/R-induced mucosal barrier dysfunction. However, the precise mechanism inducing the MMC activation remains unclear. One possible mechanism is that an increased flux of oxidants, which has been previously described at the onset of reperfusion, may be responsible for the mast cell activation [23]. Indeed, it has been reported that the administration of anti-oxidants can block the histamine release from the post-ischaemic gut [24, 25], and that this process may contribute to the pathogenesis of I/R injury. As another possibility, we have recently demonstrated an important role for complement activation and the subsequent release of anaphylatoxin; a blockade of complement activation significantly attenuated both MMC degranulation and the increase in I/R-induced epithelial permeability [16]. Based on these observations, it is conceivable that several processes may interact with each other in sequential order to induce MMC activation.
In conclusion, this study provides direct evidence for the critical role of MMC activation in the cascade of events leading to I/R-induced mucosal damage. Our results would suggest that a regulation of the MMC activation may be one of the clinical strategies for prevention of intestinal I/R injury.
References
- 1.Boros M, Kaszaki J, Nagy S. Histamine release during intestinal ischemia-reperfusion: role of irons and hydrogen peroxide. Circ Shock. 1991;35:174–80. [PubMed] [Google Scholar]
- 2.Boros M, Kaszaki J, Nagy S. Oxygen free radical induced histamine release during intestinal ischemia and reperfusion. Eur Surg Res. 1989;21:297–304. doi: 10.1159/000129042. [DOI] [PubMed] [Google Scholar]
- 3.Kubes P, Ibbotson G, Russell JM, et al. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol. 1990;259:G300–g5. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- 4.Lehr HA, Guhlmann A, Nolte D, et al. Leukotrienes as mediators in ischemia-reperfusion injury in a microcirculation model in the hamster. J Clin Invest. 1991;87:2036–41. doi: 10.1172/JCI115233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe SE, Perdue MH. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992;103:1075–95. doi: 10.1016/0016-5085(92)90047-3. [DOI] [PubMed] [Google Scholar]
- 6.Bacci S, Faussone-Pellegrini MS, Mayer B, et al. Distribution of mast cells in human ileocecal region. Dig Dis Sci. 1995;40:357–65. doi: 10.1007/BF02065422. [DOI] [PubMed] [Google Scholar]
- 7.Niwa Y, Kasugai T, Ohno K, et al. Anemia and mast cell depletion in mutant rats that are homozygous at ‘White Spotting (Ws)’ locus. Blood. 1991;78:1936–41. [PubMed] [Google Scholar]
- 8.Tsujimura T, Hirota T, Nomura S, et al. Characterization of Ws mutant allele of rats: a 12-base deletion in tyrosine kinase domain of c-kit gene. Blood. 1991;78:1942–6. [PubMed] [Google Scholar]
- 9.Tei H, Kasugai T, Tsujimura T, et al. Characterization of cultured mast cells derived from Ws/Ws mast cell-deficient rats with a small deletion at tyrosine kinase domain of c-kit. Blood. 1994;83:916–25. [PubMed] [Google Scholar]
- 10.Kanwar S, Kubes P. Mast cells contribute to ischemia-reperfusion-induced granulocyte infiltration and intestinal dysfunction. Am J Physiol. 1994;267:G316–21. doi: 10.1152/ajpgi.1994.267.2.G316. [DOI] [PubMed] [Google Scholar]
- 11.Kind PRN, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudeja PK, Brasitus TA. Inactivation of rat intestinal brush-border membrane alkaline phosphatase by oxygen free radicals. Gastroenterology. 1993;105:357–66. doi: 10.1016/0016-5085(93)90708-k. [DOI] [PubMed] [Google Scholar]
- 13.Sisley AC, Desai T, Harig JM, et al. Neutrophil depletion attenuates human intestinal reperfusion injury. J Surg Res. 1994;57:192–6. doi: 10.1006/jsre.1994.1130. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar S, Wallace JL, Befus D, et al. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222–9. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- 15.Kube P, Grisham MB, Barrowman JA, et al. Leukocyte-induced vascular protein leakage in cat mesentery. Am J Physiol. 1991;261:H1872–9. doi: 10.1152/ajpheart.1991.261.6.H1872. [DOI] [PubMed] [Google Scholar]
- 16.Kimura T, Andoh A, Fujiyama Y, et al. A blockade of complement activation prevents rapid intestinal ischaemia-reperfusion injury by modulating mucosal mast cell degranulation in rats. Clin Exp Immunol. 1998;111:484–90. doi: 10.1046/j.1365-2249.1998.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kido H, Fukusen N, Katsunuma N. Chymotrypsin- and trypsin-type serine proteases in rat mast cells: properties and functions. Arch Biochem Biophys. 1985;239:436–43. doi: 10.1016/0003-9861(85)90709-x. [DOI] [PubMed] [Google Scholar]
- 18.Kubes P, Hunter J, Granger N. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology. 1992;103:807–12. doi: 10.1016/0016-5085(92)90010-v. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H, Farhood A. Neutrophil and Kupper cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–62. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H, Bautisca AP, Spolarics Z, et al. Superoxide generation by Kupper cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–84. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H, Bautisca AP, Spolarics Z, et al. Superoxide generation by neutrophils and Kupper cells during in vitro reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–82. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vitro. FASEB J. 1990;4:3355–9. [PubMed] [Google Scholar]
- 23.Blum H, Summers JJ, Schnall MD, et al. Acute intestinal ischemia studies by phosphorous nuclear magnetic resonance spectroscopy. Ann Surg. 1986;204:83–8. doi: 10.1097/00000658-198607000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boros M, Kaszaki J, Nagy S. Oxygen free radical-induced histamine release during intestinal ischemia and reperfusion. Eur Surg Res. 1989;21:297–304. doi: 10.1159/000129042. [DOI] [PubMed] [Google Scholar]
- 25.Boros M, Kaszaki J, Nagy S. Histamine release during intestinal ischemia-reperfusion: role of iron ions and hydrogen peroxide. Circ Shock. 1991;35:174–80. [PubMed] [Google Scholar]