Abstract
IL-12 is a cytokine that induces Th1-derived cytokines (interferon-gamma (IFN-γ) and IL-2). The significance of IL-12 in human autoimmunity is no clear, and the serum levels of IL-12 in SLE are not clearly established. Therefore, we examined the levels of IL-12 in 39 patients with active SLE, with sandwich ELISA. The levels of IL-12 in patients were significantly higher than in normal subjects. Patients with high levels of IL-12 also had high levels of IFN-γ, while their levels of IL-13 were significantly lower than in patients with normal levels of IL-12. Patients with pulmonary involvement had high levels of IL-12, and steroid therapy decreased the IL-12 level in three patients. In a retrospective study of seven patients, various changes of IL-12 and IL-13 were recognized before disease flare. Thus, in SLE patients, the level of IL-12 was increased and this increase was related to the change of Th1- or Th2-derived cytokines with some organ involvement.
Keywords: IL-12, IL-13, interferon-gamma, systemic lupus erythematosus
INTRODUCTION
IL-12 is produced mainly by macrophages and dendritic cells, and induces activation of natural killer (NK) cells [1, 2], production of interferon-gamma (IFN-γ) [3] and differentiation of naive T cells to Th1 cells [4]. The role of IL-12 in human autoimmunity is not clearly established, although some reports with mice have shown this result [5].
Previous reports have shown various cytokine abnormalities in SLE sera [6–10]. For example, increased levels of IL-2 [6], IL-6 [7], IFN-γ [8] and IL-10 [9, 10] have been reported in sera. It is believed that these results relate to the activation of T and B cells. However, it is not clear how the increased production of cytokines by activated T or B cells is induced. Helper T cells may be divided into three types (Th0, Th1, Th2) according to the type of cytokine produced [11]. Th1 cells produce IFN-γ and IL-2, and Th2 cells produce IL-4, IL-5, IL-6, IL-10 and IL-13. In the lupus mice model, it was suggested that the balance of Th1/Th2 related to the pathogenesis of SLE [12, 13]. Further, Th1-derived cytokines and Th2-derived cytokines are regulated differently. In brief, Th1-derived cytokines are regulated mainly by IL-12 [4], while Th2-derived cytokines are regulated by IL-4 [14]. Therefore, it is possible that IL-12 is related to an abnormality of Th1-derived cytokines in SLE patients.
In this study, we determined the levels of IL-12 in active lupus patients, and related these results to levels of other cytokines and clinical manifestations.
PATIENTS AND METHODS
Patients
Serum samples were prepared from 39 patients with SLE (three males and 36 females, aged 23–37 years) and 12 normal subjects matched for age and sex. All patients fulfilled the American College of Rheumatism (ACR) criteria for SLE [15]. All patients had active SLE indicated by having more than 8 points on the SLE disease activity index (SLEDAI) [16]. Twenty-one patients did not receive any steroid, and other patients received < 10 mg/day for < 6 months.
Serum samples were stored in the deep freeze (–80°C) and thawed overnight before analysis.
Determination of IL-12
The levels of IL-12 were determined using a sandwich ELISA commercial kit (BioSource Int., S.A., Camarillo, CA). In brief, after coating of non-labelling anti-IL-12 antibodies and blocking, 100 μl of undiluted serum and biotinylated anti-IL-12 antibodies, reacting with a different epitope from that recognized by the coating antibody, were added to each microplate well. These two anti-IL-12 antibodies recognized the whole molecule of IL-12. After a 2-h incubation at room temperature, the plates were washed five times and avidin–peroxidase was added. After 30 min incubation at room temperature, the plate was washed five times and o-phenylenediamine (OPD) was added. After 30 min incubation at room temperature, the reaction was terminated with 2.5 m H2SO4. Then, the optical density (OD) (490 nm) was measured on an automated plate reader (Model 3550 UV Microplate Reader; BioRad, Hercules, CA). The levels of IL-12 were determined by comparison with a standard curve obtained using recombinant IL-12. This assay system can measure > 10 pg/ml of IL-12.
Other cytokines
The levels of IL-2, IFN-γ and IL-6 were determined using the sandwich ELISA kit (Otsuka Co. Ltd., Tokushima, Japan). This assay is similar to that of IL-12.
The level of IL-13 was determined using a similar sandwich ELISA. Rat anti-human IL-13 MoAbs (JES10-5A2; PharMingen, San Diego, CA), 5 μg/ml in 0.01 m PBS pH 7.2, were coated onto 96-well microtitre plates (Immulon 2 plates; Dynatech Labs Inc., Chantilly, VA) and incubated at 37°C for 2 h. Blocking was performed with BLOCK ACE (Dai Nihon Seiyaku Co. Ltd, Tokyo, Japan), at 37°C for 2 h. Then, undiluted sera were added and incubated overnight at room temperature. After washing three times with 0.05% Tween/PBS, 5 μg/ml of biotinylated rabbit anti-human IL-13 polyclonal antibodies (PharMingen, San Diego, CA) were added and incubated at room temperature for 1 h. After washing three times, OPD and 0.03% H2O2 in citrate buffer pH 5.0 were added and incubated at room temperature for 30 min. OD (490 nm) was measured, and the level of IL-13 was determined by comparison with the standard curve obtained using recombinant IL-13.
Statistical analysis
Differences in the levels of cytokines and the correlation among cytokines were assessed using Welch's rank sum test.
RESULTS
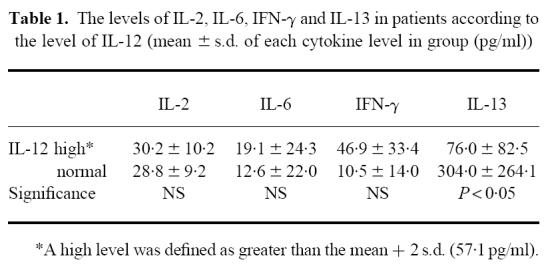
First, we examined the level of IL-12 in active SLE patients. As shown in Fig. 1, the level of IL-12 in active lupus patients was increased significantly compared with normal controls (patients 94.72 ± 91.99 pg/ml, controls 28.1 ± 14.5 pg/ml; P < 0.001). Some patients who received no steroid also had a high level of IL-12. However, since there was wide deviation in the level of IL-12 among the patients, the patients were divided into two groups—patients with a high level of IL-12 and those with a normal level. The high level group was defined as more than the mean value + 2 s.d. (57.1 pg/ml). As to the results, 22 patients (56.41%) had high levels of IL-12. Then, the levels of other cytokines in these two group were examined and compared. As shown in Table 1, the levels of IL-13 in patients with high levels of IL-12 were significantly lower than in patients with normal IL-12 levels (P < 0.05). The levels of IFN-γ in these patients tended to be higher, although this difference was not significant. There was no significant correlation between the levels of IL-2, IL-6 and IL-12. However, there was a reverse correlation between the levels of IL-13 and the levels of IFN-γ.
Fig 1.
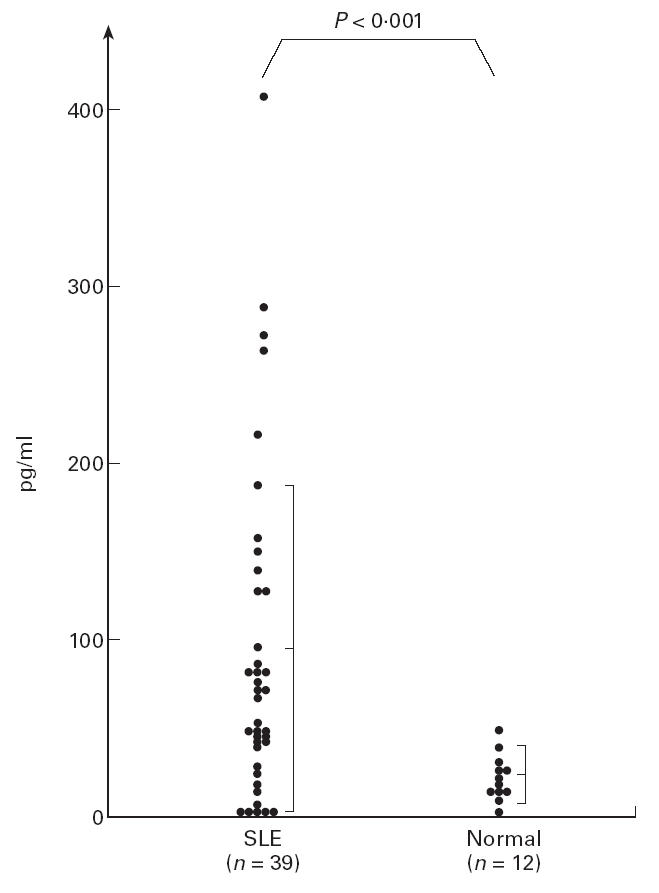
The levels of IL-12 in patients with active SLE and normal subjects. The level in SLE patients increased significantly compared with normal subjects. However, there was wide deviation among the patients.
Table 1.
The levels of IL-2, IL-6, IFN-γ and IL-13 in patients according to the level of IL-12 (mean ± s.d. of each cytokine level in group (pg/ml))
 |
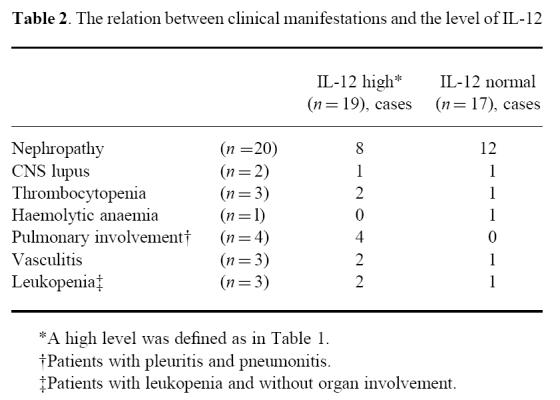
Next, we examined the relation among IL-12 levels and clinical manifestations in these two groups. The results are shown in Table 2. There was no relation to nephropathy, central nervous system (CNS) lupus or thrombocytopenia. Interestingly, however, every patient with pulmonary involvement (three patients with pleuritis and one patient with pneumonitis) had high levels of IL-12. On the other hand, there was no relation between the level of IL-12 and the dose of steroid.
Table 2.
The relation between clinical manifestations and the level of IL-12
 |
Further, the change after therapy was examined in three cases. These three patients received various doses of steroid (case 1, methyl prednisolone 200 mg/day → prednisolone 40 mg/day; case 2, prednisolone 40 mg/day; case 3, prednisolone 30 mg/day), and their disease activity decreased (less than three points of SLEDAI). In these patients, the level of IL-12 was examined closely, and simultaneously the level of IL-13 was examined at the same points, since there was a reverse correlation between the two, as previously described. As shown in Fig. 2, the level of IL-12 in all three cases decreased with steroid therapy and was accompanied by a decrease of IL-13. However, the level of IL-12 did not completely decrease. In one case with follow up of 4 months (case 1), an increase of IL-12 and IL-13 was recognized after 2 months, although the disease activity did not increase.
Fig 2.
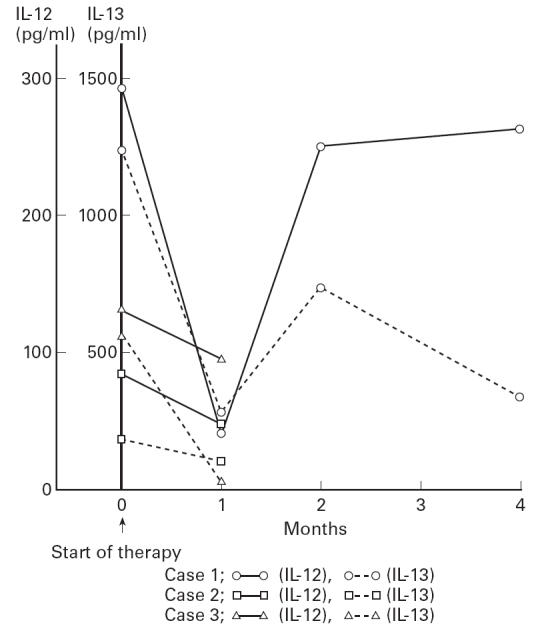
Changes of IL-12 and IL-13 with steroid therapy in three patients. The level of both cytokines decreased with therapy. However, the level of IL-12 never normalized.
Finally, we retrospectively examined the level of IL-12 before disease flare in seven patients. These patients received a maintenance dose of steroid and their disease activity increased. As shown in Fig. 3a, the level of IL-12 gradually increased before disease flare in three patients (cases 1, 2 and 3), while the levels in one patient (case 4) did not change. In the other three patients (cases 5, 6 and 7), levels increased at 1, 2 or 4 months before disease flare but decreased prior to the flare of the disease (Fig. 3b). Then, the levels of IL-13 also increased in three patients (cases 1, 4 and 6), and they showed a different change to IL-12 in three patients (cases 2, 4 and 5) (Fig. 3).
Fig 3.
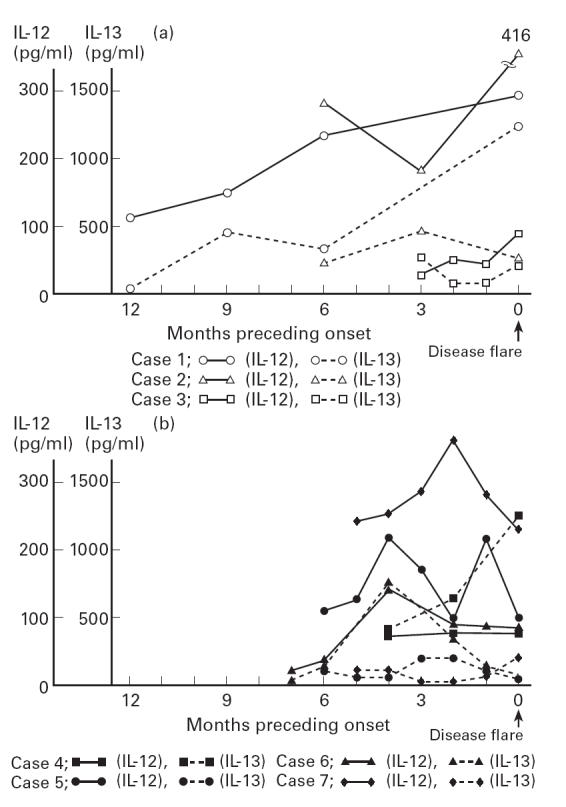
Changes of IL-12 and IL-13 before disease flare in seven patients. The levels of IL-12 in three patients (cases 1–3) increased gradually before the disease flare (a), and those in other patients increased long before flare and decreased prior to flare (b). Although three patients (cases 1, 4 and 6) showed an increase in the level of IL-13, the different change to IL-12 was also shown (cases 2, 4 and 5).
DISCUSSION
The activation of T cells or B cells is often recognized in SLE patients. Previous studies have suggested that the change of various cytokines may relate to this phenomenon. Although the cytokines related to helper T cells are divided into Th1-derived cytokines (IFN-γ, IL-2) and Th2-derived cytokines (IL-4, IL-5, IL-6, IL-10, IL-13), changes of these two cytokines were found in SLE patients [6–10]. Recently, we showed that the change of each cytokine varied among patients (unpublished data), and previous reports showed similar results [6, 7]. Thus, the levels of cytokines in SLE are never simple. Although it is interesting how these cytokines vary, the mechanism of this variation is not clear. Generally, it is believed that both Th1- and Th2-derived cytokines are regulated by other factors. Previous reports have shown the various factors such as cell surface molecules [17, 18], cytokines [4, 11, 19], the characteristics or doses of antigens [20], signal transduction [21], hormones or nervous system [22] and genetic factors [23–25]. IL-4, inducing differentiation to Th2 [23], and IL-12, inducing differentiation to Th1 cells [4], are noted as cytokines regulating Th1- or Th2-derived cytokines. It is possible that IL-4 or IL-12 relate to the variation of Th1 or Th2 cytokines in each SLE patient. Unfortunately, the increased level of IL-4 was not recognized in the previous study [26]. Therefore, we noted IL-12-regulating Th1-derived cytokines and examined these levels in lupus patients, since the previous report stressed the dominance of Th1 cells [12, 26].
The levels of IL-12 in active lupus patients are increased significantly compared with normal subjects. Since all of these patients had active disease and some patients receiving no steroid had high levels of IL-12, this increased IL-12 may reflect some form of manifestation of the disease. However, we should note that there were wide deviations in the levels of IL-12 among the patients. Although this result never related to the technical problem, we should consider the relation to the influence of natural antibody. Indeed, levels of IL-12 were normal in around half of the patients. Therefore, we divided patients into two groups: those with a high level of IL-12 and those with a normal level. Patients with high levels of IL-12 tended to have high levels of IFN-γ. This result suggests that IL-12 induced Th1-derived cytokines in some SLE patients, although there are fewer patients with a high level of Th1-derived cytokines (about 20% of patients with active disease), and most patients had high levels of Th2 cytokines in our recent study (unpublished data).
Furthermore, all patients with pulmonary involvement had a high level of IL-12, although the number of cases was small. In SLE, pulmonary involvement, including pleuritis and pneumonitis, are less frequent and patients with this involvement generally do not have renal involvement, which is the main organ involvement of SLE. Thus, patients with high levels of IL-12 have less frequent organ involvement in SLE, and our results suggest that the increased IL-12 levels and induction of Th1-derived cytokines relate to the pulmonary involvement. Further, the levels of IL-13 were significantly lower in patients with higher levels of IL-12. Although we also examined some Th2-derived cytokines (IL-4, IL-6, IL-10, IL-13), a significant increase was recognized only in IL-6 and IL-13, thus these two cytokines were used in the analysis.
Among Th2-derived cytokines, only IL-10 has been reported to inhibit IL-12 [27], and IL-12 is a strong inducer of IL-10 production [28, 29]. However, unfortunately only a few patients examined had high levels of IL-10 and we could not examine the relation between the levels of IL-10 and IL-12.
IL-13 has never been reported to inhibit IL-12 in vitro, although it has been reported that IL-13 inhibits tumour necrotizing factor (TNF), IL-1, IL-6, IL-8 and IL-10 [30, 31]. It is also reported that IL-13 induces the production of IL-12 and IFN-γ, and had effects different from those of other Th2-derived cytokines [30]. Therefore, this reverse relation between IL-12 and IL-13 can not be linked simply to the possibility that IL-12 inhibits IL-13, since this reverse relation was never recognized in all patients.
We believe that it is difficult to relate directly the increase of IL-12 in this study to the pathogenesis of SLE, since this change is not recognized in every patient, and we can not determine the origin of the elevated IL-12. However, it is possible that this increased IL-12 is indirectly related to the pathogenesis of SLE. Indeed, it is reported that the administration of anti-IL-12 antibody prolonged the onset of disease in the lupus mouse [32]. Thus, IL-12 may control the onset of the disease. We would like to note that the following results may have relevance to this possibility. First, as previously described, we demonstrated that the level of IL-12 had been increased long before the flaring of disease activity in this retrospective study of some patients, although the study of cytokines before onset is difficult to establish and changes before disease onset are not known in human SLE. However, since other patients decreased after the increase or remained unchanged, we believe that the change of IL-12 before flaring is not simply explained, and that the role of this cytokine varies. Second, although the level of IL-12 decreased with steroid therapy, the level never normalized. While most other activation markers (e.g. soluble IL-2 receptors) and cytokines (IL-6, IL-2) normalized (data not shown), the IL-12 level remained unchanged. This result suggests that several characteristics of IL-12 in SLE differ from those of other cytokines.
Although the effect of anti-IL-12 antibody has been demonstrated in the lupus mouse [32], we believe that the application of this antibody to use in humans is difficult, since the abnormality of IL-12 was not found in every patient and the level of IL-12 increases long before disease flare, as previously described. However, we believe that the importance of IL-12 in SLE can not be ignored and that we should seriously consider the influence of this cytokine.
References
- 1.Chehimi J, Valiante NM, D'Andrea A, et al. Enhancing effect of natural killer cell stimulatory factor (NKSF/interleukin-12) on cell-mediated cytotoxicity against tumor-derived and virus-infected. Eur J Immunol. 1993;23:1826–30. doi: 10.1002/eji.1830230814. [DOI] [PubMed] [Google Scholar]
- 2.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang F-P, Feng G-J, Lindop G, Stott DI, Liew FY. The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. J Exp Med. 1996;183:1447–59. doi: 10.1084/jem.183.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y-P, Perrin LH, Miescher PA. Correlation of T and B cell activities in vitro and serum IL-2 levels in systemic lupus erythematosus. J Immuno1. 1988;141:827–33. [PubMed] [Google Scholar]
- 7.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, OzeriChen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. J Immunol. 1991;147:117–23. [PubMed] [Google Scholar]
- 8.Al-Janadi M, Al-Balla S, Al-Dalaan A, Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67. doi: 10.1007/BF00920636. [DOI] [PubMed] [Google Scholar]
- 9.Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Fourrier BM, Galanaud P, Emilie D. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 10.Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer J-P, Renauld J-C. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Coffman RL. Thl and Th2 cells: different patterns of lymphokine secretion lead to different function properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Isui S. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J Clin Invest. 1996;97:1597–604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshii H, Yamamoto K, Okudaira H, et al. Age-related differential mRNA expression of T cell cytokines in NZB/NZW F1 mice. Lupus. 1995;4:213–6. doi: 10.1177/096120339500400309. [DOI] [PubMed] [Google Scholar]
- 14.Gros GL, Ben-Sasson SZ, Seder R, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL- 4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 17.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 18.Stüber E, Strober W, Neurath M. Blocking the CD40L–CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–8. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12: a cytokine produced by antigen- presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 20.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor αβ transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura T, Nakano H, Nagase H, Morokata T, Igarashi O, Oshimi Y, Miyazaki S, Nariuchi H. Early activation signal transduction pathways of Th1 and Th2 cell clones stimulated with anti-CD3. J Immunol. 1995;155:4692–701. [PubMed] [Google Scholar]
- 22.Rook GAW, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;15:301–3. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 23.Shankar AH, Titus RG. T cell and non-T cell compartments can independently determine resistance to Leishmania major. J Exp Med. 1995;181:845–55. doi: 10.1084/jem.181.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeiffer C, Murray J, Madri J, Bottomly K. Selective activation of Th1- and Th2-like cells in vivo—response to human collagen IV. Immunol Rev. 1991;123:68–84. doi: 10.1111/j.1600-065x.1991.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 25.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz DA, Wang H, Gray JD. Cytokine gene profile in circulating blood mononuclear cells from patients with systemic lupus erythematosus: increased interleukin-2 but interleukin-4 m-RNA. Lupus. 1994;3:423–8. doi: 10.1177/096120339400300511. [DOI] [PubMed] [Google Scholar]
- 27.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–82. [PubMed] [Google Scholar]
- 29.Jeannin P, Delneste Y, Seveso M, Life P, Bonnefoy J-Y. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996;156:3159–65. [PubMed] [Google Scholar]
- 30.Minty A, Chalon P, Derocq J-M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 31.Malefyt RW, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocyte. Comparison with IL-4 and modulation by IFN-γ or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 32.Nakajima A, Hirose S, Yagita H, Okumura K. Roles of IL-4 and IL-12 in the development of lupus in NZB/W F1 mice. J Immunol. 1997;158:1466–72. [PubMed] [Google Scholar]


