Abstract
The nicotinic acetylcholine receptor (AChR) is the autoantigen in the human autoimmune disease myasthenia gravis (MG). Anti-AChR antibodies in MG sera bind mainly to conformational epitopes, therefore the determination of their specificities requires the use of native AChR. Antibody competition studies suggest that most MG antibodies are directed against the extracellular part of the molecule, whereas antibodies directed against the cytoplasmic region of the AChR have not been detected. To determine whether even small quantities of such antibodies exist in MG sera, we performed competition experiments based on the inhibition by MG sera of the binding of MoAbs to the human AChR, rather than inhibition by MoAbs of the binding of MG sera performed earlier. When MoAbs directed against cytoplasmic epitopes on the α or β subunits (α373–380 and β354–360) were used as test MoAbs, 17% or 9% of MG sera inhibited the binding of the anti-α or anti-β subunit MoAbs, respectively, by ≥ 50%. Non-specific inhibition was excluded. These results suggest the presence, in several MG sera, of antibodies directed against cytoplasmic regions of the AChR; yet these antibodies seemed to represent a relatively small proportion of the total anti-AChR antibodies. The corresponding epitopes may be involved in the inducing mechanisms in certain MG cases, and knowledge of the presence of such antibodies may be useful in understanding the autoimmune mechanism involved in MG.
Keywords: acetylcholine receptor, myasthenia gravis, antibodies, cytoplasmic side
INTRODUCTION
The nicotinic acetylcholine receptor (AChR) of the neuromuscular junction is the autoantigen in the human autoimmune disease, myasthenia gravis (MG) [1]. Anti-AChR antibodies cause a reduction in the number of effective AChR at the endplate, leading to failure of synaptic transmission, manifested by muscle weakness and fatiguability [1, 2]. Anti-AChR antibodies are detectable in approx. 90% of myasthenic patients' sera. The AChR of the fish electric organ and the vertebrate neuromuscular junction is a cation channel with a molecular weight of approx. 290 000 D and composed of four subunits, present in the stoichiometry α2βγδ (embryonic) or α2βɛδ (adult). Acetylcholine and other agonists and competitive antagonists, such as α-bungarotoxin (α-BT), bind to the two α subunits, regulating the function of the ion channel [3]. The AChR subunit genes and cDNAs from a number of species have been cloned and sequenced. The three-dimensional structure of the molecule has been solved at 9 Å resolution [4].
The anti-AChR antibody response in immunized animals has been studied in detail, but relatively little is known about the fine antigenic specificities of the antibodies present in human MG sera [5]. Competition experiments, in which MoAbs were used to block the binding of MG sera to the AChR so as to define, or partially define, the epitopes recognized, suggest that most MG antibodies bind to a small region on the extracellular side of the α subunit, the main immunogenic region (MIR) [6]; however, MoAbs to other extracellular sites also inhibit the binding of significant fractions of MG antibodies [6, 7]. Competition experiments between 125I-α-BT and MG sera for binding to human AChR (blocking of 125I-α-BT binding) and electrophysiological experiments also suggest the presence of antibodies to the ligand binding site [8–10]. The presence of antibodies to the α subunit has been directly demonstrated by the use of partially matured human α subunit naturally present in TE671 cells [11] and by the use of human α subunit/Torpedoβ, γ, and δ subunit AChR hybrids [12]; the hybrids also allowed an estimate to be made of the titre of anti-α subunit antibodies in MG sera [12]. The presence of antibodies directed against other subunits has been demonstrated by the use of mouse/Torpedo and human/Torpedo AChR hybrids [13] and by the differential binding of MG sera to α2βγδ and α2βɛδ human AChRs [9, 14].
The use of synthetic peptides in epitope mapping, a very useful method in mapping the epitopes recognized by single anti-AChR MoAbs, is of questionable reliability when using MG sera (reviewed in [5]). Synthetic peptides corresponding to the extracellular region of the human AChR α subunit have been shown to bind to MG antibodies [15]. Hayashi et al. [16] tested the binding of MG antibodies to 13 synthetic human AChR peptides, including one derived from a cytoplasmic region (α304–322), and found that antibodies from many MG patients bound to α304–322 or to α41–70; however, a similar high incidence of binding to these peptides was seen using MG sera with no detectable antibodies directed against the intact AChR [16]. In contrast, Nagvekar et al. [17] could not detect any significant binding of antibodies from 17 sera of MG patients with thymoma to a pool of peptides covering the cytoplasmic region α309–417. Overall, synthetic peptides do not seem suitable for the reliable mapping of the heterogeneous conformationally dependent antibodies present in MG sera.
Antibodies directed against the cytoplasmic region of the AChR have been detected in the sera of immunized animals. When animals are injected with native AChR, the majority of their antibodies are directed against the extracellular side of the AChR, but when they are immunized with SDS-denatured AChR, or its isolated subunits, most of the antibodies produced are directed against cytoplasmic regions of the molecule [18, 19]. Antibodies directed against cytoplasmic epitopes that are produced on immunization with AChR fragments have also been detected and studied by several groups [20, 21].
No antibodies directed against the cytoplasmic region of the AChR have so far been reliably detected in MG sera (reviewed in [5]). In competition experiments, MoAbs directed against the cytoplasmic region did not inhibit the binding to the human AChR of a significant percentage of MG antibodies [22], suggesting that, if such antibodies exist, they represent a small minority of the anti-AChR antibody repertoire. However, the cytoplasmic region of the AChR is immunologically active in MG, since it contains several T cell epitopes [23, 24], and it is therefore important to determine whether MG sera contain any antibodies directed against this region. Knowledge of their presence would be instructive in understanding the nature of the immunogen (i.e. whether intact or denatured AChR).
In the present study we performed a different type of antibody competition experiment, designed to detect small quantities of anti-cytoplasmic region antibodies in MG sera, in which the human serum was used to try to block the binding of the test MoAb. Obviously, due to the specificity of the test MoAbs used, these experiments do not detect the presence of antibodies directed against all possible sites on the cytoplasmic part of the AChR, but only those directed against certain regions on the α and β subunits. However, the results show that such antibodies do indeed exist, at least in several MG sera. Since such competition experiments have always proved reliable in the past [25–29], this strongly suggests that MG sera contain antibodies directed against the cytoplasmic region of the AChR.
MATERIALS AND METHODS
AChR
The source of human AChR containing extracts was the human cell line, TE671, which expresses functional muscle-type AChR [30]. The cell line was maintained in Dulbecco's modified essential medium supplemented with 10% fetal calf serum (DMEM–FCS) at 37°C in 5% CO2 and the cells were used 4–6 days after reaching confluence. AChR-containing extracts were prepared as described by Luther et al. [31].
MoAbs and MG antisera
The MoAbs used were derived from rats immunized with SDS-denatured AChR from Torpedo electric organs, but show very strong cross-reaction with human muscle AChR; their characteristics have been described previously [28, 29, 32]. Stock solutions of MoAbs were produced from 20× ammonium sulphate-concentrated supernatants of hybridoma cultures in DMEM–10% FCS, dialysed against PBS (137 mm NaCl, 8 mm Na2HPO4, 2.7 mm KCl, 1.5 mm KH2PO4, pH 7.2) containing 0.05% (w/v) NaN3. Selected MoAbs were coupled to Sepharose–cyanogen bromide (CNBr)- activated beads (Pharmacia Biotech, Uppsala, Sweden) following the manufacturer's instructions (MoAb–Sepharose).
Serum samples were collected from Greek myasthenic patients and their titres determined by radioimmunoassay using 125I-α-BT-labelled TE671 AChR. Positive sera were defined as those with titres ≥ 0.6 nm, compared with normal human sera (NHS) with titres of ≤ 0.2 nm.
MG sera were usually proteolysed to Fab fragments by incubation with 0.2 mg/ml mercuripapain (Sigma, St Louis, MO) in 10 mml-cysteine, 2 mm EDTA for 3 h at 37°C, the reaction being stopped with 25 mm iodoacetamide; PAGE confirmed protein cleavage, including that of the immunoglobulins, had occurred. In some experiments, MoAb 124 was also cleaved into Fab fragments by 3 h treatment at 37°C with mercuripapain (0.1 mg/ml of mercuripapain and 10 mg/ml of MoAb) followed by the addition of 25 mm iodoacetamide to stop the reaction.
Antibody competition experiments
Tests of the inhibition of MoAb binding by MG serum were carried out as follows (all incubations were performed at 4°C). Thirty microlitres of 0.65 nm125I-α-BT-labelled AChR in PBS, pH 7.2, containing 0.5% Triton X-100, were incubated with 30 μl of undiluted or diluted human serum (NHS or MG serum, intact or proteolysed, dilutions in PBS), for 1 h, followed by the addition of 30 μl of a MoAb dilution (see below) in PBS pH 7.2, containing 0.5% Triton X-100 (plus 0.1 μl of normal rat serum as carrier). After a 4-h incubation, immune complexes were quantitatively precipitated by 90 min incubation with a predetermined amount of rabbit anti-rat immunoglobulin (pretreated with 1/3 volume of NHS to eliminate any cross-reactivity with human immunoglobulin). After washings with PBS pH 7.2 containing 0.5% Triton X-100, the radioactivity in the pellet was counted in a γ-counter. Background radioactivity was measured by the use of MoAb 25 which does not bind to human AChR. The dilutions of MoAb used were chosen so as to precipitate, in the presence of NHS, almost all of the labelled AChR used. The percentage residual MoAb binding in the presence of the competing MG serum was calculated as 100 × Δct/minMoAb(MG)/Δct/minMoAb(NHS), where Δct/minMoAb(MG) and Δct/minMoAb(NHS) correspond to the radioactivity precipitated by a specific MoAb in the presence of MG serum or NHS, respectively, after subtraction of the background radioactivity precipitated by control MoAb 25.
RESULTS
The ability of MG sera to block the binding of MoAbs to the cytoplasmic region of the human AChR was tested. In most experiments two representative MoAbs were used, MoAb 155 directed against the Very Immunogenic Cytoplasmic Epitope on the α-chain (α373–380; VICE-α), and MoAb 124 directed against VICE-b (β354–359). Competition experiments were performed initially using untreated MG sera, and some sera were found to block the binding of the test MoAbs, but for reasons described in Discussion, papain-treated MG sera, in which the antibodies were cleaved to Fab fragments, were used.
Papain-treated sera from 95 MG patients were tested for their ability to block the binding of the MoAbs to 125I-α-BT-labelled TE671 AChR; the results are shown in Fig. 1. About half of the sera used were chosen randomly (most sera of titres < 53 nm), while the remainder were chosen to have relatively high titres. Approximately half of the sera (46/95) caused a detectable reduction (≥ 20%) in the binding of anti-α subunit MoAb 155, whereas one-third (32/95) reduced similarly the binding of anti-β subunit MoAb 124. Efficient competition (≥ 50% inhibition of MoAb binding) was seen with 16 sera (17%) and nine sera (9%) using MoAb 155 or MoAb 124, respectively; overall, 18 sera (19%) inhibited the binding of at least one of the two MoAbs by ≥ 50%. Interestingly, none of the five sera from MG patients with thymoma exhibited significant inhibition of MoAb binding (arrows in Fig. 1). The blocking ability of the sera was only weakly dependent on their overall anti-AChR titre; however, no sera with titres < 6 nm were efficient blockers of MoAb binding.
Fig 1.
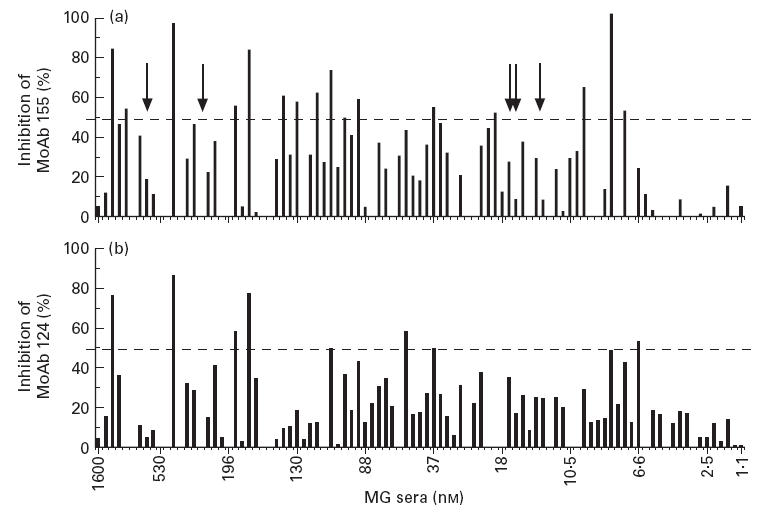
Myasthenia gravis (MG) mediated inhibition of MoAb binding to two cytoplasmic epitopes of human acetylcholine receptor (AChR). 125I-α-bungarotoxin-labelled AChR was incubated with 30 μl of one of 95 sera (proteolysed by papain to Fab fragments), then MoAb 155 (a, directed against the cytoplasmic epitope Very Immunogenic Cytoplasmic Epitope (VICE)-α) or MoAb 124 (b, directed against the cytoplasmic epitope VICE-β) was added. Subsequently, the MoAb-bound labelled AChR was immunoprecipitated using anti-rat immunoglobulin serum. The sera are arranged in order of decreasing titre and the titre, in nm, of the 10th, 20th, etc., sera (plus the first and last) are indicated. Arrows indicate sera from five MG patients with thymoma. The 50% cut-off of inhibition is shown by the horizontal lines. A small negative inhibition (1–10%) exhibited by some sera is represented as 0%. For most sera, s.d. among measurements from independent experiments did not exceed 12%. For a few sera s.d. was between 13% and 20%.
The blocking ability of the MG sera was dose-dependent. Figure 2 shows the dose dependence seen using MoAb 155 and three selected sera which were very efficient blockers. From the results of such experiments, an approximate titre can be estimated for the test MG sera in terms of antibodies directed against the corresponding cytoplasmic regions. Based on the volume of MG serum required to inhibit 40–50% of the binding of the MoAb, the estimated titres of MG antibodies to the ‘MoAb 155 region’ were 36, 3.1 and 0.84 nm for MG 1989, 3722 and 3421, corresponding to 19%, 2.8% and 0.94% of their total antibody titres (189, 110 and 89 nm), respectively.
Fig 2.
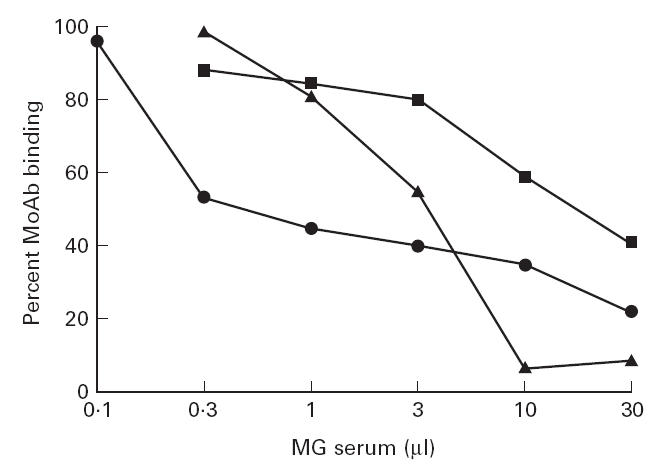
Dose response of the inhibitory effect of three papain-treated myasthenia gravis (MG) sera on the binding of anti-α subunit MoAb 155 to the acetylcholine receptor (AChR). The titres of the inhibitory MG antibodies estimated from 50% to 60% inhibition of MoAb binding are: 36 nm (MG 1989; •), 3.1 nm (MG 3722; ▴) and 0.84 nm (MG 3421; ▪). MG 1989, 3722 and 3421 are the 23rd, 35th and 39th sera in Fig. 1 (left to right).
In order to exclude any non-specific blocking peculiar to the specific MoAbs used, the ability of a few MG sera to block the binding of other MoAbs which bind to very similar epitopes on VICE-α and VICE-β was tested [28] and similar degrees of blocking were seen for a given MG serum. Thus, as shown in Fig. 3, the binding of the two anti-VICE-α MoAbs 152 and 153 was inhibited to a similar extent as that of anti-VICE-α MoAb 155, and that of anti-VICE-β MoAb 148 was inhibited to similar extent as that of anti-VICE-β MoAb 124.
Fig 3.
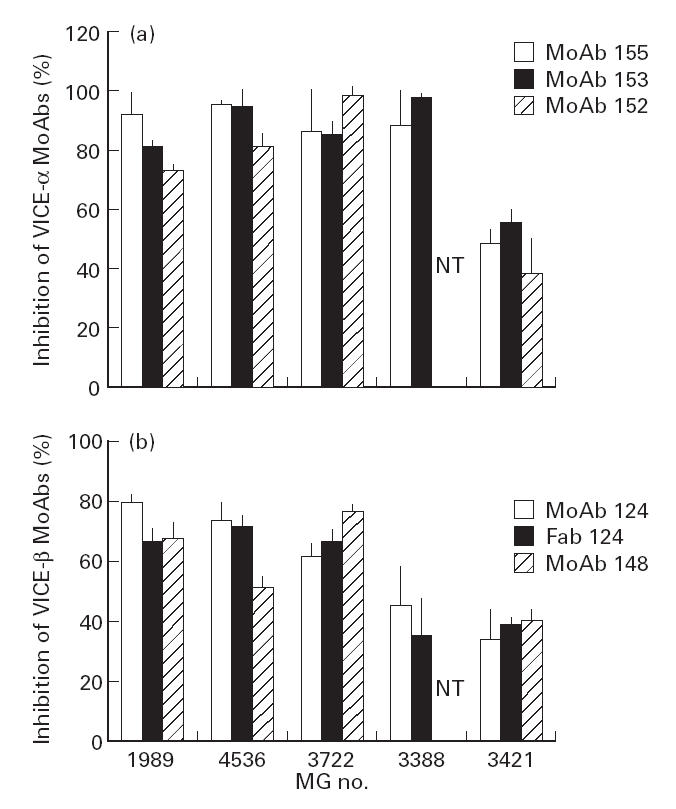
Myasthenia gravis (MG)-mediated inhibition of binding to the acetylcholine receptor (AChR) of various MoAbs and a Fab fragment. Similar inhibition by each individual serum was seen using different MoAbs directed against a single region (Very Immunogenic Cytoplasmic Epitope (VICE)-α or VICE-β), or using intact and Fab fragments of MoAb 124. MG 1989, 4536, 3722, 3388 and 3421 are the 23rd, 3rd, 35th, 76th and 39th, respectively, sera in Fig. 1. NT, Not tested.
In order to exclude the possibility that the Fab of the MG sera non-specifically inhibited cross-linking of AChRs by the bivalent MoAbs and this in turn affected precipitation, we performed two different kinds of experiments. First, we produced Fab fragments of test MoAb 124 and compared the ability of a few sera to inhibit the binding of intact MoAb 124 versus that of the Fab fragments. As shown in Fig. 3b, each of the sera tested gave a similar degree of inhibition using intact or fragmented MoAb 124. Second, instead of precipitating the test MoAbs using anti-immunoglobulin, which involves the introduction of an additional factor (the need for extensive cross-linking by the anti-immunoglobulin), the MoAbs were immobilized on Sepharose beads, thus allowing their direct and complete precipitation by simple centrifugation; again, as shown in Fig. 4, similar results were obtained using either soluble or Sepharose-bound MoAb.
Fig 4.
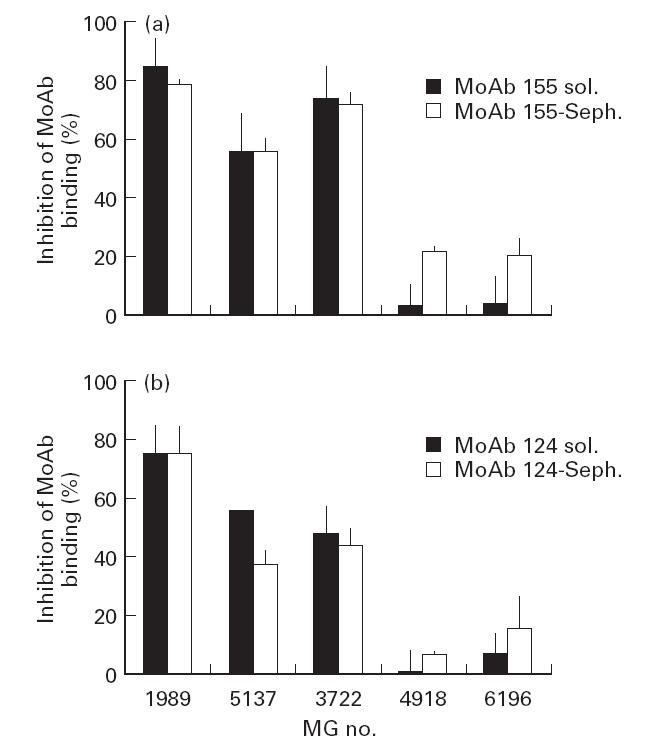
Comparison of myasthenia gravis (MG)-mediated inhibition using soluble or Sepharose-bound MoAb. Soluble MoAbs (with bound 125I-α-bungarotoxin-labelled acetylcholine receptor (AChR)) were immunoprecipitated using anti-rat immunoglobulin serum, while Sepharose-bound MoAbs were precipitated simply by centrifugation. The first three sera gave extensive inhibition of MoAb binding, whereas the last two only inhibited to a small degree. Each papain-treated MG serum gave similar inhibition using soluble or Sepharose-bound MoAb-precipitation of labelled AChR. MG 1989, 5137, 3722, 4918 and 6196 are the 23rd, 21st, 35th, 16th and 64th, respectively, sera in Fig. 1.
Finally, we investigated whether the decrease in 125I-α-BT-labelled AChR precipitation by the MoAbs, seen in the presence of MG sera, was due to competition between MG antibodies and the labelled α-BT for binding to the AChR. A titration of high doses of some MG sera resulted in some of them not being capable of precipitating all the available 125I-α-BT–AChR. To investigate whether this was due to competition between the anti-AChR antibodies and 125I-α-BT for the ligand binding site, labelled AChR was precipitated using 9 μl of low plateau MG sera in the presence of 1 μl of a high plateau MG serum (i.e. which was able to precipitate all available AChRs). The combination of the two MG sera gave similar values to that obtained by 1 μl of the high plateau serum in the presence of 9 μl of NHS (Fig. 5). This illustrates that the low plateau of these sera was not due to antibody-mediated dissociation of the radiolabelled toxin.
Fig 5.
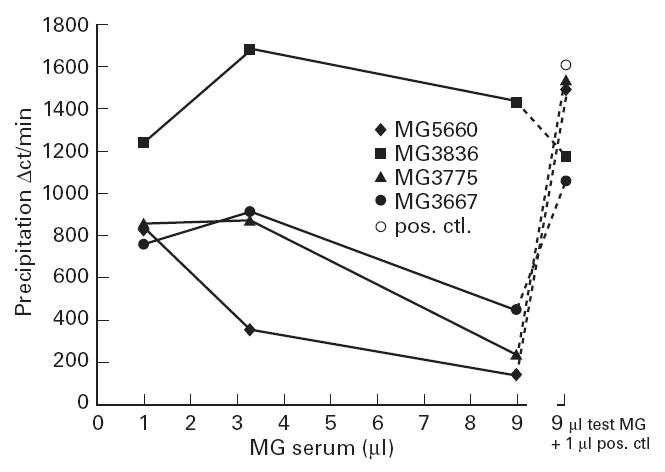
Effect of increasing volumes of myasthenia gravis (MG) sera on the MG precipitation of 125I-α-bungarotoxin (α-BT)-labelled acetylcholine receptor (AChR). The symbols on the right represent the effect of a mixture of 1 μl of a high plateau MG serum and 9 μl of test MG sera or NHS (○). Since the low plateau sera had little effect on the result obtained with the positive control high plateau serum, it is concluded that the low plateau MG sera did not release 125I-α-BT from the AChR. MG 5660, 3836, 3775 and 3667 are the 4th, 28th, 30th, and 40th, respectively, sera in Fig. 1.
DISCUSSION
In the present study we showed that several of the tested MG sera (at least 19%) contain antibodies directed against the cytoplasmic region of the human AChR. Earlier competition experiments between MoAbs and MG sera did not detect the presence of such antibodies in human MG sera [22]. However, the nature of those experiments (measuring the ability of a MoAb to inhibit the binding of MG sera to the AChR) was such that minor antibody populations in MG sera would not be detected. In the present study the reverse experiment was performed, i.e. we measured the ability of MG sera to inhibit the binding of a MoAb directed against the cytoplasmic side of the AChR. In this situation, even if only a small minority of antibodies in an MG serum are directed against the specific site recognized by the MoAb, if an increasing excess of MG serum is used, this minor antibody fraction should become detectable. Using this assay, positive sera were detected at approximately the same frequency in samples with high or relatively low anti-AChR titres (binding to at least one MoAb was inhibited by ≥ 50% by 20% of sera with titres > 110 nm and by 20% of sera with titres between 6.6 and 95 nm). Very low titre sera (< 6 nm) did not significantly inhibit the binding of anti-cytoplasmic region MoAbs; however, such sera would need to contain a high percentage of anti-cytoplasmic region antibodies for their binding to be detected in the test system. The titres for antibodies to the studied cytoplasmic regions, estimated in three selected cases, were 19%, 2.8% and 0.94% of the total anti-AChR titres of these sera. Such antibodies in MG patients probably do not play a significant pathogenic role, but their presence might be relevant to the aetiology of the disease, as discussed later.
Nagvekar et al. [17] did not observe binding of antibodies from 17 sera of thymoma MG patients to a pool of peptides covering the cytoplasmic region α309–417. Although our present experimental approach is fundamentally different from that in [17] (detection of antibodies binding to the intact AChR versus AChR peptides), it is interesting to note that none of the five sera from thymoma patients exhibited high percentages of inhibition of the cytoplasmic MoAbs (arrows in Fig. 1). Yet the number of tested thymoma sera in the present study was too small to draw definite conclusions.
Several experiments were performed in order to exclude non-specific effects; these examined the respective role of intact antibodies versus Fab fragments, possible release of 125I-α-BT from the AChR by the MG sera, possible non-specific inhibition of immunoprecipitate formation, and possible peculiar behaviour of the two test MoAbs, as described below.
Most of the competition experiments carried out in the present study were performed using papain-treated MG sera in which the antibodies were cleaved to Fab fragments, the reason being that: (i) in several cases, the MoAbs precipitated more AChR in the presence of untreated MG sera than in the presence of NHS, probably as a result of the cross-linking of several AChR molecules by the MG antibodies, resulting in the subsequent precipitation of these aggregates of AChR molecules by single MoAb molecules; (ii) Fabs are smaller than intact antibodies, thus possible steric hindrance between antibodies to neighbouring epitopes should be decreased and competition more specific; (iii) AChR cross-linking by intact MG antibodies is more likely to induce overall conformational distortions or masking of the AChRs within microaggregates. Furthermore, when a test MoAb (no 124) was proteolysed to Fab fragments, MG Fab fragments gave similar degrees of inhibition when tested on these Fab 124 fragments or on the intact MoAb (Fig. 3b).
When measuring the titre of MG sera using 125I-α-BT-labelled human AChR, some sera gave a relatively low plateau of precipitated AChR and some also gave decreased AChR binding as the volume of MG serum was increased (Fig. 5). It might be imagined that these observations could be due to MG antibody-mediated release of bound 125I-α-BT by MG antibodies which bind to the vicinity of the α-BT binding sites or cause a conformational change in the AChR. If this was so, the ‘inhibition’ of binding of the cytoplasmic MoAbs (i.e. the reduction in the amount of precipitated 125I-α-BT) could possibly be due simply to the release of 125I-α-BT from the AChR molecule. The following experiments showed that this was not the case:
1. Mixtures of low and high plateau MG sera gave almost identical results to those obtained by a high plateau serum alone, indicating that low plateau MG sera did not cause release of 125I-α-BT from the AChR (Fig. 5). The lack of ability to precipitate all available AChRs could be due to various reasons, one being that the AChR preparation may contain some partially proteolysed AChR lacking certain epitopes normally recognized by antibodies in these sera.
2. Several of the MG sera which gave good inhibition of the precipitation of radioactivity using MoAb 155 did not inhibit precipitation by MoAb 124, and vice versa (Fig. 1).
3. Several MG sera which markedly inhibited the binding of MoAbs 155 and 124 did not give low plateaus of precipitated radioactivity, whereas some MG sera with low plateaus did not appear to inhibit MoAb binding (not shown).
The possibility of non-specific interference of the MG sera with the immunoprecipitation of the AChR-bound MoAbs by soluble anti-rat immunoglobulin antibodies was excluded using Sepharose-immobilized MoAbs which were subsequently directly and quantitatively precipitated by centrifugation, avoiding interference from other factors; both systems gave the same results (Fig. 4). The use of Sepharose-immobilized MoAbs was not routinely used in the majority of the present experiments to avoid possible enhanced steric hindrance effects imposed by the Sepharose beads.
To exclude any peculiar behaviour of the two tested MoAbs, the effect of a few MG sera was tested on the binding of several other MoAbs, binding to very similar epitopes; similar results were obtained (Fig. 3).
Selection of the cut-off, i.e. the minimum degree of inhibition of MoAb binding that would definitely denote the presence of MG antibodies against the studied region, is a critical but subjective issue. Previous experience with anti-AChR antibody competition experiments [25, 33, 34] suggested that low percentages of inhibition may not necessarily be due to competition between antibodies to neighbouring epitopes, and it was concluded that an inhibition of ≥ 30% was required in order to conclude that the antibodies bound to the same or very close site(s). In the present study, in order to determine unequivocally the presence of antibodies directed against the cytoplasmic region of the AChR, we adopted a much more stringent limit, i.e. 50% inhibition. Of course, it is very likely that lower percentages of competition (e.g. between 30% and 50%) may also result from the presence of antibodies directed against the cytoplasmic region. If the 30% cut-off was adopted, the percentages of MG sera containing antibodies directed against the regions containing VICE-α, VICE-β, or either of the two, would be 34%, 22% and 41%, respectively (derived from Fig. 1).
The observed competition results do not necessarily mean that the inhibiting sera contain antibodies binding precisely to the VICE-α and VICE-β epitopes. Steric hindrance between antibodies to neighbouring epitopes is also likely to play a role, due to the bulky antibody arms, whether using whole antibody or Fab fragments. Steric hindrance, however, should occur only between antibodies binding to the same side of the AChR, and since it is well established by electron microscopy [19, 35, 36] and peptide mapping studies [28, 29, 35, 36] that the MoAbs used bind to the cytoplasmic region, the competing MG antibodies must also bind to this region.
It is unlikely that antibodies binding to the extracellular region of the AChR induce a long-distance allosteric effect affecting the conformation of the cytoplasmic side. Comparison of the results of previous competition studies between anti-AChR MoAbs and results of epitope mapping studies using synthetic peptides strongly suggest that only antibodies directed against neighbouring sites cross-compete. In addition, it should be noted that the binding of MoAbs to VICE-α and VICE-β is conformation-independent, since the MoAbs bind well to native AChR, to SDS-denatured subunits and to synthetic peptides [28, 29, 35, 36]. Thus, it is very unlikely that small conformational changes, induced by the binding of antibodies to the extracellular side, would prevent MoAb binding. Finally, the fact that the extent of inhibition was dose-dependent up to a large excess of MG antibodies (Fig. 2) over the available AChR further suggests that inhibition is induced by a minority of antibodies directed against specific sites (apparently cytoplasmic), rather than by the presence of large amounts of antibodies directed against highly immunogenic extracellular sites.
Each of the two studied immunogenic regions are of special interest. The epitope for MoAb 155 (VICE-α, α373–380) is a significant B cell epitope for experimentally induced antibodies [28], and, due to its cross-reaction with a protein (NF-M) present in MG thymomas, but not in non-MG thymomas, is considered as a possible initiator of the immune response against the AChR in thymoma-associated MG [37, 38]. Nevertheless, none of the five presently tested sera from MG patients with thymoma exhibited significant inhibition of MoAb 155 binding (Fig. 1). The epitope for MoAb 124 (VICE-β) is very immunogenic in experimental rats, since 11 of our 15 MoAbs derived from immunization of rats with Torpedo AChR β subunit bind to this region [29]. It also carries the tyrosine phosphorylation site of the β subunit and at least one MoAb (no 148) directed against this epitope can block AChR function [29, 39]. Thus, the detection of the presence of antibodies binding near these sites may be of special interest in understanding MG.
The detection in MG sera of even small quantities of antibodies directed against the cytoplasmic region of the AChR is important in understanding the autoimmune mechanisms in MG. Antibodies to the cytoplasmic region are the predominant antibody fraction when animals are immunized with denatured AChR or fragments of AChR, but not when native AChR is used as immunogen. The present results allow the hypothesis that the autoimmunization may have taken place against denatured AChR subunits. Yet the rather small amounts of anti-cytoplasmic antibodies probably suggest that both intact and denatured AChR molecules may constitute the immunogen.
Acknowledgments
This work was supported by the Greek General Secretariat of Research and Technology and the Association Française contre les Myopathies. We thank A. Kokla for excellent technical assistance, T. Barkas, A. Mamalaki, L. Jacobson, K. Poulas and D. Papanastasiou for valuable suggestions.
References
- 1.Drachman DB. Medical progress—myasthenia gravis. N Engl J Med. 1994;330:1797–810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom J, Shelton D, Fugii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–84. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 3.Galzi JL, Changeux JP. Neurotransmitter-gated ion channels as unconventional allosteric proteins. Curr Opin Struct Biol. 1994;4:554–65. [Google Scholar]
- 4.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 5.Tzartos SJ, Barkas T, Cung MT, et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol Rev. 1998;163:89–120. doi: 10.1111/j.1600-065x.1998.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 6.Tzartos SJ, Seybold M, Lindstrom J. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci USA. 1982;79:188–92. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich F, Vincent A, Newsom-Davis J. Differences in fine specificity of anti-acetylcholine receptor antibodies between subgroups of spontaneous myasthenia gravis of recent onset, and of penicillamine induced myasthenia. Autoimmunity. 1988;2:31–37. doi: 10.3109/08916938809019941. [DOI] [PubMed] [Google Scholar]
- 8.Drachman DB, Adams RN, Josifek LF, Self SG. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med. 1982;307:769–75. doi: 10.1056/NEJM198209233071301. [DOI] [PubMed] [Google Scholar]
- 9.Schuetze SM, Vicini S, Hall ZW. Myasthenic serum selectively blocks acetylcholine receptors with long channel open times at developing rat endplates. Proc Natl Acad Sci USA. 1985;82:2533–7. doi: 10.1073/pnas.82.8.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernet-der Garabedian B, Morel E, Bach JF. Heterogeneity of antibodies directed against the alpha-bungarotoxin binding site on human acetylcholine receptor and severity of myasthenia gravis. J Neuroimmunol. 1986;12:65–74. doi: 10.1016/0165-5728(86)90098-6. [DOI] [PubMed] [Google Scholar]
- 11.Conroy WG, Saedi MS, Lindstrom J. TE671 cells express an abundance of a partially mature acetylcholine receptor alpha-subunit which has characteristics of an assembly intermediate. J Biol Chem. 1990;265:21642–51. [PubMed] [Google Scholar]
- 12.Loutrari H, Kokla A, Trakas N, Tzartos SJ. Expression of human–Torpedo hybrid acetylcholine receptor for analysing the subunit specificity of antibodies in sera from patients with myasthenia gravis. Clin Exp Immunol. 1997;109:538–46. doi: 10.1046/j.1365-2249.1997.4701367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loutrari H, Tzartos SJ, Claudio T. Use of Torpedo–mouse hybrid acetylcholine receptors reveals immunodominance of the alpha-subunit in myasthenia gravis antisera. Eur J Immunol. 1992;22:2949–56. doi: 10.1002/eji.1830221129. [DOI] [PubMed] [Google Scholar]
- 14.Beeson D, Jacobson L, Newsondavis J, Vincent A. A transfected human muscle cell line expressing the adult subtype of the human muscle acetylcholine receptor for diagnostic assays in myasthenia gravis. Neurology. 1996;47:1552–5. doi: 10.1212/wnl.47.6.1552. [DOI] [PubMed] [Google Scholar]
- 15.Ashizawa T, Ruan KH, Jinnai K, Atassi MZ. Profile of the regions on the alpha-chain of human acetylcholine receptor recognized by autoantibodies in myasthenia gravis. Mol Immunol. 1992;29:1507–14. doi: 10.1016/0161-5890(92)90225-m. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M, Kohno T, Yoshinaga J, Kida K. Epitope analysis of acetylcholine receptor antibody in patients with myasthenia gravis using sensitive enzyme immunoassay. Ann NY Acad Sci. 1998;841:478–81. doi: 10.1111/j.1749-6632.1998.tb10967.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagvekar N, Jacobson LW, Willcox N, Vincent A. Epitopes expressed in myasthenia gravis thymomas are not recognized by patients' T cells or autoantibodies. Clin Exp Immunol. 1998;112:17–20. doi: 10.1046/j.1365-2249.1998.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Froehner SC. Identification of exposed and buried determinants of the membrane-bound acetylcholine receptor from Torpedo californica. Biochemistry. 1981;20:4905–15. doi: 10.1021/bi00520a016. [DOI] [PubMed] [Google Scholar]
- 19.Sargent P, Hedges B, Tsavaler L, Clemmons L, Tzartos SJ, Lindstrom J. The structure and transmembrane nature of the acetylcholine receptor in amphibian skeletal muscle as revealed by cross-recting monoclonal antibodies. J Cell Biol. 1984;98:609–18. doi: 10.1083/jcb.98.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann D, Gershoni JM, Fridkin M, Fuchs S. Antibodies to synthetic peptides as probes for the binding site on the α-subunit of the acetylcholine receptor. Proc Natl Acad Sci USA. 1985;82:3490–3. doi: 10.1073/pnas.82.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criado M, Hochschwender S, Sarin V, Fox J, Lindstrom J. Evidence for unpredicted transmembrane domains in acetylcholine receptor subunits. Proc Natl Acad Sci USA. 1985;82:2004–8. doi: 10.1073/pnas.82.7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzartos SJ, Morel E, Efthimiadis A, Bustarret AF, D'Anglejan J, Drosos A, Moutsopoulos HM. Fine antigenic specificities of antibodies in sera from patients with D-Penicillamine-induced myasthenia gravis. Clin Exp Immunol. 1988;74:80–86. [PMC free article] [PubMed] [Google Scholar]
- 23.Protti MP, Manfredi AA, Straub C, Wu X, Howard JF, Conti-Tronconi BM. Use of synthetic peptides to establish anti-human acetylcholine receptor CD4+ cell lines from myasthenia gravis patients. J Immunol. 1990;144:1711–20. [PubMed] [Google Scholar]
- 24.Conti-Fine BM, Navaneetham D, Karachunski PI, Raju R, Diethelm-Okita B, Okita D, Howard J, Wang ZY. T-cell recognition of the acetylcholine receptor in myasthenia gravis. Ann NY Acad Sci. 1998;841:283–308. doi: 10.1111/j.1749-6632.1998.tb10936.x. [DOI] [PubMed] [Google Scholar]
- 25.Kordossi AA, Tzartos SJ. Monoclonal antibodies against the main immunogenic region of the acetylcholine receptor — mapping on the intact molecule. J Neuroimmunol. 1989;23:35–40. doi: 10.1016/0165-5728(89)90070-2. [DOI] [PubMed] [Google Scholar]
- 26.Tzartos SJ, Kokla A, Walgrave S, Conti-Tronconi B. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67–76 of the α-subunit. Proc Natl Acad Sci USA. 1988;85:2899–903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saedi MS, Anand R, Conroy WG, Lindstrom J. Determination of amino acids critical to the main immunogenic region of intact acetylcholine receptors by in vitro mutagenesis. FEBS Letters. 1990;267:55–59. doi: 10.1016/0014-5793(90)80286-r. [DOI] [PubMed] [Google Scholar]
- 28.Tzartos SJ, Remoundos MS. Precise epitope mapping of monoclonal antibodies to the cytoplasmic side of the acetylcholine receptor alpha subunit — dissecting a potentially myasthenogenic epitope. Eur J Biochem. 1992;207:915–22. doi: 10.1111/j.1432-1033.1992.tb17124.x. [DOI] [PubMed] [Google Scholar]
- 29.Tzartos SJ, Valcana C, Kouvatsou R, Kokla A. The tyrosine phosphorylation site of the acetylcholine receptor beta-subunit is located in a highly immunogenic epitope implicated in channel function. Antibody probes for beta-subunit phosphorylation and function. EMBO J. 1993;12:5141–9. doi: 10.1002/j.1460-2075.1993.tb06209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoepfer R, Luther M, Lindstrom J. The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the α-subunit. FEBS Letters. 1988;226:235–40. doi: 10.1016/0014-5793(88)81430-3. [DOI] [PubMed] [Google Scholar]
- 31.Luther MA, Schoepfer R, Whiting P, et al. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989;9:1082–96. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzartos SJ, Langeberg L, Hochschwender S, Swanson L, Lindstrom J. Characteristics of monoclonal antibodies to denatured Torpedo and to native calf acetylcholine receptors: species, subunit and region specificity. J Neuroimmunol. 1986;10:235–53. doi: 10.1016/0165-5728(86)90105-0. [DOI] [PubMed] [Google Scholar]
- 33.Kordossi A, Tzartos SJ. Conformation of cytoplasmic segments of acetylcholine receptor α and β subunits probed by monoclonal antibodies. Sensitivity of the antibody competition approach. EMBO J. 1987;6:1605–10. doi: 10.1002/j.1460-2075.1987.tb02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzartos SJ. Epitope mapping by antibody competition. In: Morris GE, editor. Epitope mapping protocols book series: methods in molecular biology. Vol. 66. Totowa: Humana Press; 1996. pp. 55–66. [DOI] [PubMed] [Google Scholar]
- 35.Ratnam M, Sargent P, Sarin V, et al. Location of antigenic determinants on primary sequences of the subunits of the nicotinic acetylcholine receptor by peptide mapping. Biochemistry. 1986;25:2621–32. doi: 10.1021/bi00357a051. [DOI] [PubMed] [Google Scholar]
- 36.Ratnam M, Le Nguyen D, Rivier J, Sargent P, Lindstrom J. Transmembrane topography of the nicotinic acetylcholine receptor: immunochemical tests contradict theoretical predictions based on hydrophobicity profile. Biochemistry. 1986;25:2633–43. doi: 10.1021/bi00357a052. [DOI] [PubMed] [Google Scholar]
- 37.Marx A, O'Connor R, Geuder KI, et al. Characterization of a protein with an acetylcholine receptor epitope from myasthenia gravis-associated thymomas. Lab Invest. 1990;62:279–86. [PubMed] [Google Scholar]
- 38.Wilisch A, Schultz A, Jung A. Titin epitope in thymoma. In: Marx A, Muller-Hermelink HK, et al., editors. Epithelial tumors of the thymus. New York: Plenum Press; 1997. pp. 221–7. [Google Scholar]
- 39.Wan K, Lindstrom J. Effects of monoclonal antibodies on the function of purified acetylcholine receptor from Torpedo californica reconstituted into liposomes. Biochemistry. 1985;24:1212–21. doi: 10.1021/bi00326a024. [DOI] [PubMed] [Google Scholar]


