Abstract
The aim of the present study was to analyse the in vitro proliferation and cytokine production by alloantigen-stimulated peripheral blood mononuclear cells (PBMC) obtained from patients affected by systemic sclerosis (SSc) and patients with Raynaud's phenomenon (RP). In SSc patients the proliferation of PBMC stimulated in vitro with alloantigens was significantly increased compared with healthy subjects, while no differences were observed for RP patients. Lymphocytes from SSc patients also produced larger amounts of IFN-γ compared with healthy controls. However, patients with clinically active disease had lower IFN-γ levels than those found in clinically stable patients. Patients affected by RP showed significantly higher levels of IFN-γ than healthy subjects. Analysis at the clonal level of the lymphocyte subsets involved in alloantigen stimulation in one patient affected by active SSc, and one subject with RP confirmed the results obtained using PBMC. In particular, in the RP patient but not in the SSc patient, we observed a population of CD4+ T cells which proliferated to alloantigens in vitro and produced high levels of IFN-γ. We suggest that T lymphocytes producing high levels of IFN-γ might play a protective role in RP patients and in established scleroderma.
Keywords: systemic sclerosis, Raynaud's phenomenon, T lymphocytes, interferon-gamma
INTRODUCTION
Systemic sclerosis (SSc) is a connective tissue disease characterized by abnormalities of three systems (immune, vascular, and mesenchymal extracellular matrix) which leads to exuberant fibrosis. The first symptom of SSc is often Raynaud's phenomenon (RP), which is associated with a diffuse small vessel vasculopathy and ischaemia as well as reperfusion injury to skin and organs targeted in this disease. The interaction of immune cells with vascular endothelium, through adhesion molecules and the effect of cytokines, is one of the earlier changes in SSc [1, 2]. Dermal mononuclear cell infiltrates in SSc have been shown to be both CD4+ and CD8+ activated lymphocytes [3, 4]. Increased numbers and percentages of activated T cells have also been found in the interstitium and bronchoalveolar fluids (BALF) of SSc patients with active lung disease [5]. The migration of mononuclear cells in the perivascular space and the release of cytokines are responsible for fibroblast activation, excessive collagen and glucosaminoglycan production in SSc. Several cytokines which contribute to the worsening of the disease have been found in the sera and BALF of patients with active scleroderma [6–8]. The studies of cytokines produced in vitro by peripheral blood mononuclear cells (PBMC) in SSc patients showed a spontaneous release of the ‘fibrogenetic’ cytokines tumour necrosis factor-alpha (TNF-α) and IL-1β, and an impairment of mitogen-induced IFN-γ production [9, 10]. In particular, the production of IFN-γ is of great relevance: it is the most potent stimulator of HLA class II antigens on endothelial cells, thus up-regulating endothelial–leucocyte adhesion [11–13], but it is also a negative regulator of collagen production by fibroblasts [14–16].
Although SSc has been extensively studied, no data are available on the immune system (particularly T lymphocyte function) in patients affected only by RP. A subset of these patients have autoantibodies in the serum, as assessed by indirect immunofluorescence on the HEp2 cell line, thus indicating activation of the B cell compartment; some of these patients represent the mild end of the spectrum of SSc.
In this study we investigated the proliferation and the production of IFN-γ by alloantigen-stimulated PBMC and T cell clones obtained from SSc patients and subjects affected only by RP. In support of the use of alloantigen stimulation in vitro, scleroderma-like disease is seen in chronic graft-versus-host disease (GVDH) [17, 18].
PATIENTS AND METHODS
Patients
Forty-nine patients (nine men and 40 women, aged 50.2 ± 13.1 years (mean ± s.d.)) with SSc, according to the classification criteria proposed by LeRoy et al. [19] and followed at our out-patient clinic, were studied. Eighteen patients had diffuse SSc and 31 had limited SSc (mean disease duration 5.6 ± 5 years). Estimation of the disease activity was done on the basis of one or more of the following characteristics within 6 months preceding and 6 months following the study [20]: (i) increase in total skin thickness score by 15%; or (ii) development of new pseudo-obstruction, malabsorption, pulmonary hypertension, cardiomyopathy, dysrhythmias requiring treatment, symptomatic pericarditis; or (iii) worsening of lung function, with a 15% decrease in forced vital capacity. Several patients included in this study were receiving vasoactive agents, while no patient was treated with corticosteroids at the time of blood collection. Patients were required to discontinue aspirin, or other non-steroidal anti-inflammatory drugs for at least 14 days before entering the study. Treatment with cytotoxic drugs within 1 month before venepuncture was a criterion of exclusion. Twenty-six patients with RP (one man, 25 women, mean age 42.3 ± 15.3 years, mean duration of RP 10.4 ± 9.9 years) were studied. Sixteen patients had idiopathic RP, 10 had RP with positive autoantibodies: four with anti-nuclear, two with anti-nucleolar, and four with anti-centromere antibodies, as assessed by indirect immunofluorescence on rat liver and on the HEp2 cell line [21]. No patient had positive Scl 70 antibodies, as assessed by counterimmunoelectrophoresis, using rabbit thymus extract and human spleen extract as antigen [22].
Control subjects consisted of 60 healthy subjects (20 men, 40 women, mean age 42.2 ± 18.9 years).
T cell clones were obtained from the blood of three new subjects that had not been included in the preliminary study on allogeneic-stimulated PBMC: one patient with active diffuse SSc (a woman, with a marked worsening of skin thickness, age 45 years, duration of disease from diagnosis 27 years), one patient with idiopathic RP (a woman, age 22 years, duration of RP 5 years) and one healthy control (a woman, age 36 years).
Cell separation
Heparinized venous blood was taken from all patients and from normal volunteers, and PBMC were obtained by centrifugation on Lymphoprep (Nycomed AS, Oslo, Norway) gradients. The cells were then suspended at a concentration of 1 × 106/ml in culture medium consisting of RPMI 1640 (Gibco BRL, Paisley, UK) supplemented with 10% heat-inactivated AB human serum, glutamine 2 mm, penicillin 50 U/ml and streptomycin 50 μg/ml).
Standard allogeneic stimulators
A pool of frozen PBMC from 20 healthy subjects was employed as the standard allogeneic stimulator [23].
Primary mixed lymphocyte cultures
Cell proliferation was assessed as follows: 5 × 104 PBMC with 5 × 104 irradiated (50 Gy) pooled allogeneic stimulator lymphocytes were cultured in triplicate in 96-well round-bottomed plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) at 37°C in 5% CO2 in air for 5 days. The cells were then pulsed with 3H-thymidine (2 μCi/well) (Amersham, Aylesbury, UK) and harvested after 18 h of culture.
The production of IFN-γ was evaluated in the supernatants of primary bulk mixed lymphocyte cultures (MLC) after 5 days, as described elsewhere [24].
Cloning of alloantigen-primed T lymphocytes
Primary bulk MLC cultures were obtained from three subjects. After 5 days of incubation at 37°C 5% CO2 the blasts were harvested, washed twice in culture medium, and seeded under limiting dilution conditions in the presence of irradiated pooled allogeneic lymphocytes as feeder cells, and rIL-2 100 U/ml (Cetus, Emeryville, CA) [25]. Twelve clones for the healthy subject, 15 for the SSc patient and 16 for the RP patient were studied. The reactivity of each clone to allogeneic stimulus was evaluated incubating 5 × 104 T cells from each clone with 5 × 104 irradiated (50 Gy) pooled allogeneic stimulator lymphocytes in duplicate for 2 days. The cells were then pulsed with 3H-thymidine (2 μCi/well) and harvested after 18 h of culture. The stimulation index (SI) was calculated as the ratio ct/min of alloantigen-stimulated clone:ct/min clone alone. The production of IFN-γ was evaluated in the supernatants of alloantigen-stimulated T cell clones after 2 days of culture.
IFN-γ assay
The amounts of IFN-γ were estimated by the commercial immunoenzymatic kit CytElisa IFN-γ (CYTImmune Sciences Inc., College Park, MD). The average sensitivity was 0.72 pg/ml. Cytokine concentrations were assayed in duplicate. The production of IFN-γ by the irradiated pool of allogeneic lymphocytes was below the sensitivity of the kit.
Statistical analysis
Data are expressed as mean ± s.e.m. Comparisons were made using Student's t-test.
RESULTS
Table 1 shows that PBMC from SSc patients proliferated vigorously in response to the alloantigen stimulus. In these patients the proliferative response was higher than in healthy controls (P < 0.001), with no differences in the absence of stimulus (data not shown). No difference in proliferation was observed between clinically stable patients and those with active disease (Table 1), or between patients according to the clinical subsets (e.g. diffuse versus limited SSc). Patients affected by RP did not show any difference in the proliferative response to alloantigen stimulation compared with the healthy subjects (Table 1).
Table 1.
In vitro proliferation and IFN-γ production by alloactivated peripheral blood mononuclear cells (PBMC) from systemic sclerosis (SSc) and Raynaud's phenomenon (RP) patients compared with healthy controls
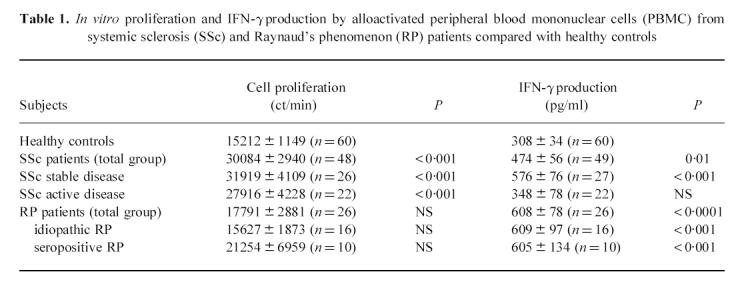 |
The in vitro production of IFN-γ is shown in Table 1. PBMC from SSc patients produced higher amounts of IFN-γ than healthy controls (P = 0.01). The analysis of the levels of IFN-γ produced by SSc patients according to disease activity showed that only patients with stable disease at the time of blood collection had significantly higher amounts of IFN-γ than those found in healthy controls. Patients with active disease at the time of the study had similar levels to those observed in healthy subjects. No differences were observed between diffuse and limited SSc (data not shown). As shown in Table 1, PBMC obtained from patients with RP produced increased levels of IFN-γ compared with healthy subjects (P < 0.0001), with no differences between patients with idiopathic RP and those found positive for antinuclear antibodies.
T cell clones were obtained from alloantigen-stimulated PBMC from three subjects (one healthy control, one patient with active SSc, one patient with idiopathic RP) that had never been studied before for proliferation and IFN-γ production in vitro. The frequencies of T cell clones showing high proliferation to allogeneic stimulation (SI > 2) were 50% (6/12) in the healthy control, 46.7% (7/15) in the SSc patient and 62.5% (10/16) in the patient with RP. T cell clones with SI > 2 obtained from all the subjects had a CD4+ phenotype and belonged to the Th1 subset, as demonstrated by the absence of IL-4 released in the supernatants (data not shown).
T cell clones obtained from the patient affected by active SSc produced amounts of IFN-γ similar to those obtained from the healthy subject, while the clones from the patient with RP produced significantly increased levels (Fig. 1). The mean level of IFN-γ produced by total clones (corrected by dividing the amount of IFN-γ produced by each clone by its proliferation (ct/min) after stimulation) was 0.012 ± 0.003 pg/ml (n = 12) from healthy controls versus 0.08 ± 0.02 pg/ml (n = 16) in RP clones (P = 0.007). The difference in IFN-γ production observed between RP and healthy clones was due mostly to the clones showing a SI > 2 (Fig. 1). The production of IFN-γ corrected for the proliferation was 0.016 ± 0.005 pg/ml (n = 6) in healthy versus 0.07 ± 0.01 pg/ml (n = 10) in RP clones (P = 0.002). The clones from the RP patient with a SI < 2 after alloantigen stimulation showed a slight increase in IFN-γ production when compared with the ‘healthy’ clones, even though the difference was not statistically significant (Fig. 1). The clones from the SSc patient produced a similar amount of IFN-γ to that produced by the clones from the healthy subject, even considering the two functionally distinct subsets (clones with SI > 2 and those with SI < 2).
Fig 1.
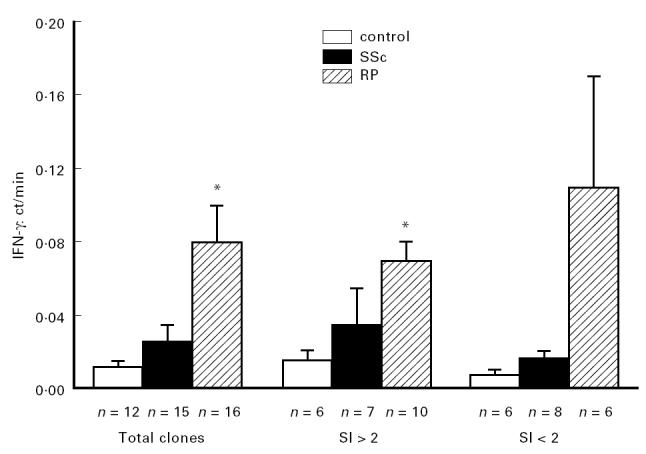
IFN-γ production by alloactivated T cell clones, according to the different functional subsets. The data are expressed as mean ± s.d. of the ratio IFN-γ:ct/min of each clone. *P < 0.01.
DISCUSSION
We have analysed the proliferation and IFN-γ production in alloactivated T lymphocytes in SSc and RP patients. The use in vitro of an oligoclonal stimulation of T cells by alloantigens is relevant from several points. First, the proliferation observed after allogeneic stimulation represents the result of the interaction among various heterogeneous subpopulations of T cells and alloantigen-specific helper, inducer, suppressor, and cytolytic functions are all activated in this reaction [26–28]. Second, chronic GVHD induced in non-irradiated F1 hybrid mice is a model for the study of the autoimmune responses that characterize human connective tissue diseases, and it has been suggested that the mechanisms involved in the immunological dysregulations of GVHD may also play a role in the induction and persistence of systemic autoimmune diseases. Recent papers have reported the occurrence of autoantibodies that have established associations with human connective tissue disease (such as anti-synthetases, anti-U-3RNP, and anti-NOR:90) in mice with chronic GVHD [29].
In this study we demonstrated that alloantigens represent a good trigger of lymphocyte proliferation in vitro in SSc patients. These results are in contrast with the data obtained by other investigators using polyclonal mitogen to induce lymphocyte proliferation [30, 31], but similar to those reported by Morse & Bodi [32] using oligoclonal stimulation. This supports the hypothesis that PBMC from patients with SSc are better responders in vitro to alloantigens than those from healthy subjects. The analysis of the production of IFN-γin vitro by alloantigen-stimulated lymphocytes showed increased amounts of this cytokine in the supernatants from SSc patients in comparison with healthy subjects. This is a surprising result, since an impairment in IFN-γ production was reported by all investigators who studied this cytokine [9, 10]. The discrepancy could be partially explained by the different model of activation used in vitro (polyclonal rather than allogeneic stimulation) and/or the population of patients selected for the studies. In our study, patients with stable SSc showed increased levels of IFN-γ released in the supernatants, while patients with active disease had levels similar to those observed in healthy subjects. These data suggest that a subset of T lymphocytes responsible for IFN-γ production could play a critical role in the different phases of the disease, although serial data on the same patients will be needed to confirm this possibility.
We have also investigated the characteristics of alloantigen response in vitro in patients affected by RP. PBMC from RP patients, both idiopathic and seropositive, did not show increased proliferation compared with healthy subjects, but there was increased production in vitro of IFN-γ. These results support the hypothesis that a functionally distinct subset of T lymphocytes producing large amounts of IFN-γ might be involved early in RP. The observation that in SSc there are high levels of IFN-γ, but only in patients with stable disease, suggests a possible role for this subset of lymphocytes in the control of disease progression. The analysis, at the clonal level, of the subsets of T lymphocytes activated during alloantigen stimulation confirmed the results obtained with PBMC. In particular, T cell clones from a RP patient, that were able to proliferate in vitro when triggered with alloantigens, showed a significant increased production of IFN-γ when compared with clones from healthy subjects. These clones belonged to the CD4+ Th1 subset. This suggests a protective role for this IFN-γ-producing CD4+ Th1 population against the progression towards connective tissue disease in RP patients. A long-term study on a number of seropositive RP patients, with clinical follow up and evaluation of IFN-γ production is now needed to clarify the role of IFN-γ in disease progression.
Acknowledgments
We wish to thank Dr Noemi Greppi, Centro Trasfusionale e di Immunologia dei Trapianti IRCCS Ospedale Maggiore di Milano, for providing the buffy coats used as a source of pooled allogeneic stimulator lymphocytes and Santo Scrofani for expert technical assistance. This work has been partially supported by CNR grant number 95.02435.CT04.
References
- 1.Kahaleh B. Immunologic aspects of scleroderma. Curr Opin Rheumatol. 1993;5:760–5. doi: 10.1097/00002281-199305060-00011. [DOI] [PubMed] [Google Scholar]
- 2.Postlethwaite AE. Connective tissue metabolism including cytokines in scleroderma. Curr Opin Rheumatol. 1993;5:766–72. doi: 10.1097/00002281-199305060-00012. [DOI] [PubMed] [Google Scholar]
- 3.Prescott RJ, Freemont AJ, Jones CJP, Hoyland J, Fiending P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–63. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 4.Ferrarini M, Steen V, Medsger TA, Jr, Whiteside TL. Functional and phenotypic analysis of T lymphocytes cloned from the skin of patients with systemic sclerosis. Clin Exp Immunol. 1990;79:346–52. doi: 10.1111/j.1365-2249.1990.tb08094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 1996;22:695–708. doi: 10.1016/s0889-857x(05)70296-9. [DOI] [PubMed] [Google Scholar]
- 6.Kahaleh BM, LeRoy EC. Interleukin-2 in scleroderma: correlation of serum level with the extent of skin involvement and disease duration. Ann Intern Med. 1989;110:446–50. doi: 10.7326/0003-4819-110-6-446. [DOI] [PubMed] [Google Scholar]
- 7.White Needleman B, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumour necrosis factor α, and interferon-γ levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35:67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 8.Bolster MB, Ludwika A, Sutherland SE, Strange C, Silver RM. Cytokine concentrations in bronchoalveolar lavage fluid of patients with systemic sclerosis. Arthritis Rheum. 1997;40:743–51. doi: 10.1002/art.1780400422. [DOI] [PubMed] [Google Scholar]
- 9.Kantor TV, Friberg D, Medsger TA, Jr, Buckingham RB, Whiteside TL. Cytokine production and serum levels in systemic sclerosis. Clin Immunol Immunopathol. 1992;65:278–85. doi: 10.1016/0090-1229(92)90158-k. [DOI] [PubMed] [Google Scholar]
- 10.Prior C, Haslam PM. In vivo levels and in vitro production of interferon-gamma in fibrosing interstitial lung disease. Clin Exp Immunol. 1992;88:280–7. doi: 10.1111/j.1365-2249.1992.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pober JS, Collins T, Gimbrone MA, et al. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983;305:726–9. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10:370–5. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- 13.Dobrina A, Schwartz BR, Carlos TM, Ochs HHD, Beatty PG, Harlan JM. CD11/18-independent neutrophil adherence to inducible endothelial-leukocyte adhesion molecules in vitro. Immunology. 1989;67:502–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Varga J, Olsen A, Herhal J, Constantine G, Rosembloom J, Jimenez SA. IFN-γ reverses the stimulation of collagen but not fibronectin gene expression by TGF-β. Eur J Clin Invest. 1990;20:487–92. doi: 10.1111/j.1365-2362.1990.tb01890.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosembloom J, Feldman G, Freundlich B, Jimenez S. Inhibition of excessive scleroderma fibroblast collagen production by recombinant interferon. Arthritis Rheum. 1986;29:851–6. doi: 10.1002/art.1780290706. [DOI] [PubMed] [Google Scholar]
- 16.Bryckaert M, Fontenay M, Liote' F, Bellucci S, Carriou R, Tobelem G. Increased mitogenic activity of scleroderma serum: inhibitory effect of human recombinant interferon-gamma. Ann Rheum Dis. 1994;53:776–9. doi: 10.1136/ard.53.11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claman HN. On scleroderma. JAMA. 1989;262:1206–9. doi: 10.1001/jama.262.9.1206. [DOI] [PubMed] [Google Scholar]
- 18.Foegh ML. Chronic rejection-graft arteriosclerosis. Trasplant Proc. 1990;22:119–20. [PubMed] [Google Scholar]
- 19.LeRoy EC, Black C, Fleischsmayer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–8. [PubMed] [Google Scholar]
- 20.Steen VD, Engel EE, Charley MR, Medsger TA. Soluble serum interleukin 2 receptors in patients with systemic sclerosis. J Rheumatol. 1996;23:646–9. [PubMed] [Google Scholar]
- 21.Tan EM, Rodnam GP, Garcia I, Moroi Y, Fritzler MJ, Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis. Lancet. 1963;2:1188–90. doi: 10.1002/art.1780230602. [DOI] [PubMed] [Google Scholar]
- 22.Riboldi P, Asero R, Origgi L, Crespi S. The SL-Ki system in connective tissue diseases: incidence and clinical associations. Clin Exp Rheumatol. 1987;5:29–33. [PubMed] [Google Scholar]
- 23.Coppola C, Della Bella S, Molteni M, Mascagni B, Grassi C, Scorza R. Allogeneic in vitro testing: analysis of response variability. J Chemother. 1991;3:251–4. [Google Scholar]
- 24.Della Bella S, Molteni M, Mascagni B, Zulian C, Compasso S, Scorza R. Cytokine production in scleroderma patients: effects of therapy with either iloprost or nifedipine. Clin Exp Rheumatol. 1997;15:135–41. [PubMed] [Google Scholar]
- 25.Moretta A, Pantaleo G, Moretta L, Cerottini JC, Mingari MC. Direct demonstration of the clonogenic potential of every human peripheral blood T cell. J Exp Med. 1983;157:743–54. doi: 10.1084/jem.157.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damle NK, Englemann EG. Immunoregulatory T cell circuits in man. Alloantigen-primed inducer T cells activate alloantigen-specific suppressor T cells in the absence of the initial antigenic stimulus. J Exp Med. 1983;158:159–73. doi: 10.1084/jem.158.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanier LL, Engleman EG, Gatemby P, Bablock GF, Warner NL, Herzenberg LA. Correlation of functional properties of human lymphoid cell subsets and surface marker phenotypes using multiparameter analysis and flow cytometry. Immunol Rev. 1983;74:143–60. doi: 10.1111/j.1600-065x.1983.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 28.Damle NK, Engleman EG. Role of suppressor T cells and suppressor circuits. In: Oppenheim JJ, Shevach EM, editors. Immunophysiology. Oxford: Oxford University Press; 1990. pp. 405–17. [Google Scholar]
- 29.Gelpi C, Martinez MA, Vidal S, Targoff IN, Rodriguez-Sanchez JL. Autoantibodies to a transfer RNA-associated protein in a murine model of chronic graft versus host disease. J Immunol. 1994;152:1989–99. [PubMed] [Google Scholar]
- 30.Lockshin MD, Markenson JA, Fuzesi L, Kazanjian-Aram S, Joachim C, Ordene M. Monocyte induced inhibition of lymphocyte response to phytohemagglutinin in progressive systemic sclerosis. Ann Rheum Dis. 1983;42:40–44. doi: 10.1136/ard.42.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degiannis D, Seibold JR, Czarnecki M, Raskova J, Raska K. Soluble and cellular markers of immune activation in patients with systemic sclerosis. Clin Immunol Immunopathol. 1990;56:259–70. doi: 10.1016/0090-1229(90)90147-i. [DOI] [PubMed] [Google Scholar]
- 32.Morse JH, Bodi BS. Autologous and allogeneic mixed lymphocyte reactions in progressive systemic sclerosis. Arthritis Rheum. 1982;25:390–5. doi: 10.1002/art.1780250405. [DOI] [PubMed] [Google Scholar]


