Abstract
In peripheral blood progenitor cell (PBPC) collections from patients with solid tumour or haematological malignancy, monocytes were separated into two subpopulations. The majority of monocytes expressed CD14 at a high density without CD16 antigen (the CD14+CD16− monocytes). The remaining monocytes co-expressed CD14 and CD16 (the CD14+CD16+ monocytes). These CD14+CD16+ monocytes amounted to 20.6 ± 15.8%, while those in peripheral blood (PB) obtained from healthy volunteers were 7.3 ± 3.1% (P < 0.05). When subdividing the CD14+CD16+ monocytes into CD14brightCD16dim and CD14dimCD16bright cells, both populations were found to be increased in PBPC collections. Since typical CD14+CD16+ monocytes are the CD14dimCD16bright population, we compared the additional surface antigens on CD14dimCD16bright monocytes with those of CD14+CD16−monocytes. In PBPC collections, the CD14dimCD16bright monocytes exhibited lower levels of CD11b, CD15, CD33 and CD38 expression and higher levels of CD4, CD11a, CD11c and MHC class II, and also revealed a higher percentage of CD4+ cells and a lower percentage of CD15+ cells and CD38+ cells, compared with the CD14+CD16− monocytes. When compared with the CD14dimCD16bright monocytes in PB, those in PBPC collections exhibited higher expression of CD4 and lower expression of CD11b, and also showed higher percentages of CD4+ cells and CD38+ cells and a lower percentage of CD11b+ cells. These results suggest that PBPC collections may be rich in the CD14+CD16+ monocytes in which the proportion of the immature population is increased. It is likely that these monocytes participate in the haematological and immune recovery after PBPC transplantation.
Keywords: CD16 antigen, monocytes, peripheral blood progenitors, phenotype
INTRODUCTION
Autologous peripheral blood progenitor cell transplantation (PBPCT) has been used in a manner similar to autologous bone marrow transplantation (BMT) as a method to restore haematopoietic function following the administration of high-dose chemotherapy to treat solid tumours or haematological malignancies [1, 2]. Recently, allogeneic PBPCT has been also used as a substitute for allogeneic BMT in patients with haematological diseases [3]. Leukapheresis collections for peripheral blood progenitor cells (PBPC) contain many monocytes and T lymphocytes that are thought to be major cellular sources of cytokines [4]. It is supposed that these cells play an important role in haematological recovery or immune response to malignant cells in the transplant patients.
The expression of CD14 and CD16 antigens suggests at least two subsets of monocytes with distinct functional properties [5, 6]. The majority of blood monocytes are CD16− and exhibit strong CD14 expression (the CD14+CD16− monocytes) [6]. The other subpopulation of monocytes shows a low level of CD14 expression and is CD16+ (the CD14+CD16+ monocytes), and these CD14+CD16+ cells represent about < 10% of total blood monocytes [7, 8]. The functional significance of this small CD16+ population remains unclear. Since some cytokines are capable of inducing the CD14+CD16+ monocytes in vivo, it is conceivable that these cells increase in number in diseases that involve cytokine production [6]. In fact, an elevation of CD14+CD16+ monocytes in peripheral blood (PB) was reported in patients with bacterial sepsis [9], tuberculosis [10], AIDS [11] or cancer [12]. The CD14+CD16+ monocytes also enhance cytokine production [6], and may participate in immune responses to infection or malignancy.
In this study, we show an increase in CD14+CD16+ monocytes in PBPC collections obtained from patients with malignancy. To assess the role of these monocytes in specific or unspecific immune response to malignancy or in the regulation of haematopoiesis after PBPCT, the surface antigens associated with differentiation and function on the CD14+CD16+ monocytes were analysed by flow cytometry.
MATERIALS AND METHODS
Mononuclear cell preparation
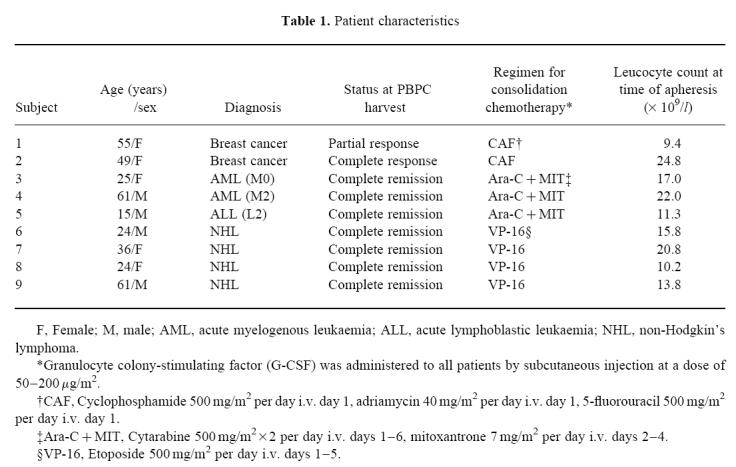
The clinical characteristics of the nine patients evaluated appear in Table 1. For the mobilization of PBPC, two patients with breast cancer were treated with CAF chemotherapy which consisted of cyclophosphamide 500 mg/m2 (day 1), adriamycin 40 mg/m2 (day 1) and 5-fluorouracil 500 mg/m2 (day 1), and seven patients with haematological malignancy (three with acute leukaemia and four with malignant lymphoma) were treated with the Ara-C + MIT regimen (cytarabine 500 mg/m2 × 2 (days 1–6) and mitoxantrone 7 mg/m2 (days 2–4)), or the high-dose VP16 regimen (etoposide 500 mg/m2, days 1–5). Granulocyte colony-stimulating factor (G-CSF; Filgrastim; Kirin Brewery, Tokyo, Japan) was administered to all patients at a dose of 50–200 μg/m2 by subcutaneous injection, from leucocyte suppression (≤ 1 × 109/l) until apheresis completion. After they had given their informed consent, single apheresis collections for PBPC were prepared using a continuous-flow blood cell separator (Cobe spectra; Cobe BCT, Lakewood, CO). We examined fresh PBPC collections from the first apheresis. PB samples were obtained from healthy volunteers, and mononuclear cells (MNC) were separated by density gradient centrifugation, and used directly.
Table 1.
Patient characteristics
 |
Immunofluorescence staining of monocytes
Table 2 summarizes the MoAbs used. PBPC collections or MNC from PB were washed twice with cold Dulbecco's PBS, and incubated for 30 min with appropriately diluted MoAbs according to the results of their titration or with the isotype control antibodies obtained from the same source. Cells were then washed twice with PBS and prepared for analysis.
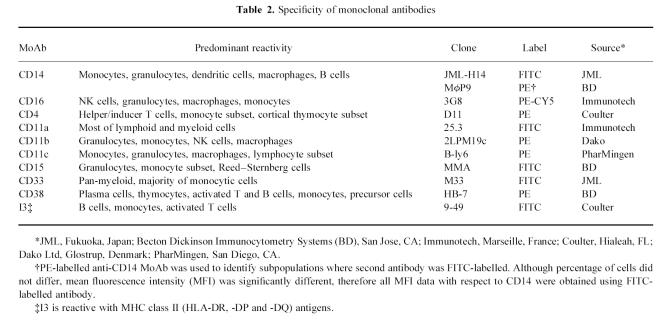
Table 2.
Specificity of monoclonal antibodies
Flow cytometric analysis
Monocyte phenotypic profiles were obtained by flow cytometry using a FACScan Analyser (Becton Dickinson, Mountain View, CA). A minimum of 50 000 events were measured for each sample. The acquisition modes of forward and side scatter (FSC, SSC) were linear, and those of the three fluorescent colours were logarithmic. The monocyte gate was defined by FSC and SSC in data analysis, using the Lysis II software (Becton Dickinson), and was adjusted to contain > 95% CD14+ cells in all samples tested. CD14+CD16− or CD14dimCD16bright monocytes were selected within the monocyte gate (Fig. 1). The expressions of third surface markers were investigated in the histogram mode, and the results were expressed as the mean fluorescence intensity (MFI) on the logarithmic fluorescence scale and the proportion of positive cells.
Fig 1.
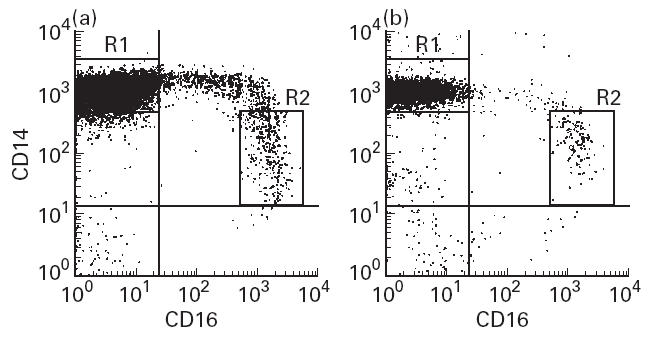
Fluorescence characteristics of monocytes obtained from peripheral blood progenitor cell (PBPC) collections (a) and peripheral blood (PB) (b). MNC were stained with anti-CD14–FITC and anti-CD16–PE-CY5 conjugate. The expression profiles of CD14 (ordinate) and CD16 (abscissa) within the monocyte gate are represented. The R1 gate delineates the CD14+CD16− cells within the monocyte gate, and the R2 gate the CD14dimCD16bright cells. The percentages of the CD14+CD16+ monocytes in representative cases were 16.1% (a) and 5.9% (b), in which those of the CD14dimCD16bright population were 5.5% (a) and 3.3% (b), respectively.
Statistical analysis
Statistically significant differences were assessed using the Mann–Whitney U-test or the Wilcoxon signed rank test.
RESULTS
In PBPC leukapheresis collections obtained from patients with solid tumour or haematological malignancy, monocytes were apparently separated into two subpopulations: CD14+CD16− and CD14+CD16+ monocytes (Fig. 1). The CD14+CD16+ monocytes in PBPC collections accounted for 20.6 ± 15.8% (mean ± s.d.), and those in PB obtained from healthy volunteers (7.3 ± 3.1%), this difference being significant (P < 0.05). Although typical CD14+CD16+ monocytes expressed lower levels of CD14 and higher levels of CD16 (the CD14dimCD16bright cells), the CD14+CD16+ monocytes with low-density expression of CD16 antigen (the CD14brightCD16dim cells) as well as the CD14dimCD16bright cells increased in PBPC collections. The CD14 expression of the CD14brightCD16dim monocytes was equal to that of CD14+CD16− monocytes.
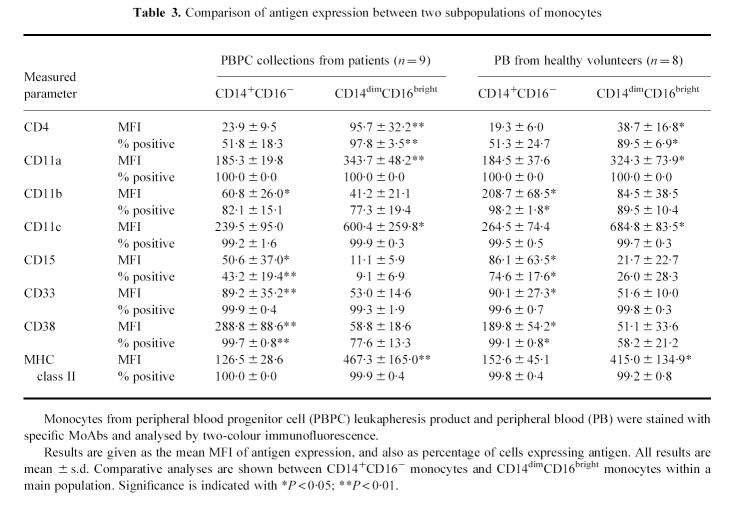
Co-expression of various antigens associated with monocyte differentiation and function was also examined. We limited our investigation to the differences between the CD14+CD16− monocytes and the typical CD14+CD16+ (CD14dimCD16bright) monocytes. Three-colour immunofluorescence was obtained by staining with CD14–FITC, CD16–PE-CY5 and a third PE-conjugated MoAb, or with CD14–PE, CD16–PE-CY5 and a FITC-conjugated MoAb. Figure 2 shows an example of the cluster distributions in the surface antigens seen in a PBPC collection. A summary of phenotypic data on the CD14+CD16− monocytes and the CD14dimCD16bright monocytes is presented in Table 3. To provide normal expression of surface antigens on blood monocytes, PB from eight healthy volunteers was processed and stained with the same MoAb panel (Table 3).
Fig 2.
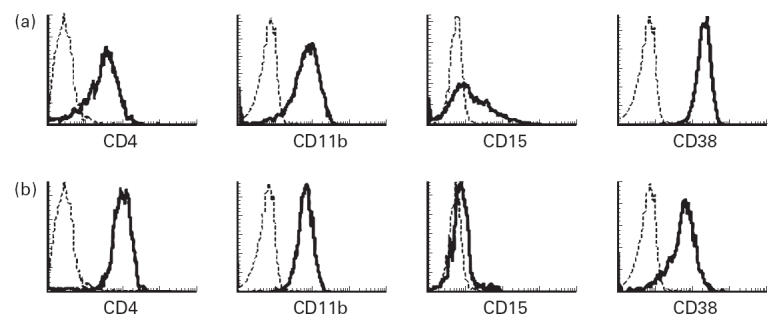
Representative histograms of some phenotypic markers on monocytes from a peripheral blood progenitor cell (PBPC) collection. The fluorescence intensity (abscissa) is expressed on a logarithmic scale. The histogram profiles of CD4, CD11b, CD15 and CD38 antigens within the CD14+CD16− monocyte gate (a) or the CD14dimCD16bright monocyte gate (b) are represented as bold lines. The broken histograms represent the binding of the isotypic control MoAb.
Table 3.
Comparison of antigen expression between two subpopulations of monocytes
The CD14dimCD16bright monocytes in PBPC collections exhibited lower levels of CD11b, CD15, CD33 and CD38 expression, and higher levels of CD4, CD11a, CD11c and MHC class II, and also revealed a higher percentage of CD4+ cells and lower percentages of CD15+ cells and CD38+ cells, compared with CD14+CD16− monocytes (Table 3). There were no differences between the CD14+CD16− monocytes and the CD14dimCD16bright monocytes in the proportions of the other surface antigens.
When compared with the monocytes obtained from healthy volunteers' blood, the CD14dimCD16bright monocytes in PBPC collections exhibited higher expression of CD4 and lower expression of CD11b, and showed higher percentages of CD4+ cells and CD38+ cells and a lower percentage of CD11b+ cells (Table 3). The CD14+CD16− monocytes in PBPC collections expressed lower CD11b and higher CD38, and showed lower proportions of CD11b+ cells and CD15+ cells (Table 3). All of these results have significant differences (P < 0.05).
DISCUSSION
In PBPC collections as well as in PB, two subpopulations of monocytes were revealed. The CD14+CD16+ monocytes in PB from healthy subjects account for < 10% [7, 8]. From the analysis of the monocyte subpopulation in this study, PBPC collections may contain more CD14+CD16+ monocytes than PB. An elevation of blood CD14+CD16+ monocytes has been reported in patients with various diseases [9–12], but there were no obvious differences between the diseases or treatments in our study, although the number of patients was small.
The CD14+CD16+ monocytes can be subdivided into CD14brightCD16dim and CD14dimCD16bright cells; the typical CD14+CD16+ monocytes in PB are the CD14dimCD16bright population [5, 6]. In PBPC collections both populations increased. Similar results were observed in patients or normal volunteers treated with M-CSF [12, 13]. The CD16dim monocytes were also detected in fetuses and neonates [14, 15]. These CD16dim monocytes may represent intermediate forms that go on to down-regulate CD14 in order to develop into the CD14dimCD16bright cells [6], suggesting that PBPC collections are rich in immature CD14+CD16+ monocytes.
Additional markers on the monocytes obtained from PBPC collections were analysed (Table 3). The CD33 and the CD38 antigens which are highly expressed on myelomonocytic precursor cells decrease with differentiation [16, 17]. The CD15 antigen is generally expressed on most terminally differentiated myeloid cells, but its expression on macrophages is at a lower level [18, 19]. On alveolar macrophages and in vitro differentiated macrophages, lower expressions of CD11b, CD15 and CD33, and higher expression of MHC class II were found when compared with blood monocytes [8, 18]. In this study, the CD14dimCD16bright monocytes expressed lower levels of CD11b, CD15, CD33 and CD38 antigens and higher MHC class II than the CD14+CD16− monocytes, suggesting that CD14dimCD16bright monocytes are more differentiated toward tissue macrophages.
The CD14dimCD16bright monocytes in PBPC collections had higher CD4, CD11a, CD11c and MHC class II expression compared with CD14+CD16− monocytes (Table 3). Since macrophages reveal no enhancement of CD11a and CD11c expression [18], the CD14+CD16+ monocytes may be more efficient in integrin- dependent adhesion processes than macrophages. The higher expression of MHC class II suggests that the CD14dimCD16bright monocytes are activated [20] and potent in antigen presentation [7]. The CD4 antigen is found on almost 30% of blood monocytes, with most monocytes expressing cytoplasmic CD4 [21]. A subset of the CD14dim monocytes expresses low levels of CD4 surface antigen [7]. We speculate that the up-regulation of these surface antigens may be involved in cell activation or maturation into macrophages.
The production of mature monocytes/macrophages is regulated by haematopoietic growth factors, or colony-stimulating factors [22]. The production of these factors is induced by the chemotherapy for PBPC mobilization in patients during haematopoietic recovery from myelosuppression. Furthermore, the administration of G-CSF to healthy volunteers results in a persistent and selective up-regulation of CD16 on monocytes [23], and a large increase in monocytic progenitors is reported in PBPC collections mobilized with G-CSF and chemotherapy [24]. Thus, in this study, the chemotherapy for PBPC mobilization and the administration of G-CSF may alter monocyte populations in PB, resulting in an elevation of CD16+CD14+ cells.
When compared with the monocytes from PB, those from PBPC collections have some differences in surface antigens (Table 3). From the changes in CD4, CD11b and CD38 antigens on the CD14+CD16+ monocytes, and in CD11b, CD15 and CD38 on the CD14+CD16− monocytes, the monocytes in PBPC collections may be more immature in differentiation and function than those from PB.
In polymerase chain reaction analysis, CD14+CD16+ monocytes from PB produced high levels of tumour necrosis factor-alpha (TNF-α), IL-1 and IL-6, whereas IL-10 was low or absent [25]. We also observed, using flow cytometry, that the percentage of the CD14+CD16+ monocytes expressing IL-6 or TNF-α was elevated in both PBPC collections and PB compared with that of the CD14+CD16− monocytes (unpublished data). These findings suggest that CD14+CD16+ monocytes may be involved in the activation of the cytokine network. In this study the CD14+CD16+ monocytes, especially immature CD14+CD16+ monocytes, were increased in the PBPC collections. Thus, it is likely that these CD14+CD16+ monocytes influence the cytokine network and participate both in the haematological recovery and in the immune response to residual malignant cells after PBPCT in the transplant patients.
References
- 1.Bezwoda WR, Seymour L, Dansey RD. High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial. J Clin Oncol. 1995;13:2483–9. doi: 10.1200/JCO.1995.13.10.2483. [DOI] [PubMed] [Google Scholar]
- 2.Schimtz N, Linch DC, Dreger P, et al. Randomized trial of filgrastim-mobilized peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–7. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 3.Körbling M, Przepiorka D, Huh YO, et al. Allogenic blood stem cell transplantation for refractory leukemia and lymphoma: potential advantage of over marrow allografts. Blood. 1995;85:1659–65. [PubMed] [Google Scholar]
- 4.Takamatsu Y, Akashi K, Harada M, et al. Cytokine production by peripheral blood monocytes and T cells during haemopoietic recovery after intensive chemotherapy. Br J Haematol. 1993;83:21–7. doi: 10.1111/j.1365-2141.1993.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock HWL, Passlick B, Flieger D. The monoclonal antimonocyte antibody My4 stains B lymphocytes and two distinct monocytes subsets in human peripheral blood. Hybridoma. 1988;7:521–7. doi: 10.1089/hyb.1988.7.521. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler-Heitbrock HWL. Heterogeneity of human blood monocytes: the CD14+CD16+ subpopulation. Immunol Today. 1996;17:424–8. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 7.Passlick B, Flieger D, Ziegler-Heitbrock HWL. Identification and characterization of a novel monocytes subpopulation in peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock HWL, Fingerle G, Ströbel M, Schraut W, Stelter F, Schütt C, Passilick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 9.Fingerle G, Pforte A, Passlick B, Blumenestein M, Ströbel M, Ziegler-Heitbrock HWL. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 10.Vanham G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locher C, Vanham G, Kestens L, Kruger M, Ceuppens JL, Vingerhoets J, Gigase P. Expression patterns of Fcγ receptors, HLA-DR and selected adhesion molecules on monocytes from normal and HIV-infected individuals. Clin Exp Immunol. 1994;98:115–22. doi: 10.1111/j.1365-2249.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleh MN, Goldman SJ, Lobuglio AF, et al. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85:2910–7. [PubMed] [Google Scholar]
- 13.Schmid I, Baldwin GC, Jacobs EL, Isacescu V, Neagos N, Giorgi JV, Glaspy JA. Alterations in phenotype and cell-surface antigen expression levels of human monocytes: differential response to in vivo administration of rhM-CSF or rhGM-CSF. Cytometry. 1995;22:103–10. doi: 10.1002/cyto.990220205. [DOI] [PubMed] [Google Scholar]
- 14.Mawas F, Wiener E, Ryan G, Soothill PW, Rodeck CH. The expression of IgG Fc receptors on circulating leucocytes in the fetus and new-born. Transfus Med. 1994;4:25–33. doi: 10.1111/j.1365-3148.1994.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 15.Maeda M, Van Schie RCAA, Yüksel B, Greenough A, Fanger MW, Guyre PM, Lydyard PM. Differential expression of Fc receptors for IgG by monocytes and granulocytes from neonates and adults. Clin Exp Immunol. 1996;103:343–7. doi: 10.1046/j.1365-2249.1996.d01-615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leukemia Res. 1984;8:521–34. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 17.Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human CD38, a glycoprotein in search of function. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 18.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 19.Lo SK, Golenbock DT, Sass PM, Maskati A, Xu H, Silverstein RY. Engagement of the Lewis X antigen (CD15) results in monocytes activation. Blood. 1997;89:307–14. [PubMed] [Google Scholar]
- 20.River A, Pène J, Pabesandratana H, Chanez P, Bousquet J, Campbell AM. Blood monocytes of untreated asthmatics exhibit some features of tissue macrophages. Clin Exp Immunol. 1995;100:314–8. doi: 10.1111/j.1365-2249.1995.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filion LG, Izaguirre CA, Garber GE, Huebsh L, Aye MT. Detection of surface and cytoplasmic CD4 on blood monocytes from normal and HIV-1 infected individuals. J Immunol Methods. 1990;135:59–69. doi: 10.1016/0022-1759(90)90256-u. [DOI] [PubMed] [Google Scholar]
- 22.Hassan NF, Chehimi J, Ho WZ, Campbell DE, Douglas SD. Effect of hematopoietic growth factors on human blood monocytes/macrophages in in vitro culture. Clin Diagn Lab Immunol. 1994;1:620–5. doi: 10.1128/cdli.1.6.620-625.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohsaka A, Saionji K, Kuwaki T, Takeshima T, Igari J. Granulocyte colony-stimulating factor administration modulates the surface expression of effector cell molecules on human monocytes. Br J Haematol. 1995;89:465–72. doi: 10.1111/j.1365-2141.1995.tb08350.x. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda T, Okamura S, Shimoda K, Takamatsu Y, Inaba S, Harada M, Niho Y. Predominance of myeloid antigens in CD34-positive peripheral blood stem cells over those in bone marrow after administration of granulocyte colony-stimulating factor. Eur J Haematol. 1994;52:201–6. doi: 10.1111/j.1600-0609.1994.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 25.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HWL. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]