Abstract
Intestinal intraepithelial lymphocytes (IEL) constitute the first lymphoid compartment to encounter dietary antigens and intestinal pathogens. IEL are proposed to be involved in the defence against bacterial and viral invasion and to play an important role in mucosal immunity. Fas (CD95/APO-1) is a surface receptor that induces apoptotic cell death upon ligation with Fas ligand (FasL). The aim of this study was to examine the expression and function of Fas and FasL on freshly isolated normal human colonic IEL. The expression and function of Fas and FasL on IEL isolated from 40 normal colonic specimens were examined by flow cytometry, reverse transcriptase-polymerase chain reaction, immunohistochemistry, and DNA-release cytotoxicity assay. Virtually all CD3+ IEL (95.2 ± 4.3%) expressed Fas and were sensitive to agonistic anti-Fas antibody, whereas only 56.6 ± 8.4% of peripheral T lymphocytes expressed Fas and were resistant to the antibody. We also detected FasL mRNA and protein (40.1 ± 4.2%) on IEL, and found that IEL exerted FasL-mediated cytotoxicity against Fas-expressing target cells. These findings suggest that human IEL are activated in situ but are tightly regulated by the constitutive expression of functional Fas and FasL to maintain homeostasis of the mucosal immune system.
Keywords: Fas, Fas ligand, intestinal intraepithelial lymphocytes, apoptosis
INTRODUCTION
Intestinal intraepithelial lymphocytes (IEL) comprise mainly T cells bearing T cell receptor (TCR) γδ, are rich in CD8+ T cells, and represent a unique population in comparison with peripheral T cells [1, 2]. The function of IEL is proposed to play a part in the surveillance of epithelium, and an involvement in the first line of defence against bacterial and viral invasion. Intestinal mucosa are always exposed to constant challenge from an abundance of dietary antigens and intestinal pathogens. This continual antigen stimulation may activate IEL [3, 4]. However, the activation dose not normally result in inflammatory tissue damage, so it is suggested that some regulating mechanisms in the intestine may exist.
Fas is a 48-kD type I membrane protein of the tumour necrosis factor (TNF) receptor family [5]. The ligation of Fas stimulates an intracellular cascade of events that leads to the induction of apoptosis. Whereas freshly isolated peripheral blood T cells express Fas, these cells are insensitive to Fas-mediated apoptosis [6, 7]. Fas ligand (FasL) is a 40-kD type II membrane protein homologous with TNF [8]. FasL on T cells is rapidly induced after activation. Chronically activated T cells constitutively express functional Fas, and the FasL induction of these cells by stimulation with phytohaemagglutinin (PHA), anti-CD3 antibody, or anti-TCR antibody produces rapid cell death by apoptosis, a process termed activation-induced cell death (AICD) [9]. AICD has been proposed to be one mechanism for the regulation of an immune response and the elimination of autoreactive T cells [10, 11]. Thus, the Fas–FasL interaction is considered to contribute to the process of lymphocyte homeostasis. In this study, we investigated the expression and function of Fas and FasL on freshly isolated human colonic IEL.
MATERIALS AND METHODS
Cells
The mouse T lymphoma cell line WR19L and its human Fas cDNA transfectant WR19L-12a [5] were kindly provided by Dr S. Yonehara (Kyoto University, Kyoto, Japan). The mouse T lymphoma cell line L5178Y and its human FasL cDNA transfectant hFasL/L5178Y [12] were kindly provided by Dr H. Yagita (Juntendo University, Tokyo, Japan).
Tissue and blood samples
Human colon samples and autologous peripheral blood mononuclear leucocytes (PBL) were obtained from 40 adult patients (age 66.7 ± 10.8 years, range 42–87 years) undergoing surgical resection for colorectal carcinoma. Normal colonic specimens were obtained at least 5 cm away from macroscopically detectable lesions. Heparinized peripheral blood was also obtained just before surgery.
Lymphocyte isolation
PBL were isolated by standard Ficoll density gradient centrifugation. IEL were obtained by a modification of the procedure of Taunk et al. [13]. Surgical specimens were washed, minced and subjected to three 30-min incubations with 1 mm dithiothreitol and 0.75 mm EDTA in calcium/magnesium-free Hanks' balanced salt solution containing 5% fetal bovine serum (FBS). The crude preparation was passed through a no. 150 stainless steel wire mesh to obtain a single-cell suspension. The lymphocytes were obtained by a three-step discontinuous Percoll density gradient centrifugation and suspended in RPMI 1640 containing 25 mm HEPES, 2 mml-glutamine, 50 μm 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS.
Flow cytometry
MoAbs used were PE-conjugated anti-human CD3, CD8, FITC-conjugated anti-human CD4, TCRαβ, TCRγδ (Becton Dickinson, San Jose, CA), FITC-conjugated anti-human Fas (UB2; MBL, Nagoya, Japan), and PE-conjugated anti-human FasL (NOK-1; kindly provided by Dr H. Yagita). Isotype-matched mouse IgG1 MoAb was used as a control. Freshly isolated IEL and PBL were incubated with saturating concentrations of MoAb at 4°C for 30 min and analysed on a FACSCalibur using he CELLQuest program (Becton Dickinson).
Agonistic anti-Fas antibody-induced cytotoxicity assay
Na51CrO7-labelled cells were incubated with the indicated concentration of anti-human Fas MoAb (CH-11; MBL) at 2 × 104 cells/well in 96-well round-bottomed plates (Becton Dickinson Labware, Lincoln Park, NJ) in triplicate cultures at 37°C for 10 h. The supernatant from each well was harvested and counted in a γ-counter (Aloka, Tokyo, Japan). The percentage of cytotoxicity was calculated according to the formula; 100 × (experimental ct/min – spontaneous ct/min)/(total ct/min – spontaneous ct/min), where spontaneous ct/min is that of culture in medium alone, and total ct/min is in 1% Triton-X.
Reverse transcriptase-polymerase chain reaction
Polyadenylated RNA was directly isolated using a QuickPrep Micro mRNA purification Kit (Pharmacia, Uppsala, Sweden) according to the manufacturer's instructions. mRNA were extracted from 3 × 106 cells of IEL or PBL, and one tenth of the purified mRNA was used for reverse transcriptase-polymerase chain reaction (RT-PCR). cDNA was synthesized from the mRNA by extension of an oligo d(T)16 primer (Perkin Elmer, Norwalk, CT) with 2.5 U MuLV reverse transcriptase (Perkin Elmer). Detection of FasL mRNA was performed using AmpliTaq DNA polymerase (Perkin Elmer) and primers with the following sequences; 5′-TGATGCTGTGTGCATCTGGC and 5′-AGGCACAGTTCTTCCCTGTC [14], with a total of 33 amplification cycles in a automated DNA Thermal Cycler (Perkin Elmer). Each cycle consisted of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and polymerization at 72°C for 2 min. Analysis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA served as a control for sample loading and integrity [15]. Amplification products electrophoresed on a 2% agarose were visualized with ethidium bromide staining.
Immunohistochemistry
Cryostat sections of surgically resected normal colon were cut from quick-frozen tissues embedded in OCT compound (Miles, Elkhart, IN) and mounted on silanized glass slides (Dako, Glostrup, Denmark). After fixation with 100% acetone at 4°C for 9 min, endogenous peroxidase was quenched with 0.5% periodic acid solution (Merck, Darmstadt, Germany) for 10 min and sections were blocked with 10% normal goat serum. The slides were incubated overnight at 4°C with anti-human Fas MoAb (UB2) at 0.1 μg/ml or rabbit anti-FasL polyclonal IgG (N-20; Santa Cruz Biotechnology, Santa Cruz, CA) at 0.1 μg/ml in PBS containing 1% bovine serum albumin (BSA). Bound primary antibodies were visualized by the avidin–biotin complex immunoperoxidase methods, using the Vectastain ABC Elite peroxidase kit and 3, 3′-diaminobenzidine as a chromogen (Vector Labs, Burlingame, CA). Slides were counterstained with methyl green.
DNA-release cytotoxicity assay
Target cell death resulting from culture with effector cells in the wells of 96-well round-bottomed plates (Becton Dickinson Labware) were quantified by measurement of target cell DNA fragmentation [16]. WR19L and WR19L-12a (1 × 106 cells/ml) were labelled with 3H-thymidine at 5 μCi/ml at 37°C for 3 h. After three washes, 2 × 104 cells/100 μl of labelled target cells and 1 × 105 cells/100 μl IEL or PBL were mixed and incubated at 37°C for 10 h in round-bottomed plates in triplicate culture in either the presence or absence of phorbol 12-myristate 13-acetate (PMA; 10 ng/ml) and ionomycin (500 ng/ml) (Sigma Chemical Co., St Louis, MO). The fragmented DNA was washed out and cells were collected on a glassfibre filter by a cell harvester (Labo Science Co., Tokyo, Japan). The radioactivity of the intact chromosomal DNA retained on each filter was measured by liquid scintillation counting. The percentage of 3H-thymidine release was calculated as follows; ((ct/min without effector – ct/min with effector)/ct/min without effector) × 100.
Statistical analysis
Student's t-test was used to determine the significance of differences. P < 0.05 was regarded as significant.
RESULTS
Phenotype of IEL
Flow cytometric analysis of IEL freshly isolated from normal human colon showed that a majority (> 60%) of these lymphocytes expressed CD3, and that this percentage was comparable to the level seen with autologous PBL. The proportions of T cells expressing CD8 and TCRγδ in IEL were significantly greater than those in PBL and, conversely, CD4+ and TCRαβ+ T cell populations were smaller in IEL (Table 1). This is in keeping with the results of previous studies [1, 2].
Table 1.
Expression of T cell markers on freshly isolated colonic intestinal intraepithelial lymphocytes (IEL) and peripheral blood lymphocytes (PBL)
Fas protein expression on IEL
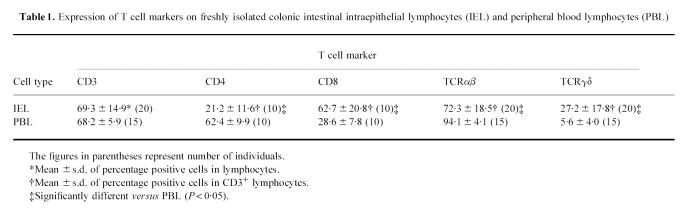
Fas protein expression on freshly isolated IEL was analysed by flow cytometry. As shown in Fig. 1a, virtually all IEL (95.2 ± 4.3%, n = 5) intensely expressed Fas protein. This expression of Fas on IEL was confirmed by immunohistochemical staining, showing that positively stained cells were located adjacent to and slightly deeper than the lining of epithelial cells (Fig. 1b). In contrast, Fas protein expression on PBL from autologous patients was observed in only about a half (56.6 ± 8.4%, n = 5) of the lymphocytes. The difference in the percentage of positive cells between PBL and IEL was statistically significant (P < 0.0001).
Fig 1.
Expression of Fas protein on freshly isolated colonic intestinal intraepithelial lymphocytes (IEL). (a) IEL and peripheral blood lymphocytes (PBL) were stained with PE-conjugated anti-human CD3 MoAb and FITC-conjugated anti-human Fas MoAb (UB2) (open histograms) or control MoAb (filled histograms), and CD3+-gated cells were analysed by FACSCalibur. Mouse T lymphoma cell line (WR19L) and its human Fas cDNA transfectant (WR19L-12a) were used as negative and positive control, respectively. Data from a representative donor out of five are shown. (b) Normal colonic specimen was stained with UB2. Fas (arrow) is detected in a lymphocyte located adjacent to and slightly deeper than the lining of epithelial cells. The scale bar represents 50 μm.
Function of Fas expressed on IEL
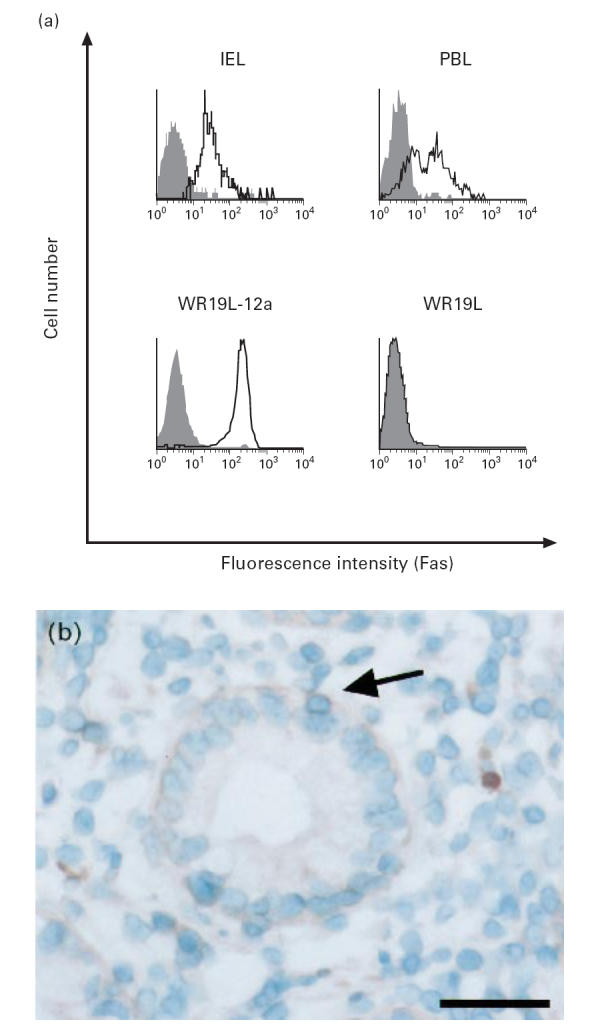
To examine whether the Fas protein expressed on IEL is functional, we assessed the sensitivity of IEL to agonistic anti-human Fas MoAb (CH-11) which induced apoptotic cell death in cells expressing functional Fas [6, 7]. As shown in Fig. 2, incubation of freshly isolated IEL in the presence of CH-11 induced significant cell death in a dose-dependent manner. This is in contrast to the observation that PBL were hardly sensitive to the antibody. These results indicate that freshly isolated human colonic IEL, but not PBL, express functional Fas receptor.
Fig 2.
Cytotoxicity of agonistic anti-human Fas MoAb against intestinal intraepithelial lymphocytes (IEL). 51Cr-labelled IEL, peripheral blood lymphocytes (PBL), WR19L and WR19L-12a were incubated at 37°C for 10 h in the presence of indicated concentrations of anti-human Fas MoAb CH-11. Mean ± s.d. of triplicate cultures of a representative donor out of four is shown.
FasL mRNA expression in IEL
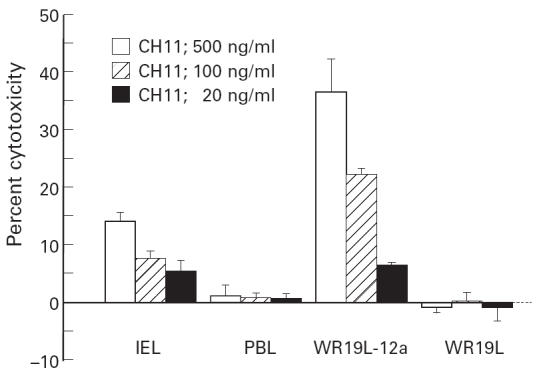
FasL mRNA was extracted from freshly isolated colonic IEL and PBL, and analysed by RT-PCR. As shown in Fig. 3, a 298-base pair FasL transcript was detected by RT-PCR in IEL, but not in PBL, indicating that freshly isolated colonic IEL express Fas ligand mRNA. FasL mRNA was also not detected in fresh PBL treated with the same isolation procedure as IEL (data not shown). As the starting materials for this assay contained the same number of IEL and PBL, the differences in FasL mRNA expression are likely to be qualitative and not quantitative.
Fig 3.
FasL mRNA expression detected by reverse transcriptase-polymerase chain reaction (RT-PCR). A 298-base pairs (bp) human FasL-specific sequence and a 312-bp glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequence were amplified from mRNA isolated from intestinal intraepithelial lymphocytes (IEL), peripheral blood lymphocytes (PBL), mouse T lymphoma cell line L5178Y and its human FasL cDNA transfectant hFasL/L5178Y. Samples were electrophoresed on agarose gel and visualized by staining with ethidium bromide. FasL-specific bands are detected in IEL and hFasL/L5178Y but not in PBL and L5178Y.
FasL protein expression on IEL
Freshly isolated colonic IEL were analysed by flow cytometry using an anti-human FasL MoAb (NOK-1). A substantial proportion (40.1 ± 4.2%, n = 5) of IEL expressed FasL and the mean fluorescence intensity (MFI) was similar to that obtained with the human FasL cDNA transfectant hFasL/L5178Y (Fig. 4a). As FasL is rapidly inducible by exogenous stimulation, it is possible that the detected FasL might have been induced by the isolation procedures. To investigate such a possibility, the in situ expression of FasL in normal colon was examined using immunohistochemical staining. FasL was detected in IEL and lamina propria lymphocytes (LPL) (Fig. 4b). No FasL protein was detected in fresh PBL by flow cytometry (Fig. 4a) nor by immunohistochemistry (data not shown).
Fig 4.
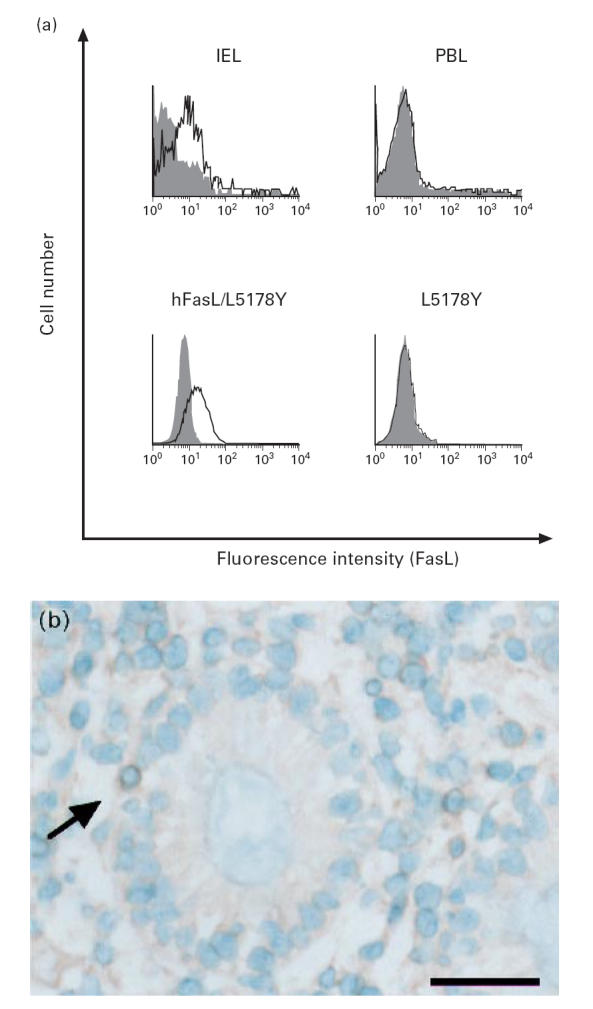
Expression of FasL protein on freshly isolated colonic intestinal intraepithelial lymphocytes (IEL). (a) IEL and peripheral blood lymphocytes (PBL) were stained with FITC-conjugated anti-human CD3 MoAb and PE-conjugated anti-human FasL MoAb NOK-1 (open histograms) or control MoAb (filled histograms), and CD3+-gated cells were analysed by FACSCalibur. Mouse T lymphoma cell line L5178Y and its human FasL cDNA transfectant hFasL/L5178Y were used as negative and positive control, respectively. Data from a representative donor out of five are shown. (b) Normal colonic specimen was stained with rabbit anti-FasL polyclonal antibody N-20. FasL (arrow) is detected in a lymphocyte located adjacent to and slightly deeper than the lining of epithelial cells. The scale bar represents 50 μm.
Function of FasL expressed on IEL
We examined the functional expression of FasL on freshly isolated colonic IEL by DNA-release cytotoxicity assay (Fig. 5). IEL exerted substantial cytotoxicity against a human Fas cDNA transfectant (WR19L-12a) compared against a parental cell line (WR19L). This level of IEL cytotoxicity was comparable to that obtained using a human FasL cDNA transfectant (hFasL/L5178Y). The cytotoxic activity of fresh PBL was not detected in such an assay, but was induced by stimulation with PMA and ionomycin. Fresh PBL treated with the identical isolation procedure to that of IEL exhibited negligible cytotoxicity comparable with untreated PBL (data not shown). These results indicate that colonic IEL constitutively express functional FasL which induces apoptotic cell death in Fas-expressing target cells by Fas–FasL-mediated cytotoxicity.
Fig 5.
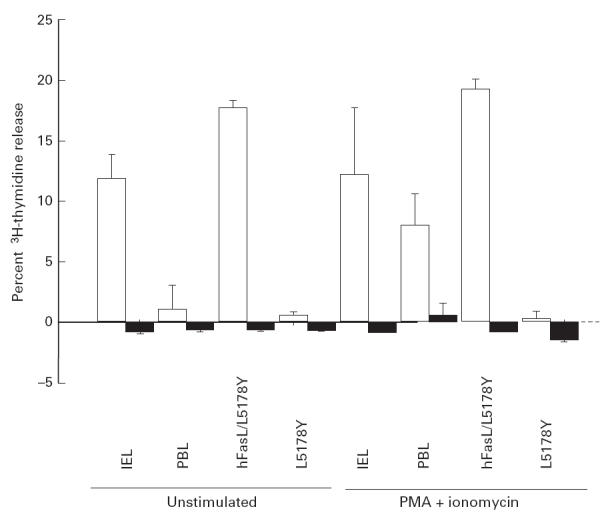
FasL-mediated cytotoxicity of normal human intestinal intraepithelial lymphocytes (IEL). Freshly isolated IEL, autologous peripheral blood lymphocytes (PBL), hFasL/L5178Y and L5178Y cells were incubated with 3H-thymidine-labelled WR19L-12a (□) or WR19L (▪) at 37°C with or without phorbol 12-myristate 13-acetate (PMA) (10 ng/ml) and ionomycin (500 ng/ml) for 10 h at an effector:target ratio of 5. Mean ± s.d. of triplicate cultures of a representative donor out of six is shown.
DISCUSSION
This study examines the expression and function of Fas and FasL on human colonic IEL. We show that both Fas and FasL are constitutively expressed on normal colonic IEL at the mRNA as well as the protein level and are functional for Fas–FasL-mediated apoptosis. Furthermore, our data confirm previous results showing that IEL contain a large proportion of T lymphocytes expressing CD8 and TCRγδ in comparison with PBL [1, 2]. These results indicate that IEL represent a characteristic lymphoid population and that our isolation procedure supplied cell populations from the appropriate compartment.
The spontaneous cytotoxicity of normal human IEL has long been the subject of controversy. Our results are consistent with previous reports which show that human IEL exert spontaneous cytotoxicity against tumour target cells [13, 17]. On the other hand, it is reported that human IEL exhibit cytotoxicity only after in vitro activation with mitogens, IL-2 [18], or ligation of CD2 and CD3 [19]. In these studies, the cytotoxic function was evaluated by 51Cr-release assay, which may be less sensitive in detecting Fas–FasL-mediated cytotoxicity compared with the 3H-thymidine release assay used in this study. Chott et al. [20] examined the expression of cytotoxicity-related molecules on normal human IEL and were unable to detect molecules such as granzyme B, TNF-α or FasL on in situ, but isolated, IEL. It is possible that the cytotoxic function is induced by in vitro activation due to the isolation procedures used. However, this is unlikely to be the case because FasL was detected in normal human IEL by in situ immunohistochemical staining in our present study. Furthermore, we treated PBL with an identical procedure to that used in IEL isolation and failed to detect the phenotypic and functional expression of FasL on these PBL (data not shown). These results strongly suggest that normal human IEL express functional FasL in vivo and kill Fas-expressing target cells.
Whereas freshly isolated peripheral blood T lymphocytes express Fas, these cells are insensitive to Fas-mediated apoptosis [6, 7] as shown in our data. These cells acquire sensitivity to the Fas signalling pathway only after prolonged activation [7]. FasL is not expressed on resting T cells but is rapidly induced by activation [21]. The finding that IEL express both functionally active Fas and FasL suggests their activated state in situ is probably due to continuous exposure to dietary antigens and intestinal flora.
AICD, which is induced by the expression of both Fas and FasL in the same population of lymphocytes, is considered to be one mechanism for the down-regulation of ongoing immune responses to terminate or prevent excessive responses by deletion of activated T cells [10, 11, 22]. The expression of functional Fas and FasL on IEL may prevent deleterious activation of IEL and maintain lymphocyte homeostasis in the normal intestine.
The mechanisms and cellular interactions involved in oral tolerance are not well understood. T cells bearing γδ T cell receptor, most of which reside in intestinal mucosa as IEL, play a critical role in the induction and maintenance of oral tolerance to soluble antigens [23]. Recently, it has been reported that FasL is required to be functionally expressed on tolerance-inducing cells for the induction of allogeneic tolerance [24]. These findings suggest that FasL functionally expressed on IEL may be implicated in unresponsiveness to dietary antigens and prevention of inflammatory tissue damage.
In conclusion, we have demonstrated that human normal IEL functionally express both Fas and FasL. The co-expression of these molecules may be involved in the regulation of immune responses to a variety of dietary antigens and contribute to the maintenance of lymphocyte homeostasis in the digestive tract.
Acknowledgments
We thank Ms Kyoko Nasu and Dr Tohru Tanida for excellent technical assistance.
References
- 1.Deusch K, Lüling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur J Immunol. 1991;21:1053–9. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 2.Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984;25:32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smart CJ, Heatley RV, Trejdosiewicz LK. Expression of CD6 and the UCHL1-defined CD45 (p180) antigen by human colonic T lymphocytes. Immunology. 1989;66:90–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Evert EC. Do the CD45RO+CD8+ intestinal intraepithelial T lymphocytes have the characteristics of memory cells? Cell Immunol. 1993;147:331–40. doi: 10.1006/cimm.1993.1073. [DOI] [PubMed] [Google Scholar]
- 5.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (Anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–56. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–8. [PubMed] [Google Scholar]
- 7.Klas C, Debatin K-M, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–30. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994;6:1567–74. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 9.Kabelitz D, Pohl T, Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993;14:338–9. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 11.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 12.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taunk J, Roberts AI, Ebert EC. Spontaneous cytotoxicity of human intraepithelial lymphocytes against epithelial cell tumors. Gastroenterology. 1992;102:69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- 14.Racila E, Hsueh R, Marches R, Tucker TF, Krammer PH, Scheuermann RH, Uhr JW. Tumor dormancy and cell signaling: anti-μ-induced apoptosis in human B-lymphoma cells is not caused by an APO-1–APO-1 ligand interaction. Proc Natl Acad Sci USA. 1996;93:2165–8. doi: 10.1073/pnas.93.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–9. [PubMed] [Google Scholar]
- 16.Matzinger P. The jam test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 17.Targan S, Britvan L, Kendal R, Vimadalal S, Soll A. Isolation of spontaneous and interferon inducible natural killer like cells from human colonic mucosa: lysis of lymphoid and autologous epithelial target cells. Clin Exp Immunol. 1983;54:14–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert EC. Intra-epithelial lymphocytes: interferon-gamma production and suppressor/cytotoxic activities. Clin Exp Immunol. 1990;82:81–85. doi: 10.1111/j.1365-2249.1990.tb05407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rüthlein J, Heinze G, Auer IO. Anti-CD2 and anti-CD3 induced T cell cytotoxicity of human intraepithelial and lamina propria lymphocytes. Gut. 1992;33:1626–32. doi: 10.1136/gut.33.12.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chott A, Gerdes D, Spooner A, Mosberger I, Kummer JA, Ebert EC, Blunberg RS, Balk SP. Intraepithelial lymphocytes in normal human intestine do not express proteins associated with cytolytic function. Am J Pathol. 1997;151:435–42. [PMC free article] [PubMed] [Google Scholar]
- 21.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–6. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/ (Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 23.Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. γδ T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–8. [PubMed] [Google Scholar]
- 24.George JF, Sweeney SD, Kirklin JK, Simpson EM, Goldstein DR, Thomas JM. An essential role for Fas ligand in transplantation tolerance induced by donor bone marrow. Nature Med. 1998;4:333–5. doi: 10.1038/nm0398-333. [DOI] [PubMed] [Google Scholar]