Abstract
PBC is characterized by T cell-mediated destruction of the biliary epithelial cells lining the small intrahepatic bile ducts. The E2 and E3 binding protein (E3BP (protein X)) components of pyruvate dehydrogenase complex (PDC) are disease-specific autoantigens in PBC. Attempts to localize the T cell autoepitopes within PDC-E2 have, however, generated contradictory results. One study has suggested the presence of T cell epitopes throughout PDC-E2, whilst another has identified a single dominant 14 amino acid T cell epitope (p163) spanning the lipoic acid binding lysine residue in the inner lipoyl domain (ILD) of PDC-E2. The aim of the current study was to determine the prevalence of T cell responses to p163 and PDC-E2 ILD, and the role played by lipoylation of these antigens in their immunogenicity, in a UK PBC population. We found that the majority of the PBC patients showing a 6-day peripheral blood T cell proliferative response to native human PDC also responded, in a MHC class II-restricted fashion, to biochemically purified PDC-E2 and E3BP (which co-purify) (9/10 positive (SI > 2.76), mean SI 5.74 ± 5.04 (PDC-E2/E3BP) versus 6.67 ± 3.84 (PDC), P = NS), implying that the important PBC-specific T cell epitopes are contained within the PDC-E2 or E3BP components of PDC. Only a minority of patients responsive to PDC, however, responded to either lipoylated recombinant PDC-E2 ILD (4/10 positive, mean SI 1.98 ± 1.24, P < 0.005 versus PDC response) or lipoylated p163 (4/12 positive, mean SI 1.90 ± 1.58, P < 0.001). The lipoylation state did not affect the T cell response to either ILD or p163. Our findings suggest that in some UK patients with PBC there are immunodominant T cell autoepitopes within PDC-E2/E3BP which are outside the ILD of PDC-E2.
Keywords: liver cirrhosis, biliary, inner lipoyl domain, T lymphocyte, autoimmunity
INTRODUCTION
PBC is a chronic cholestatic liver disease with an autoimmune aetiology, characterized by a high incidence (> 95%) of serum autoantibodies directed against mitochondrial self-antigens (anti-mitochondrial antibodies (AMA)) [1, 2]. The classical pathological lesion seen in the liver in PBC is damage to, and ultimately destruction of, the biliary epithelial cells (BEC) lining the small intrahepatic bile ducts [3]. BEC damage is accompanied by a portal tract mononuclear cell infiltrate rich in activated T cells [4, 5], leading to the suggestion that autoreactive T cell responses play an important role in the pathogenesis of PBC [6]. The important autoantigens in PBC, which react with AMA, are all members of the 2-oxo acid dehydrogenase family of multienzyme complexes, in particular the E2 component and E3 binding protein (E3BP, previously known as protein X) of pyruvate dehydrogenase complex (PDC) [7]. PDC and related complexes (oxoglutarate dehydrogenase complex (OGDC) and branched chain oxo-acid dehydrogenase complex (BCOADC) to which autoantibody responses are seen in PBC, albeit at lower frequency than to PDC) all play critical roles in intermediary metabolism. Aberrant BEC surface expression of PDC-E2/E3BP, which appears to be restricted to patients with PBC, may play a role in the breakdown of self-tolerance to these antigens by allowing autoantigen expressed on the cell surface to enter the MHC class II-specific antigen-processing pathway [8].
In light of the apparent importance of autoreactive T cell responses in the pathogenesis of PBC, several studies have attempted to characterize T cell responses to PDC in PBC patients [9–14]. Peripheral blood [11–14] and liver-derived [9, 12] T cell proliferative responses to recombinant PDC-E2, and a preparation containing PDC-E2 and E3BP (which co-purify biochemically), have been demonstrated in a significant proportion of PBC patients. We have previously reported that equivalent responses were not seen either to bovine or to human autoantigen in 34 normal subjects (24 of whom were age- and sex-matched) and 46 non-autoimmune chronic liver disease controls (18 of whom were age- and sex-matched) [11, 14]. Moreover, this difference between PBC patients and controls appears to be autoantigen-specific, in that we were unable to demonstrate any difference in the T cell proliferative response to a physiological control antigen (purified protein derivative (PPD)) between PBC patients and controls [15].
The aim of this study was to further our understanding of the epitope specificity of the T cell response to human PDC in PBC. One previous study suggested that there is a single dominant T cell autoepitope within PDC-E2 wholly contained within a 14 amino acid peptide (p163) spanning the lysine residue within the inner lipoyl domain (ILD) of PDC-E2 which binds a cofactor, lipoic acid, critical for enzymatic function [13] [Fig. 1]. This conclusion was based on the peptide specificities of six peripheral blood-derived CD4+ T cell clones from four Japanese PBC patients. We set out here to examine to what extent this apparent restriction of epitope specificity was universally applicable by studying whether proliferative T cell responses are restricted to the ILD of PDC-E2 in general, and to p163 in particular, in a series of Northern European PBC patients.
Fig 1.

Schematic representation of the structure of human (a) PDC-E2 and (b) E3BP. The sequences of peptide p163 from PDC-E2, and the equivalent peptide from E3BP, and their localization within the lipoyl domains are shown. The lipoic acid binding lysine residue is marked K. A high level of sequence identity is observed between the inner lipoyl domain of PDC-E2 and the single lipoyl domain of E3BP. Within p163 and the homologous peptide from E3BP sequence identity is represented by the boxed residues, sequence similarity by *. The three hatched residues, identical in the two sequences and containing the lipoic acid binding residue in each, are the residues identified by Shimoda et al. as the minimal conserved residues for the PDC-E2-derived p163 epitope [13].
Another question we have attempted to answer is whether the lipoylation state of the ILD of PDC-E2 impinges on the autoreactive T cell response to this protein. In the studies of Shimoda et al. [13] the lysine residue of p163 was unlipoylated. We have previously demonstrated, however, that PBC patients' AMA have a higher relative affinity for the human lipoylated ILD than the unlipoylated form using immunoblotting, enzyme-linked immunosorbent inhibition experiments and antibody affinity measurements [16]. A recent study was not able to replicate our results using porcine PDC-E2, but AMA affinity was not examined [17].
MATERIALS AND METHODS
Subjects and study design
Proliferative responses to whole human PDC, its purified E2/E3BP subunits and lipoylated and unlipoylated recombinant human PDC-E2 ILD, were compared in 27 PBC patients. Responses to human PDC and lipoylated and unlipoylated peptide (p163), spanning the ILD lipoic acid binding site, were compared in 32 PBC patients. All responses measured were those of T cells present in the peripheral blood mononuclear cell (PBMC) fraction.
All subjects had biopsy-proven PBC together with cholestatic liver function tests and positive serum anti-PDC-E2/E3BP antibodies by ELISA [18]. All PBC patients were attending the Freeman Hospital PBC clinic and gave informed consent.
Cell preparation
Blood (20 ml) was taken aseptically by venepuncture and added to heparinized tubes (200 U of preservative-free heparin (Sigma, Poole, UK)). The mononuclear cell fraction was separated by standard Lymphoprep density centrifugation (Nycomed, Oslo, Norway) using Leucosep tubes (Greiner, Gloucester, UK) at 800 g for 20 min at 20°C with no braking. PBMC were collected from the interface and washed three times in RPMI 1640 medium, counted and cultured as below.
Antigen preparation
Human PDC was extracted from heart muscle tissue obtained from explanted organs at cardiac allograft. Purification was achieved using a modification [19] of the method described by Stanley & Perham [20]. The final preparation was stored in 20 mm HEPES, 150 mm NaCl and 30% (v/v) glycerol, pH 7.4 (buffer A). This material revealed the five polypeptides characteristic of mammalian PDC when analysed by SDS–PAGE [21]. A fraction containing PDC-E2 and E3BP (protein X) was separated from E1 and E3 by resolution of the complex using gel filtration on a preparative Superose 6 (Pharmacia, Uppsala, Sweden; HR 16/50) column at pH 9.0 in the presence of 1 m NaCl [22] and collected by ultracentrifugation at 250 000 g for 2 h at 2°C in a Beckman L5-65B ultracentrifuge. Purities of all antigen preparations were analysed by SDS–PAGE, and protein concentrations were measured by the method of Bradford [23]. All enzyme preparations were stored at −20°C before use.
Recombinant human inner lipoyl domain was prepared as previously reported [24]. Briefly, the previously cloned human E2p cDNA (kindly provided by Professor M. E. Gershwin, University of California at Davis) was used to construct and express a subgene encoding the ILD (bases 1176–1481). Following polymerase chain reaction (PCR) and subcloning of this region into the prokaryotic expression vector pGEX-2T [23] the resulting plasmid pGLIP-2T encodes a recombinant glutathione-S-transferase (GST) lipoyl domain fusion protein. Escherichia coli was used as the host for the recombinant plasmid and the over-expressed fusion protein was affinity-purified from crude bacterial lysates on a column of immobilized glutathione [25, 26]. Lipoyl domain was separated from the solid-phase GST by cleavage with thrombin at an engineered cleavage site. Two forms of lipoyl domain are obtained with a lipoylated form migrating faster than an unlipoylated form on non-denaturing PAGE [24]. Separation of these products was carried out using anion-exchange chromatography on Mono Q (Pharmacia).
The peptide GDLLAEIETDKATI, which corresponds to human PDC-E2 ILD amino acids 163–176 (p163), was synthesized by the Molecular Biology Facility, University of Newcastle-upon-Tyne, on an Applied Biosystems model 431A peptide synthesizer. Purity was confirmed by reversed-phase high performance liquid chromatography (HPLC) amino acid analysis, and identity confirmed by mass spectroscopy. A modified peptide having lipoic acid covalently attached to lysine 173 was also synthesized (p163-L). Human PDC-E2 p163 shows 10/14 amino acid residue identity and 2/14 amino acid similarity with the equivalent sequence of the lipoic acid binding domain of PDC-E3BP [27] (Fig. 1).
Culture
PBMC were cultured in 96-well U-bottomed plates (Costar, Cambridge, MA) at a density of 2 × 105 cells/well in 100 μl culture medium (RPMI 10) consisting of RPMI 1640 (Hyclone, Cramlington, UK) supplemented with l-glutamine (Gibco, Paisley, UK; 2 mm final concentration) and 10% pooled human AB serum (heat-inactivated at 56°C for 1 h). Antigens were diluted in RPMI 10 and following filter sterilization added to cells in a volume of 100 μl at the following range of final concentrations: PDC 50–200 μg/ml, E2/E3BP 20–80 μg/ml, ILD (± lip) 10–40 μg/ml and p163 (± lip) 10–20 μg/ml. Triplicates of wells were set up for each subject and antigen. For each patient three control wells were set up containing cells only. Cells and antigen were co-cultured for 6 days at 37°C under 5% CO2 in a humidified incubator.
Proliferation assay
Antigen responses were measured by proliferation assay after 6 days in culture. 3H-thymidine (37 kBq; Amersham, Aylesbury, UK) was added to each well in 30 μl of RPMI 10. After a further 16 h of culture the plates were filter harvested semiautomatically, dried, and counted on a Canberra-Packard Matrix 96 counter. Results are expressed as stimulation indices (SI), the SI being the ratio of mean ct/min in the antigen-containing wells to mean ct/min in control wells. An SI > 2.76 (mean control ct/min + 2 s.d. [11]) was taken as indicating a positive response to antigen. For each subject/antigen combination the results given are for the maximum proliferative response. MHC restriction of the response to antigen was assessed by the addition to cultures of blocking anti-class I (W6 32) and class II (Hb 145) MoAbs. Antibody preparations were dialysed before use. In class I and II restriction experiments cells were precultured at 37°C for 1 h with the antibody of interest at a range of concentrations. Blocking protocols were initially optimized using control antigens before being refined and used in autoantigen response.
Statistical analysis
Proliferative responses to different antigen combinations in the same subjects were compared by a non-parametric paired t-test. Correlations between responses to lipoylated and unlipoylated peptide and recombinant polypeptide were compared by Spearman's rank correlation. Frequencies of positive responses in different groups were compared by Fisher's exact test.
RESULTS
Recombinant ILD
PBMC proliferative T cell responses to native human PDC, biochemically purified PDC-E2/E3BP and lipoylated (native form) recombinant human ILD were initially compared in 27 PBC patients. When PBMC were cultured with the native purified human autoantigens PDC or PDC-E2/E3BP, significant proliferation was seen in subjects with a frequency and magnitude of response similar to that previously reported by our group [11, 14]. In our earlier studies we demonstrated that the T cell response to PDC-E2/E3BP is largely absent from normal (n = 34) and non-PBC chronic liver disease (n = 46) controls [11, 14] and that the responding cells are IL-2-secreting, CD4+ T cells [15, 28]. The frequencies of positive response were 10/27 (37%) and 9/27 (33%) for PDC and PDC-E2/E3BP, respectively. Mean SIs in response to PDC and PDC-E2/E3BP in the whole PBC patient group were 3.39 ± 3.44 and 2.89 ± 3.74 (P = NS), respectively. Mean SI in response to PDC-E2/E3BP was not significantly different from that seen to whole complex in the 10/27 patients showing a positive proliferative response to PDC (PDC, mean SI 6.67 ± 3.84; PDC-E2/E3BP, mean SI 5.74 ± 5.04) [Fig. 2]. Positive response to PDC-E2/E3BP was not seen in any patient in the absence of a positive response to whole PDC. The response to PDC-E2/E3BP appeared to be MHC class II-restricted, co-culture with by anti-class II but not class I MoAbs significantly reducing the proliferative response to antigen (Fig. 3).
Fig 2.
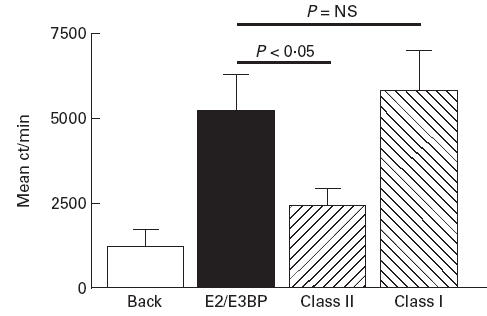
Comparative peripheral blood T cell proliferative responses to whole human PDC (PDC), biochemically purified human PDC-E2/E3BP (E2/E3BP) and lipoylated recombinant human PDC-E2 inner lipoyl domain (recom) in a series of PBC patients responding to whole human PDC. The patient who responded to whole PDC but not PDC-E2/E3BP did not respond to recombinant inner lipoyl domain.
Fig 3.
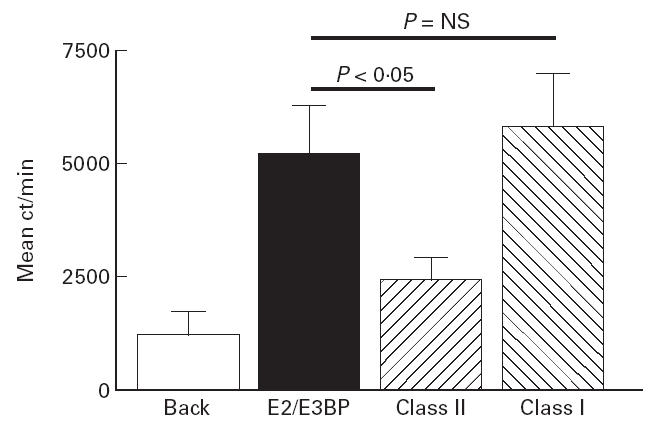
Blocking effects of addition of anti-class I and class II MoAbs on the peripheral blood T cell proliferative response to PDC-E2/E3BP in a representative PBC patient.
In contrast to the whole PDC-E2/E3BP preparation, responses to recombinant, lipoylated PDC-E2 ILD were of significantly lower magnitude (mean SI 1.98 ± 1.24, P < 0.005), and less frequently positive (4/10 (40%) positive, P = 0.01) than those seen to whole complex in the 10/27 patients responsive to PDC (Fig. 2). A positive response to recombinant ILD was not seen in any patient in the absence of a positive response to PDC. The single patient who showed a response to PDC but not PDC-E2/E3BP did not respond to recombinant ILD.
Peptide 163
In order to localize further the important T cell epitopes within PDC we studied proliferative responses to lipoylated p163, the peptide suggested by Shimoda et al. to be (in an unlipoylated form) an important T- cell autoepitope within PDC [13]. Responses to PDC and lipoylated p163 were compared in 32 PBC patients (mean SI to PDC 3.58 ± 3.40). The response seen to PDC in this subject group was identical to that seen in the subject group used for the study of responses to PDC-E2/E3BP and recombinant ILD. Twelve of 32 (38%) of the patients had a positive response to whole complex (mean SI to PDC in positive group 6.67 ± 3.84). Within the group of PDC patients responsive to whole complex only 4/12 (33%, P < 0.005) responded to lipoylated p163 (mean SI 1.90 ± 1.58, P < 0.001) (Fig. 4).
Fig 4.
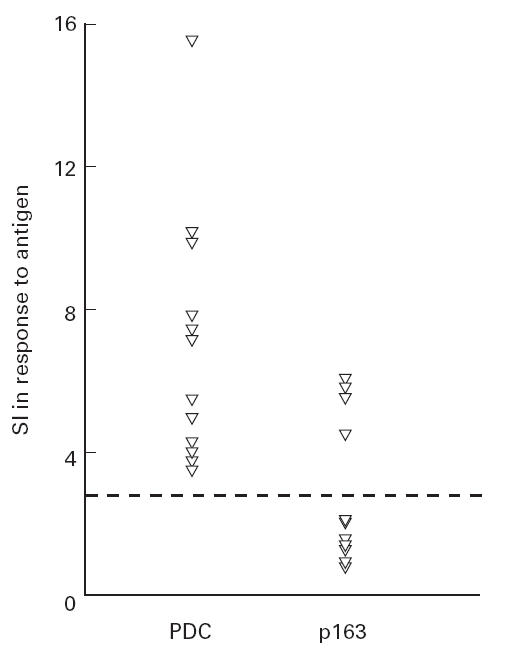
Comparative peripheral blood T cell responses to human PDC (PDC) and lipoylated p163 in a series of PBC patients responding to PDC.
No significant differences were seen between the responses to lipoylated recombinant ILD and p163 in either the whole subject groups (mean SI 1.98 ± 1.24 versus 1.90 ± 1.58, P = NS) or in the subgroups of patients showing a positive response to PDC (mean 2.65 ± 1.75 versus 2.70 ± 2.06, P = NS).
Lipoylation status
In order to study the role played by the lipoic acid co-factor in the T cell epitope spanning the lipoic acid binding residue, we compared responses to lipoylated recombinant domain and p163 with those seen to equivalent unlipoylated homologues. Significant correlations were seen between the responses seen to lipoylated and unlipoylated recombinant ILD (r = 0.92, P < 0.0005) (Fig. 5) and to lipoylated and unlipoylated p163 (r = 0.69, P < 0.01) (Fig. 6) in PDC-responsive PBC patients. Mean SIs in response to lipoylated and unlipoylated domain in PDC-responsive patients were 2.65 ± 1.75 and 3.22 ± 2.61, respectively (P = NS). Mean SIs in response to lipoylated and unlipoylated p163 in PDC-responsive patients were 2.70 ± 2.06 and 2.34 ± 1.81, respectively (P = NS). These findings suggest that lipoic acid does not play a significant role in in vitro T cell responses to the ILD of PDC-E2.
Fig 5.
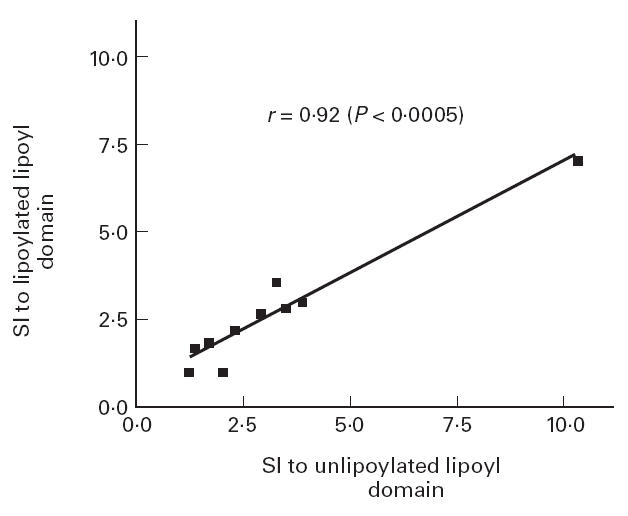
Correlation between the response to lipoylated and unlipoylated recombinant PDC-E2 inner lipoyl domain in PBC patients responsive to PDC.
Fig 6.
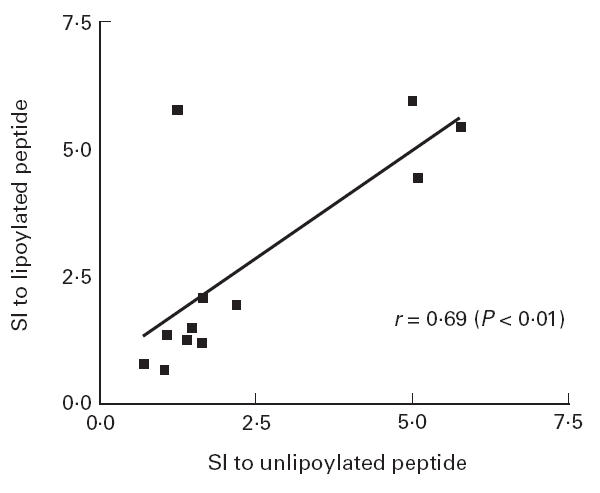
Correlation between the response to lipoylated and unlipoylated p163 in PBC patients responsive to PDC. A significant correlation is seen.
DISCUSSION
In previous studies we have demonstrated that peripheral blood MHC class II-restricted CD4+ T cell proliferative responses to purified whole PDC-E2/E3BP (protein X) both of bovine [11] and human origin [14] are restricted to PBC patients, suggesting a role in the disease process. Studies using limited tryptic digestion to split native PDC-E2 into catalytic and lipoyl binding domains (Fig. 1), to which autoreactive T cell responses were studied in PBC patients [14], demonstrated that most PBC patients responsive to PDC-E2 showed a response to both the catalytic and the lipoyl domains, suggesting that there are T cell autoepitopes throughout PDC-E2. The T cell cloning experiments of Shimoda et al., in contrast, suggested that there may be a single dominant peptide epitope (p163) spanning the lipoyl binding lysine within the ILD [13]. The main aim of this present study was to address the apparently contradictory findings of these methodologically distinct studies.
When we compared peripheral blood T cell responses of PBC patients to purified whole human PDC, PDC-E2/E3BP and recombinant lipoylated (native form) human PDC-E2 ILD, highly similar responses were seen to whole human PDC and PDC-E2/E3BP, suggesting that the T cell response to the whole complex is largely directed against PDC-E2 and/or E3BP. In only one patient did the response appear to be against a component of PDC not contained within the PDC-E2/E3BP preparation. Previous studies suggest that this response is likely to be directed against E1 [11, 14]. Positive responses were seen to recombinant human PDC-E2 ILD, however, in only a minority of patients responding to PDC and PDC-E2/E3BP. These findings suggest that the PDC-E2 ILD contains T cell autoepitopes of significance for some patients. In other patients, however, additional epitopes, which appear to lie outside the ILD, are of importance. This is in keeping with the initial primary response data of Shimoda et al. who demonstrated primary responses to pools of peptides spanning the outer lipoyl and catalytic domains as well as the ILD [13]. No significant differences were seen between the T cell responses to lipoylated ILD and (lipoylated) p163. This suggests that the limited response seen to the ILD is largely directed against p163.
Taken together, these data suggest that there are significant T cell epitopes within the PDC-E2/E3BP preparation which, unlike p163, lie outside the ILD. This is in contrast to the main immunodominant B cell epitopes, which are restricted to the ILD (although ILD-specific autoantibodies show some cross-reactivity with the structurally homologous outer lipoyl domain) [29–31]. These additional T cell autoepitopes may be located either within the binding or catalytic domains of PDC-E2, or within E3BP, which shows structural homology with PDC-E2 in its lipoyl domain (Fig. 1) and with which anti-PDC-E2 autoantibody responses are cross-reactive [32]. The possibility that there are significant T cell epitopes within E3BP may be of significance in the light of recent data suggesting that the autoantigen present on the surface of BEC of affected patients, but not normal individuals, may be E3BP rather than PDC-E2 itself [33]. One potential explanation for the absence of response to peptide p163 in the majority of our PBC patients could lie in the MHC class II restriction of the response to peptide. Shimoda et al. demonstrated that the only class II allele common to their four patients responsive to p163 was HLA DRB40101 (DR53) [13], an allele seen in between 70% and 80% of the normal Japanese population (but not seen at increased incidence in the Japanese PBC population). This led them to suggest that presentation of p163 in these patients is DRB40101-restricted. It should be noted, however, that p163 contains a number of additional class II binding motifs for other alleles including DR1, DR3, DR7 and DR8, the only class II allele to show a significant disease association in Western PBC populations [34, 35]. If p163 does have the capacity to bind to multiple class II gene products [36, 37], the relatively high frequency of p163 responses seen even in our population may result from this phenomenon in addition to presentation by DRB40101. Another possible explanation for the lack of response to p163 would be a low frequency of p163-specific T cells in the peripheral circulation. However, the magnitude of positive responses to p163, when seen, was comparable to that seen to the whole complex, whilst no responses to p163 (or to recombinant ILD) were seen in patients not responding to PDC, making this explanation less likely.
The differences between the findings of this study and previously published data regarding peptide epitopes derived from T cell cloning provide support for the view that caution must be applied in interpreting data from T cell cloning experiments [38]. The act of cloning involves the selection of the progeny of a single precursor because of vigorous in vitro growth characteristics, and imposition of culture conditions which can themselves skew the response phenotype [38]. This may lead to apparently important observations being made regarding the specificity and phenotype of cloned T cells which in reality relate primarily to the cloning response and have little relevance to the in vivo situation. Indeed, Ichiki and colleagues have recently reported that T cell lines reactive to p163 can be derived from healthy subjects using ‘primary IL-2 stimulation’, although they found that the frequency of such lines was higher in PBC patients who have HLA-DR53 [39]. The use of primary responses of the type described here has the advantage that the responding cells have not been subjected to cloning selection bias, and are therefore at least one step closer to the in vivo state.
The presence or absence of lipoic acid appeared to have no affect on the T cell responses to lipoyl domain or immunodominant peptide. There are two potential explanations for this finding. The first is that the lipoic acid residue does not form part of the T cell epitope interacting either with the T cell receptor or MHC. The second potential explanation is that lipoic acid is cleaved during antigen processing. The lack of effect of lipoylation of p163 (which as a peptide is likely to bypass normal antigen-processing pathways) suggests that the former interpretation is correct.
In conclusion, our studies show that the state of lipoylation of PDC-E2 has no effect on the T cell response to this autoantigen. In addition, although p163 represents an important T cell autoepitope in some PBC patients, our findings support the conclusion that there are immunodominant T cell epitopes which are outside the ILD of PDC-E2. This should be appreciated, if as has been proposed [13] peptide-based tolerization revolving around p163 is attempted in PBC.
Acknowledgments
J.M.P. was supported by a project grant from the Wellcome Trust. D.E.J.J. was supported by a Medical Research Council Clinician Scientist Fellowship.
References
- 1.Kaplan M. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–9. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 2.Jones DEJ, Bassendine MF. Primary biliary cirrhosis. J Int Med. 1997;241:127–30. doi: 10.1046/j.1365-2796.1997.127138000.x. [DOI] [PubMed] [Google Scholar]
- 3.Scheuer PJ. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkland A, Festin R, Mendel-Hartvig I, et al. Blood and liver infiltrating lymphocytes in primary biliary cirrhosis: increase in activated T and natural killer cells and recruitment of primed memory T-cells. Hepatology. 1991;13:1106–11. doi: 10.1002/hep.1840130617. [DOI] [PubMed] [Google Scholar]
- 5.Leon MP, Spickett G, Jones DEJ, et al. CD4+ T-cell subsets defined by isoforms of CD45 in primary biliary cirrhosis. Clin Exp Immunol. 1995;99:233–9. doi: 10.1111/j.1365-2249.1995.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van de Water J, Shimoda S, Niho Y, et al. The role of T-cells in primary biliary cirrhosis. Semin Liver Dis. 1997;17:105–13. doi: 10.1055/s-2007-1007188. [DOI] [PubMed] [Google Scholar]
- 7.Bassendine MF, Jones DEJ, Yeaman SJ. Biochemistry and autoimmune response to the 2-oxoacid dehydrogenase complexes in primary biliary cirrhosis. Semin Liver Dis. 1997;17:49–60. doi: 10.1055/s-2007-1007182. [DOI] [PubMed] [Google Scholar]
- 8.Joplin R, Gershwin ME. Ductular expression of autoantigens in primary biliary cirrhosis. Semin Liver Dis. 1997;17:97–103. doi: 10.1055/s-2007-1007187. [DOI] [PubMed] [Google Scholar]
- 9.Van de Water J, Ansari A, Surh CD, et al. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T-cell response of primary biliary cirrhosis. J Immunol. 1991;146:89–94. [PubMed] [Google Scholar]
- 10.Löhr H, Fleischer B, Gerken G, et al. Autoreactive liver-infiltrating T cells in primary biliary cirrhosis recognise inner mitochondrial epitopes and the pyruvate dehydrogenase complex. J Hepatol. 1993;18:322–7. doi: 10.1016/s0168-8278(05)80276-4. [DOI] [PubMed] [Google Scholar]
- 11.Jones DEJ, Palmer JM, Yeaman SJ, et al. T-cell responses to the components of pyruvate dehydrogenase complex in primary biliary cirrhosis. Hepatology. 1995;21:995–1002. [PubMed] [Google Scholar]
- 12.Van de Water J, Ansari A, Prindiville T, et al. Heterogeneity of autoreactive T-cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723–33. doi: 10.1084/jem.181.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimoda S, Nakamura M, Ishibashi H, et al. HLA DRB4, 0101-restricted immunodominant T-cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune disease. J Exp Med. 1995;181:1835–45. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DEJ, Palmer JM, Yeaman SJ, et al. T-cell responses to native human proteins in primary biliary cirrhosis. Clin Exp Immunol. 1997;107:562–8. doi: 10.1046/j.1365-2249.1997.3101202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DEJ, Palmer JM, Leon MP, Yeaman SJ, Bassendine MF, Diamond AG. T-cell responses to Tuberculin-purified protein derivative in primary biliary cirrhosis: evidence for defective T-cell function. Gut. 1997;40:277–83. doi: 10.1136/gut.40.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn J, Diamond AG, Palmer JM, et al. Lipoylated and unlipoylated domains of human PDC-E2 as autoantigens in primary biliary cirrhosis: significance of lipoate attachment. Hepatology. 1993;18:1384–91. [PubMed] [Google Scholar]
- 17.Koike K, Ishibashi H, Koike M. Immunoreactivity of porcine dihydrolipoamide acetyl- and succinyl-transferases (PDC-E2, OGDC-E2) with primary biliary cirrhosis sera: characterisation of the autoantigenic region and effects of enzymatic delipoylation and re-lipoylation. Heaptology. 1998;27:1647–74. doi: 10.1002/hep.510270602. [DOI] [PubMed] [Google Scholar]
- 18.Heseltine L, Turner IB, Fussey SPM, et al. Primary biliary cirrhosis: quantitation of autoantibodies to purified mitochondrial enzymes and correlation with disease progression. Gastroenterology. 1990;99:1786–92. doi: 10.1016/0016-5085(90)90488-m. [DOI] [PubMed] [Google Scholar]
- 19.Palmer JM, Bassendine MF, James OFW, et al. Human pyruvate dehydrogenase complex as an autoantigen in primary biliary cirrhosis. Clin Sci. 1993;85:289–93. doi: 10.1042/cs0850289. [DOI] [PubMed] [Google Scholar]
- 20.Stanley CJ, Perham RN. Purification of the 2-oxo acid dehydrogenase complexes from ox heart by a new method. Biochem J. 1980;191:147–54. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman SJ. The mammalian 2-oxo acid dehydrogenases: a complex family. Trends Biochem Sci. 1986;11:293–6. [Google Scholar]
- 22.Cook KG, Bradford AP, Yeaman SJ. Resolution and reconstitution of bovine kidney branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1985;225:731–5. doi: 10.1042/bj2250731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradford AP, Howell S, Aitken A, et al. Primary structure around the lipoate attachment site on the E2 component of bovine heart pyruvate dehydrogenase complex. Biochem J. 1987;245:919–22. doi: 10.1042/bj2450919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn J, Diamond AG, Masters AK, et al. Expression and lipoylation in Escherichia coli of the inner lipoyl domain of the E2 component of the human pyruvate dehydrogenase complex. Biochem J. 1993;289:81–85. doi: 10.1042/bj2890081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DB, Johnson S. Single step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 26.Miles JS, Guest JR. Subgenes expressing single lipoyl domains of the pyruvate dehydrogenase complex of Escherichia coli. Biochem J. 1987;245:869–74. doi: 10.1042/bj2450869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RA, Bowker-Kinley MM, Wu P, et al. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. J Biol Chem. 1997;272:19746–51. doi: 10.1074/jbc.272.32.19746. [DOI] [PubMed] [Google Scholar]
- 28.Jones DEJ. T-cell autoimmunity in primary biliary cirrhosis. Clin Sci. 1996;91:551–8. doi: 10.1042/cs0910551. [DOI] [PubMed] [Google Scholar]
- 29.Van de Water J, Gershwin ME, Leung P, et al. The autoepitope of the 74 KD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791–9. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fussey SPM, Bassendine MF, James OFW, et al. Characterisation of the reactivity of autoantibodies in primary biliary cirrhosis. FEBS Letters. 1989;246:49–53. doi: 10.1016/0014-5793(89)80251-0. [DOI] [PubMed] [Google Scholar]
- 31.Surh CD, Coppel R, Gershwin ME. Structural requirement for autoreactivity on human pyruvate dehydrogenase — E2, the major autoantigen of primary biliary cirrhosis. J Immunol. 1990;144:3367–74. [PubMed] [Google Scholar]
- 32.Yeaman SJ, Fussey SPM, Danner DJ, et al. Primary biliary cirrhosis: identification of two major mitochondrial autoantigens. Lancet. 1988;i:1067–70. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]
- 33.Joplin R, Wallace LL, Lindsay JG, et al. The human biliary epithelial cell plasma membrane antigen in primary biliary cirrhosis—pyruvate dehydrogenase X? Gastroenterology. 1997;113:1727–34. doi: 10.1053/gast.1997.v113.pm9352878. [DOI] [PubMed] [Google Scholar]
- 34.Underhill JA, Donaldson PT, Bray GP. Susceptibility to primary biliary cirrhosis is associated with the HLA-DR8-DQB1*0402 haplotype. Hepatology. 1992;16:1404–8. doi: 10.1002/hep.1840160616. [DOI] [PubMed] [Google Scholar]
- 35.Gregory WL, Mehal WZ, Dunn AN, et al. Primary biliary cirrhosis: contribution of HLA class II allele DR8. Q J Med. 1993;86:393–9. [PubMed] [Google Scholar]
- 36.Chicz RM, Urban RG, Lane WS, et al. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC related molecules and are heterogenous in size. Nature. 1992;358:764–8. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 37.Chicz RM, Urban RG, Gorga JC, et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen SBA, Webb LMC, Feldmann M. The method of deriving human T-cell clones alters the proportion of IL-10-producing cells. Immunology. 1996;87:343–7. doi: 10.1046/j.1365-2567.1996.503575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichiki Y, Shimoda S, Hara H. Analysis of T-cell receptor β of the T-cells reactive to the human PDC-E2, 163–176 peptide in the context of HLA–DR53 in patients with primary biliary cirrhosis. Hepatology. 1997;26:728–33. doi: 10.1053/jhep.1997.v26.pm0009303504. [DOI] [PubMed] [Google Scholar]


