Abstract
The roles of enteric viruses and food antigens as possible triggers in human insulin-dependent diabetes mellitus and the evidence that mucosal-associated homing receptors are important in both human and experimental diabetes prompted us to undertake an immunohistochemical study of intestinal specimens from patients with IDDM. We studied jejunal morphology and immunohistochemistry in 26 patients with IDDM, 13 of whom had the HLA-DQB1*0201 gene and therefore a higher risk of coeliac disease. The findings were compared with those in specimens from age-matched controls. Villous structure and the density of the intraepithelial lymphocytes were normal in every biopsy specimen. The extent of positivity with anti-DR and -DP antibodies in the villous epithelium was significantly greater in the specimens from patients than in those from controls (P = 0.0002 in both comparisons). The crypts were also more positive: for DR P = 0.0001, and for DP P = 0.002. The densities of T cells, CD4+, CD8+, and T cell receptor α/β+ and γ/δ+ cells in the epithelium and lamina propria were similar in patients and controls, but the patients had significantly more α4/β7 integrin+ cells in the lamina propria (P = 0.006). No difference was seen between HLA-DQB1*0201-positive and -negative patients. These findings reflect a stage of inflammation in the structurally normal intestines of patients with IDDM and suggest secretion of inflammatory Th1-type cytokines in the intestine.
Keywords: insulin-dependent diabetes mellitus, immunohistochemistry, HLA class II antigens, α4/β7 integrin
INTRODUCTION
The progressive extinction of insulin-secreting β cells in the pancreatic islets, mediated by cytotoxic T cells and leading to diabetes both in man and non-obese diabetic (NOD) mice has been shown to be produced by programmed cell death, apoptosis [1–3]. Transgenic NOD mice made to express Fas ligand showed heightened sensitivity to diabetogenic T cells due to increased apoptosis [1], while, on the other hand, NOD mice lacking Fas expression were protected from diabetes [4]. One mechanism for the expression of Fas in human β cells may be induction of nitric oxide production by IL-1β [3]. While the initiator(s) of the autoreactive T cells in diabetes remain enigmatic [5, 6], in the mouse model glutamic acid decarboxylase (GAD)-reactive CD4+ Th1 cells alone are capable of inducing diabetes [7]. We recently showed that the reactivity of the peripheral lymphocytes of patients with IDDM to GAD65 was markedly reduced when the α4/β7 integrin+ cells were depleted [8], suggesting that GAD-reactive cells may originate in the intestine. The mucosal homing pattern of infiltrating lymphocytes and the appearance of mucosal addressin in the endothelium of vessels in the pancreatic islets during the development of diabetes in NOD mice suggest that the antigens encountered in the intestine may be relevant to the autoimmune process [9–12]. A cross-reactive initial stimulation for both T and B cells may be caused by food antigens, such as cow's milk [13], or by infectious agents, recent data indicating the strongest candidate to be enterovirus [14, 15]. B cells, too, are essential for the development of autoimmune diabetes in NOD mice [16, 17]; and therefore, B cells originating from the gut may also be important as antigen-presenting cells in the pancreas.
In the present study we investigated lymphocytes in the jejuna of 26 patients with IDDM, measuring their subgroups, activation markers and homing receptors, and looked for vascular integrins and expression of class II antigens on the epithelium as markers of immune activation.
PATIENTS AND METHODS
Patients
Patients were recruited from those attending the diabetes clinic of the Children's Hospital, University of Helsinki. They had all participated in an epidemiological study on childhood diabetes in Finland [18]. The study was designed to look for latent coeliac disease (CoD) in IDDM. We therefore invited equal numbers of patients who were either positive or negative for the HLA-DQB1*0201 gene allele; 13 patients in each group agreed to participate. None of the patients had gastrointestinal symptoms or growth retardation, and serologic tests for CoD (IgA- and IgG-antigliadin and IgA-reticulin antibodies) were negative [19].
Findings in the histochemical study were compared with those for age-matched controls studied in the same laboratory. The numbers of control biopsies available for the different stains ranged from eight to 24 owing to the loss of 16 control specimens (as the freezer had accidentally warmed up). None of the controls had gastrointestinal symptoms; their jejuna were studied because of retardation of growth. The morphology of the jejunum was found to be normal in every case.
Immunohistochemical methods
Jejunal biopsy specimens were taken with a Crosby–Kugler capsule from the ligament of Treitz or distal to it. Specimens were divided for routine histology and immunohistochemical study. The part for morphology was embedded in paraffin and stained with haematoxylin–eosin; the other part for immunohistochemistry was fixed in acetone for 10 min, then in chloroform for 30 min [20]. The specimens were evaluated without knowledge of the background of the specimen. The numbers of stained cells were counted under a light microscope with a calibrated graticule at ×1000 magnification. In the surface epithelium, the positive cells along the basement membrane of the epithelium were counted from the length of at least 30 fields (1.8 mm) and their densities expressed as cells/mm. In the lamina propria at least 30 fields between the epithelium and muscularis mucosae (0.1 mm2) were measured and cell densities expressed as cells/mm2. The proportion of epithelial cells staining with antisera to HLA-DR and HLA-DP was measured with point counting, with a grid with 100 points. At least 1500 points falling on epithelial cells were counted in each specimen, each point registered as positive or negative, and the percentage of positive points was calculated. With the same technique, the proportions of the lamina propria occupied by cells positive for intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) antisera were estimated. Proliferative (Ki-67+) cells in the crypts were calculated as percentages of the crypt epithelial cells, at least 200 crypt cells being counted in each specimen. In the same specimens the densities of cells having α/β or γ/δ T cell receptors, and the CD3, CD4, CD8 or CD38 antigens were counted both in the epithelium and in the lamina propria.
Monoclonal antibodies
MoAbs to constant fragments of HLA-DR and -DP chains purchased from Becton Dickinson (Mountain View, CA) were used at dilutions of 1:1000 and 1:40, respectively. The anti-DQ MoAb (Serotec Ltd, Oxford, UK) reacting with a monomorphic determinant present on all HLA-DQ molecules [21] was used at a dilution of 1:400. The MoAb Ki-67 (DAKO-PC; Dakopatts, Glostrup, Denmark) recognizing a nuclear antigen present only in proliferating cells [22] was used at a dilution of 1:10. The MoAb TCRδ1 (T Cell Diagnostics, Inc., Woburn, MA), and the antibody βF1 (T Cell Diagnostics) were used at dilutions of 1:100. The MoAbs anti-Leu-4 (anti-CD3; Becton Dickinson), T4 (anti-CD4; Coulter Immunology, Hialeah, FL) and OKT8 (anti-CD8; Ortho Diagnostic Systems, Raritan, NJ) were used at a dilution of 1:400. Antiserum to CD38, anti-Leu-17, was obtained from Becton Dickinson and used at a dilution of 1:50. Antibodies to CD11a, recognizing the lymphocyte functional antigen-1 (LFA-1; Immunotech, Marseilles, France), were used at a dilution of 1:50, antibodies to its ligand, ICAM-1 (CD54) (T Cell Diagnostics) were used at a dilution of 1:2000; and antibodies to VCAM-1 (CD106) (T Cell Diagnostics) at a dilution of 1:1000. The MoAb to α4/β7 integrin (ACT-1) was received from Leukosite Inc. (Cambridge, MA) and used at a dilution of 1:200.
Determination of HLA antigens
The HLA-DQ alleles were determined in the context of a nationwide study on genetic and environmental factors in childhood diabetes in Finland, as described elsewhere [23].
Statistical analysis
Cell densities and the percentages of positive stainings in patients and controls were compared by non-parametric tests (Mann–Whitney U-test and χ2 test) because of the non-linear distribution of the parameters. The values for patients either positive or negative for the HLA-DQB1*0201 allele were compared by the same tests.
Ethical considerations
Specimens from controls were taken for diagnostic purposes. The indication for the biopsy, i.e. the high incidence of asymptomatic CoD, was explained to patients with IDDM and their parents, who gave their consent for taking the biopsy specimen for this study. The study plan was accepted by the Ethics Committee of the Children's Hospital, University of Helsinki.
RESULTS
Histology of jejunal specimens
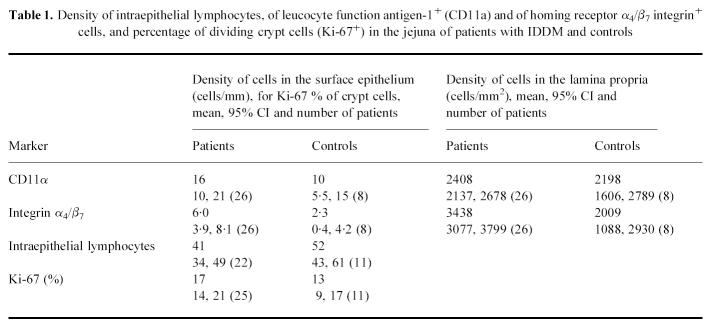
The morphology of all biopsy specimens was normal. The percentages of proliferative cells in the crypts of the specimens from patients and controls were similar, indicating normal turnover rates of epithelial cells (Table 1).
Table 1.
Density of intraepithelial lymphocytes, of leucocyte function antigen-1+ (CD11a) and of homing receptor α4/β7 integrin+ cells, and percentage of dividing crypt cells (Ki-67+) in the jejuna of patients with IDDM and controls
Intraepithelial lymphocytes
The density of intraepithelial lymphocytes was similar in patients and controls (Table 1). No difference was found between the patients with the DQB1*0201 allele and those without it.
The densities of CD3+, α/β TCR+, γ/δ TCR+, CD8 and CD4+ intraepithelial lymphocytes were similar in patients and controls. The density of α4/β7 integrin+ cells tended to be greater in the patients (P = 0.07) (Table 1).
Lymphocytes in the lamina propria
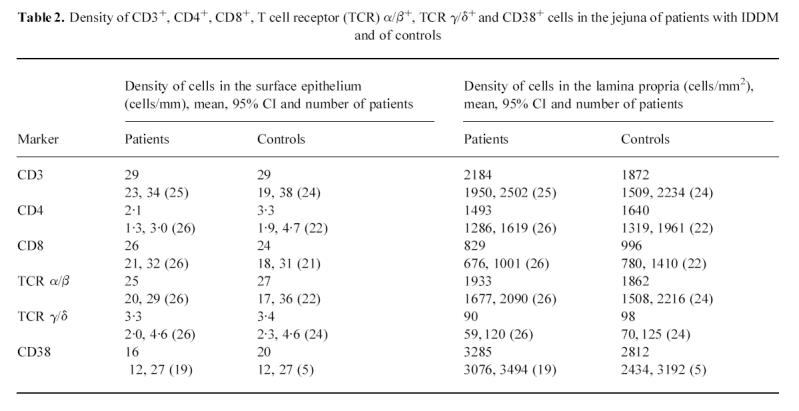
The densities of CD3+, CD4+, CD8+, CD11a+, TCR α/β and γ/δ+ cells in patients and controls did not differ; the density of CD38+ cells being somewhat higher in the patients (P = 0.065) (Tables 1 and 2). The densities of α4/β7 integrin+ cells, however, were significantly greater in the specimens from the patients than in those from the controls (P = 0.006) (Table 1).
Table 2.
Density of CD3+, CD4+, CD8+, T cell receptor (TCR) α/β+, TCR γ/δ+ and CD38+ cells in the jejuna of patients with IDDM and of controls
 |
Staining with HLA class II antibodies
In biopsy specimens from patients with IDDM, the percentage of epithelium stained with anti-HLA-DR antiserum was significantly greater than in those from the controls (P = 0.0002) (Table 3). In 10 out of 24 patients the crypts stained positive with HLA-DR antiserum to a small extent (Fig. 1), while none of the eight control specimens showed positive staining (P = 0.001, χ2 test). The area stained with anti-HLA-DP antiserum was similarly more extensive in the specimens from patients than in those from controls (P = 0.0002) (Table 3). In many specimens from patients the epithelium of the villi was positive with anti-HLA-DP antibodies from the tip to the base (Fig. 2), but in the controls only granular spots of some cells in the apex of the villi were positive (Fig. 3). Of 22 patients, 19 had some staining of the crypt epithelium (Fig. 4), while two out of seven control specimens also showed weak staining (P = 0.002).
Table 3.
Staining of the jejunal epithelium with HLA-DR and -DP antibodies, and of the structures in the lamina propria with anti-intercellular adhesion molecule (ICAM) and -vascular cell adhesion molecule (VCAM) antibodies in jejuna of patients with IDDM and controls
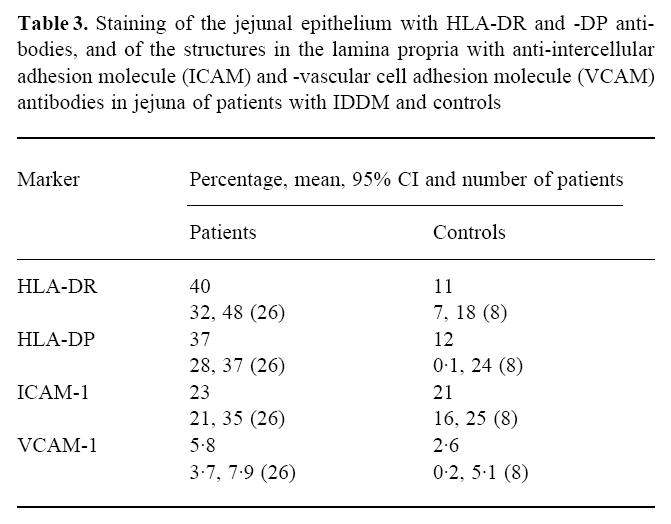 |
Fig 1.
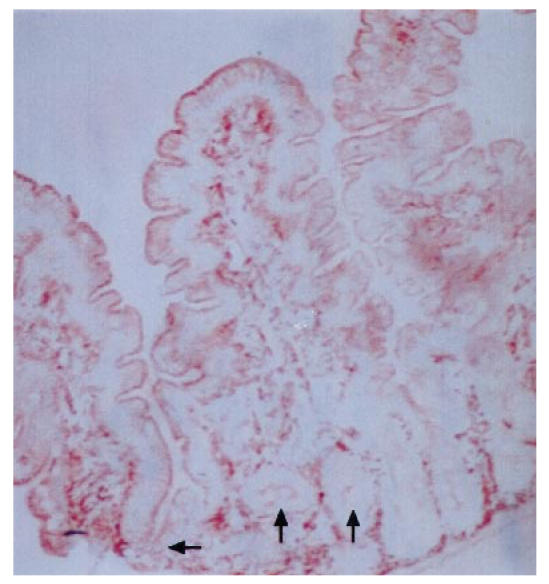
Biopsy specimen from a patient with IDDM treated with antibodies to HLA-DR, original mag. ×10. Positive staining is seen in the apical parts of epithelial cells throughout the villi and also in many crypts, indicated by arrows.
Fig 2.
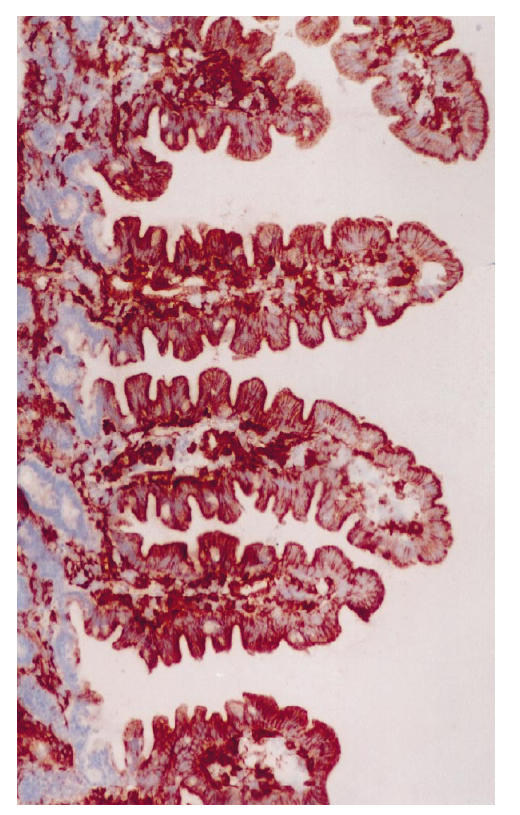
Biopsy specimen from a patient with IDDM treated with antibodies to HLA-DP, original mag. ×10. Intensive, positive staining is seen throughout the epithelial cells of villi; on average, 81% of the area of epithelial cells was positive.
Fig 3.
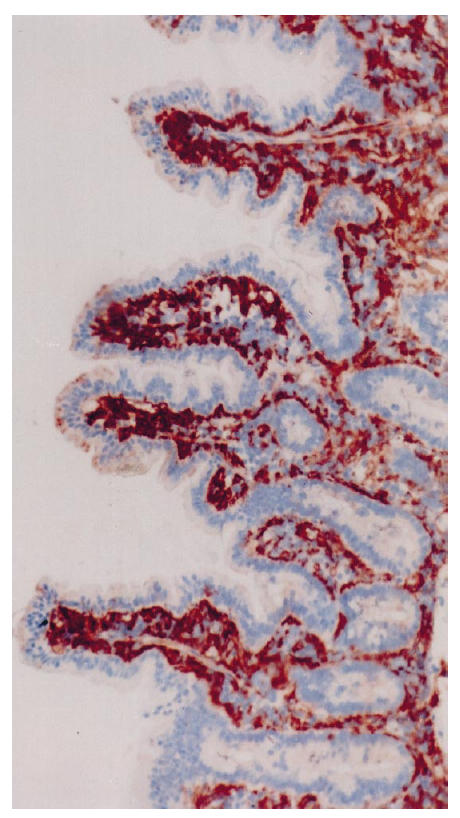
Biopsy specimen from a control patient treated with antibodies to HLA-DP, original mag. ×10. Only a few positive granules are seen in the apical parts of the epithelial cells at the tip of villi; on average, 1% of the area of epithelial cells was positive.
Fig 4.
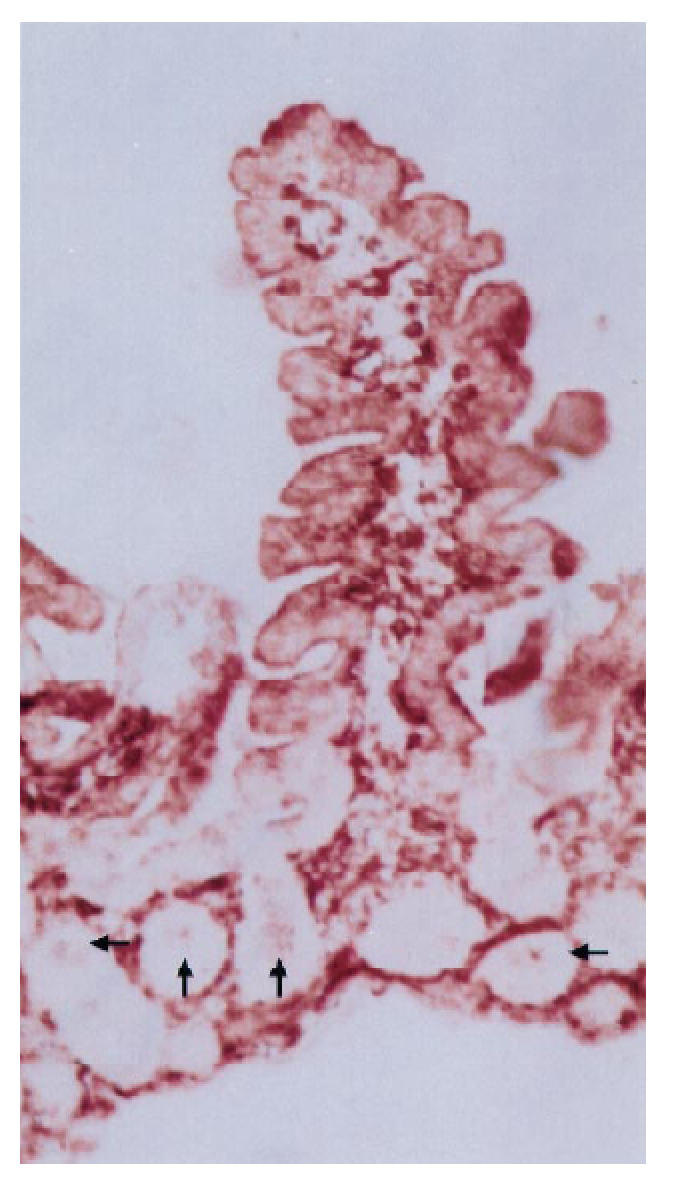
Biopsy specimen from a patient with IDDM treated with antibodies to HLA-DP, original mag. ×10. In addition to positive staining in epithelial cells of villi, cells in several crypts are stained (indicated with arrows).
Staining of the epithelial cells with anti-DQ antiserum was seen in two of the 11 controls studied, while 19 of 26 patients had similar positive staining (P = 0.001); in all, the staining was weak and seen only in the apical parts of the villi.
When the patients were divided according to the presence or absence of HLA-DQB1*0201 allele, no difference was seen in the staining pattern for HLA-DR or -DP (data not shown).
Staining with anti-ICAM-1 (CD54) and VCAM-1 (CD106) antibodies
In both patients and controls, anti-CD54 antibodies were bound to a large proportion, > 20%, of the surface of the lamina propria, the majority of positive staining was on cells, but some was also seen extracellularly (Table 3). The percentage of structures in the lamina propria positive for anti-CD106 antibodies was much smaller, in patients about 6%, and in controls 3% (P = 0.1).
DISCUSSION
In patients with IDDM, compared with age-matched controls, we found a marked extension of the expression of both HLA-DR and HLA-DP antigens in the surface epithelium of the jejunum. HLA-DQ was also more frequently expressed in the patients' jejuna than in control specimens. In the normal human jejunum, HLA-DR and -DP antigens are present only in the upper part of the villi [24], as seen in the controls of the present study. In inflammatory conditions, such as active CoD, DR and DP expression in the epithelium is greatly extended, involving not only the villi but also the crypts [25–27]. The major inducers of this extensive expression of HLA class II molecules are interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), as shown in tissue culture experiments [28, 29]. Thus, in the jejunum of a patient with diabetes, the same Th1-dependent cytokine secretion pattern prevails as is essential for the development of diabetes in the pancreas [30]. In such an environment, in vitro experiments have demonstrated that the antigen presentation by epithelial cells is enhanced and the cells are more leaky [29]. Both changes may relate to the pathogenesis of IDDM.
Another striking finding in the jejuna of our patients with IDDM was the increased density of mononuclear cells bearing α4/β7 integrin, significantly in the lamina propria and to a lesser extent in the epithelium. The α4/β7 integrin is a homing receptor of lymphocytes, directing them to mucosal tissues, especially to the intestine, by binding to mucosal vascular addressin cellular adhesion molecule-1 [31]. Subpopulations of CD4+ memory cells [32] and B cell blasts [33] express particularly high levels of α4/β7 integrin. In addition, we have shown that B cells activated by oral vaccines [34] or by enteric infections also express this receptor [35]. Therefore, the present finding in the jejuna of patients with IDDM, that expression of α4/β7 integrin by mononuclear cells is increased, suggests that in these patients more T and B memory cells have accumulated in the intestinal mucosae. The necessity of B cells for the initiation of experimental diabetes has recently been demonstrated [16, 17]. We previously showed that α4/β7+ cells were responsible for the greater part of T cell stimulation by GAD65 [8], showing the importance of mucosally activated T cells in the autoimmune response in IDDM. In the NOD mouse model, the endothelium of the pancreatic venules expresses the mucosal addressin cellular adhesion molecule-1, and mononuclear cells infiltrating the pancreas bear the homing receptor interacting with it, integrin α4β7 [9]. Another marker suggestively different in patients and controls was CD38; its antibodies recognized the majority of T and B cells. This molecule is also involved in the trafficking of lymphocytes through high endothelial venules [36].
In the present study we set out to find markers of latent CoD [37]. The great majority of patients with IDDM have normal jejunal morphology, but the risk of CoD is greatly increased [19, 38, 39]. This increase is seen particularly in those patients with IDDM whose HLA class II antigens are similar to those of patients with CoD [39]. Recently it was shown that, of the patients with IDDM found to have CoD, a significant proportion had developed it only after the onset of clinical diabetes [19, 40]. All our patients with IDDM were on a normal gluten-containing diet. In Finland, young patients with IDDM have been shown to have a higher dietary intake of cereal proteins than control children [41]. It is to be expected that patients liable to develop CoD will show subtle changes, best distinguished by immunohistochemical methods, similar to those described in many family members of patients with CoD [42, 43]. A constant finding in the jejunal epithelium of such patients is a markedly higher density of T cell receptor γ/δ+ cells [20, 44, 45]. This is definitively a marker of latent CoD [46], and is associated with the disease-specific HLA-DQ genes in the family members of patients with CoD [42]. Among the patients with IDDM, and even among those having the DQB1*0201 allele, a high density of γ/δ+ cells was not more frequent than in the controls. This is probably due to the relative rarity of latent CoD in IDDM among those having already had diabetes for 5 years. In our study [19], 1.2% of patients had unrecognized silent CoD when IDDM became clinically manifest and an equally high percentage developed CoD within 2 years, resulting in an incidence of 2.4% in patients having had diabetes for 2–3 years. As the incidence in Finnish adults with IDDM is only slightly higher, 4.1% [47], the lat-ent CoD in the study population may be expected to be quite low.
Thus even when routine histology shows nothing abnormal, immunohistochemistry suggests a jejunal abnormality in many patients with IDDM. The antigen presentation by the epithelial cells of the jejunum may be enhanced in these patients compared with that of non-diabetic subjects, and the lamina propria contains more mononuclear cells with mucosal homing receptors. These changes suggest that a similar cytokine secretion pattern prevails in the jejunum as in the prediabetic pancreas. It may relate to disturbed induction of tolerance to oral antigens in the gut.
Acknowledgments
We are grateful for the skilful technical assistance of Ms Sirkku Kristianssen. Financial support for this study was received from the Sigrid Juselius Foundation, Finland, and the University of Helsinki, and OMFB-CIMO. Monoclonal antibody ACT-1 (anti-α4/β7) was a gift from Leukosite Inc., Cambridge, MA.
References
- 1.Chervonsky AV, Wang Y, Wong FS, Visintin I, Janeway CA, Matis LA. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 2.Obrien RL, Born WK. Direct evidence for an immunological role of lymphocytes bearing T-cell receptor-gamma-delta. Year in Immunology. 1990;6:51–68. 1989–90. [PubMed] [Google Scholar]
- 3.Stassi G, Maria RD, Trucco G, Rudert W, Testi R, Galluzzo A, Giordano C, Trucco M. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med. 1997;186:1193–200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itoh N, Imagawa A, Hanafusa T, et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1997;186:613–8. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 6.Schloot NC, Roep BO. Islet antigen-specific T cell clones in autoimmune diabetes: from mice to men. Diabet Met Rev. 1997;13:127–38. doi: 10.1002/(sici)1099-0895(199709)13:3<127::aid-dmr192>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Zekzer D, Wong FS, Ayalon O, Millet I, Altieri M, Shintani S, Solimena M, Sherwin RS. GAD-reactive CD4+ Th1 cells induce diabetes in NOD/SCID mice. J Clin Invest. 1998;101:68–73. doi: 10.1172/JCI119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paronen J, Klemetti P, Kantele JM, Savilahti E, Perheentupa J, Åkerblom HK, Vaarala O. Glutamate decarboxylase reactive peripheral blood lymphocytes from patients with insulin-dependent diabetes mellitus express gut-specific homing receptor α4β7-integrin. Diabetes. 1997;46:583–8. doi: 10.2337/diab.46.4.583. [DOI] [PubMed] [Google Scholar]
- 9.Hänninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA. Vascular addressins are included on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–15. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepault F, Cagnerault M-C, Faveeuw C, Bazin H, Boitard C. Lack of l-selectin expression by cells transferring diabetes in NOD mice: insights into the mechanisms involved in diabetes prevention by Mel-14 antibody treatment. Eur J Immunol. 1995;25:1502–7. doi: 10.1002/eji.1830250605. [DOI] [PubMed] [Google Scholar]
- 11.Fabien N, Bergerot I, Orgiazzi J, Thivolet C. Lymphocyte function associated antigen-1, integrin α4, and l-selectin mediate T-cell homing to the pancreas in the model of adoptive transfer of diabetes in NOD mice. Diabetes. 1996;45:1181–6. doi: 10.2337/diab.45.9.1181. [DOI] [PubMed] [Google Scholar]
- 12.Hänninen A, Salmi M, Simell O, Jalkanen S. Mucosa-associated (β7-integrin high) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes. 1996;45:1173–80. doi: 10.2337/diab.45.9.1173. [DOI] [PubMed] [Google Scholar]
- 13.Vaarala O, Klemetti P, Savilahti E, Reijonen H, Ilonen J, Åkerblom HK. Cellular immune response to cow milk betalactoglobulin in patients with newly diagnosed insulin dependent diabetes. Diabetes. 1996;45:178–82. doi: 10.2337/diab.45.2.178. [DOI] [PubMed] [Google Scholar]
- 14.Dahlquist G, Frisk G, Ivarsson S, Svanberg L, Forsgren M, Diderholm H. Indications that maternal coxsackie B virus infection during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia. 1995;38:1371–3. doi: 10.1007/BF00401772. [DOI] [PubMed] [Google Scholar]
- 15.Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Diabetes. 1995;44:652–7. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 16.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new ‘speed congenic’ stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–53. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–6. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 18.Tuomilehto J, Lounamaa R, Tuomilehto-Wolf E, et al. Epidemiology of childhood diabetes in Finland—background of a nationwide study of type I (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:70–76. doi: 10.1007/BF00400854. [DOI] [PubMed] [Google Scholar]
- 19.Saukkonen T, Savilahti E, Reijonen H, Ilonen J, Tuomilehto-Wulf E, Åkerblom HK. Coeliac disease: frequent occurrence after clinical onset of insulin dependent diabetes mellitus. Diabetic Med. 1996;13:464–70. doi: 10.1002/(SICI)1096-9136(199605)13:5<464::AID-DIA101>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Savilahti E, Arato A, Verkasalo M. Intestinal γ/δ receptor bearing T lymphocytes in celiac disease and inflammatory bowel diseases in children. Constant increase in celiac disease. Pediatr Res. 1990;28:579–81. doi: 10.1203/00006450-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Spits H, Borst J, Giphart M, Coligan J, Terhorst C, De Vries JE. HLA-DQ antigen can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984;14:299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- 22.Gerdes J, Lemke H, Baisch H, Wacker H-H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 23.Ilonen J, Reijonen H, Herva E, et al. Rapid HLA-DQB1 genotyping for four alleles in the assessment of risk for IDDM in the Finnish population. Diabetes Care. 1996;19:795–800. doi: 10.2337/diacare.19.8.795. [DOI] [PubMed] [Google Scholar]
- 24.Scott H, Solheim BG, Brandtzaeg P, Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Gastroentrol. 1980;12:77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 25.Scott H, Brandtzaeg P, Solhei BG, Thorsby E. Relations between HLA-DR-like antigens and secretory component (SC) in jejunal epithelium of patients with coeliac disease or dermatitis herpetiformis. Clin Exp Immunol. 1981;44:233–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Marley NJ, Macartney JC, Ciclitira PJ. HLA-DR, DP and DQ expression in the small intestine of patients with coeliac disease. Clin Exp Immunol. 1987;70:386–93. [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly J, Weir DG, Feighery C. Differential expression of HLA-DR gene products in the normal and coeliac small bowel. Tissue Antigens. 1988;29:151–60. doi: 10.1111/j.1399-0039.1988.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 28.Sturgess RP, Hooper LB, Spencer J, Hung CH, Nelufer JM, Ciclitira PJ. Effects of interferon-gamma and tumour necrosis factor-alpha on epithelial HLA class-II expression on jejunal mucosal biopsy specimens cultured in vitro. Scand J Gastroenterol. 1992;27:907–11. doi: 10.3109/00365529209000161. [DOI] [PubMed] [Google Scholar]
- 29.Terpend K, Boisgerault F, Blaton MA, Desjeux JF, Heyman M. Protein transport and processing by human HT29-19A intestinal cells: effect of interferon gamma. Gut. 1998;42:538–45. doi: 10.1136/gut.42.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Herrath MG, Oldstone MB. Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–9. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picker LJ. Control of lymphocyte homing. Curr Opin Immunol. 1994;6:394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 32.Schweighoffer T, Tanaka Y, Tidswell M, et al. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol. 1993;151:717–29. [PubMed] [Google Scholar]
- 33.Farstad IN, Halstensen TS, Lazarovits AI, Norstein J, Fausa O, Brandtzaeg P. Human intestinal B-cell blasts and plasma cells express the mucosal homing receptor integrin α4β7. Scan J Immunol. 1995;42:662–72. doi: 10.1111/j.1365-3083.1995.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 34.Kantele A, Kantele JM, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher CE, Mäkelä PH. Homing potentials of circulating lymphocytes in human depend on the site of activation. Oral, but not parental, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol. 1997;158:574–9. [PubMed] [Google Scholar]
- 35.Kantele JM, Arvilommi H, Kontiainen S, Salmi M, Jalkanen S, Savilahti E, Westerholm M, Kantele A. Mucosally activated circulating human B cells in diarrhea express homing receptors directing them back to gut. Gastroenterol. 1996;110:1061–7. doi: 10.1053/gast.1996.v110.pm8612994. [DOI] [PubMed] [Google Scholar]
- 36.Dianzani U, Malavasi F. Lymphocyte adhesion to endothelium. Crit Rev Immunol. 1995;15:167–200. doi: 10.1615/critrevimmunol.v15.i2.40. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson A, Arranz E, Omahony S. Clinical and pathological spectrum of coeliac disease active, silent, latent, potential. Gut. 1993;34:150–1. doi: 10.1136/gut.34.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mäki M, Hällström O, Huupponen T, Vesikari T, Visakorpi JK. Increased prevalence of coeliac disease in diabetes. Arch Dis Child. 1984;59:739–42. doi: 10.1136/adc.59.8.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savilahti E, Simell O, Koskimies S, Rilva A, Åkerblom HK. Celiac disease in insulin-dependent diabetes mellitus. J Pediatr. 1986;108:690–3. doi: 10.1016/s0022-3476(86)81042-3. [DOI] [PubMed] [Google Scholar]
- 40.Mäki M, Huupponen T, Holm K, Hällström O. Seroconversion of reticulin antibodies predicts coeliac disease in insulin dependent diabetes mellitus. Gut. 1995;36:239–42. doi: 10.1136/gut.36.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virtanen SM, Räsänen L, Mäenpää J, Åkerblom HK. Dietary survey of Finnish adolescent diabetics and non-diabetic controls. Acta Paediatr Scand. 1987;76:801–8. doi: 10.1111/j.1651-2227.1987.tb10568.x. [DOI] [PubMed] [Google Scholar]
- 42.Holm K, Mäki M, Savilahti E, Lipsanen V, Laippala P, Koskimies S. Intraepithelial gammadelta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339:1500–3. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 43.Holm K, Savilahti E, Mäki M, Koskimies S, Lipsanen V. Immunohistochemical changes in the jejunum in first degree relatives of patients with coeliac disease marker DQ genes. HLA class II antigen expression, interleukin-2 receptor positive cells and dividing crypt cells. Gut. 1994;35:55–60. doi: 10.1136/gut.35.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer J, Isaacson PG, Diss TC, MacDonald TT. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor γ/δ heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989;19:1335–8. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- 45.Halstensen TS, Scott H, Brandtzaeg P. Intraepithelial T cells of the TcR γ/δ+CD8− and Vδ/Jδ1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989;30:665–72. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 46.Mäki M, Holm K, Collin P, Savilahti E. Increase of γ/δ T cell receptor bearing lymphocytes in normal small bowel mucosa in latent coeliac disease. Gut. 1991;32:1412–4. doi: 10.1136/gut.32.11.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collin P, Salmi O, Hällström O, Oksa H, Oksala H, Mäki M. High frequency of coeliac disease in adult patients with type-1 diabetes. Scand J Gastroenterol. 1989;24:81–84. doi: 10.3109/00365528909092243. [DOI] [PubMed] [Google Scholar]