Abstract
It has been suggested that vitamin B12 (vit.B12) plays an important role in immune system regulation, but the details are still obscure. In order to examine the action of vit.B12 on cells of the human immune system, lymphocyte subpopulations and NK cell activity were evaluated in 11 patients with vit.B12 deficiency anaemia and in 13 control subjects. Decreases in the number of lymphocytes and CD8+ cells and in the proportion of CD4+ cells, an abnormally high CD4/CD8 ratio, and suppressed NK cell activity were noted in patients compared with control subjects. In all 11 patients and eight control subjects, these immune parameters were evaluated before and after methyl-B12 injection. The lymphocyte counts and number of CD8+ cells increased both in patients and in control subjects. The high CD4/CD8 ratio and suppressed NK cell activity were improved by methyl-B12 treatment. Augmentation of CD3−CD16+ cells occurred in patients after methyl-B12 treatment. In contrast, antibody-dependent cell-mediated cytotoxicity (ADCC) activity, lectin-stimulated lymphocyte blast formation, and serum levels of immunoglobulins were not changed by methyl-B12 treatment. These results indicate that vit.B12 might play an important role in cellular immunity, especially relativing to CD8+ cells and the NK cell system, which suggests effects on cytotoxic cells. We conclude that vit.B12 acts as an immunomodulator for cellular immunity.
Keywords: vitamin B12, NK cell, CD8, immunomodulation
INTRODUCTION
Vitamin B12 (Vit.B12) has various effects on biological processes in vivo. It is well known that megaloblastic anaemia and peripheral nerve disturbances are caused by lack of vit.B12. In the immune system, an important role of vit.B12 has been reported. Vit.B12 enhanced T cell proliferative responses to concanavalin A (Con A) and immunoglobulin synthesis of B cells by pokeweed mitogen (PWM) [1]. It has been reported that vit.B12 deficiency caused suppression of protective immune responses to viruses and bacteria in an animal model [2].
In human immunity, the action of vit.B12 is still obscure, probably because it is impossible to study the action of vit.B12 using artificially deficient human model systems. However, we occasionally encounter patients with vit.B12 deficiency disorders, such as megaloblastic anaemia, and it is possible to observe the change of immunological parameters after vit.B12 administration in such patients. A few investigations and case reports of immunological abnormalities in vit.B12-deficient megaloblastic anaemia patients have been reported [3–7], but there have been no systematic studies.
In order to investigate the biological actions of vit.B12 on cells of the human immune system, lymphocyte subsets and NK cell activities were examined in patients with vit.B12 deficiency anaemia, and the changes of immune parameters after vit.B12 administration were evaluated.
SUBJECTS AND METHODS
Subjects
Eleven newly diagnosed Japanese patients with vit.B12 deficiency anaemia were admitted to our hospital between December 1990 and June 1993 (age 36–83 years, median 65 years; six males and five females). Seven of 11 patients had pernicious anaemia (PA) and four had post-gastrectomy megaloblastic anaemia (PGMA). All patients showed low serum levels of vit.B12 (< 85 pg/ml; normal range 230–820 pg/ml). Diagnosis was made based on medical history, macrocytic anaemia in peripheral blood, erythroblastosis with megaloblastic changes in bone marrow, low serum levels of vit.B12, the presence of anti-intrinsic factor antibody, antiparietal antibody and clinical responsiveness to vit.B12 therapy.
Thirteen haematologically and immunologically normal volunteers were included as a control group (age 26–92 years, median 72 years; five males, eight females). None showed low serum levels of vit.B12 or anaemia. All tests, including any sampling of blood, were performed with informed consent and with our hospital ethical committee's approval.
Study design
Leucocyte and lymphocyte numbers, percentage and absolute numbers of CD4+ cells and CD8+ cells, CD4/CD8 ratio and NK cell activity were evaluated in all patients at diagnosis and compared with the values in control subjects.
In order to examine the immunomodulatory effect of vit.B12, methylcobalamin was administrated to all patients and to eight of 13 volunteers as follows. Methyl-vit.B12 (500 μg/day; methyl-B12; mecobalamin; Eisai, Tokyo, Japan) was injected intramuscularly every other day for 2 weeks and immunophenotyping of peripheral lymphocytes and NK cell activity were evaluated as before treatment. At that time, all patients and control subjects showed high serum levels of vit.B12 (> 3000 pg/ml). After 2 weeks treatment, patients were treated with vit.B12 1000 μg every 3 months as out-patients; all of them were quite well and anaemia had improved. After 1–2 years of follow up, NK cell activity was estimated in seven of 11 patients who showed high serum levels of vit.B12 (> 3000 pg/ml).
Surface marker analysis
Heparinized peripheral blood was obtained and the mononuclear cell (MNC) fraction was collected by centrifugation on lymphocyte separation medium (Gunma Immunology Institute, Fujioka, Japan). After washing with PBS, MNC were used for further analysis. Commercial MoAbs used for immunophenotyping were as follows: CD3 (OKT3–FITC), CD4 (OKT4–FITC), CD8 (OKT8–FITC) (Ortho, Raritan, NJ) for both patient group and control group before and after methyl-B12 treatment, CD56 (NKH1–FITC) (Coulter, Hialeah, FL) for nine of 11 patients before and after treatment. Additionally, two-colour analyses, using CD3 (Leu-4–PE) × CD57 (Leu-7–FITC), CD3 (Leu-4–PE) × CD16 (Leu-11a–FITC), CD57 (Leu-7–FITC) × CD16 (Leu-11c–PE) (Becton Dickinson, Mountain View, CA), were done in eight of 11 patients and 10 of 13 control subjects for evaluation of NK cell subsets. All phenotyping was performed using a FACScan (Becton Dickinson).
NK cell activity and other tests for immune response
NK cell activity was estimated in all cases and all control subjects before and after 2 weeks of methyl-B12 treatment by the standard 51Cr-release assay using K562 cells as target cells (effector:target ratio was 20:1) and results were expressed as percentage cell lysis.
In five patients, phytohaemagglutinin (PHA)-, Con A- and PWM-stimulated lymphocyte blast formation were measured, and antibody-dependent cell-mediated cytotoxicity (ADCC) was also evaluated in four patients. In all patients and control subjects serum levels of IgG, IgA and IgM were evaluated before and after methyl-B12 administration.
Statistical analysis
Statistical analysis were conducted with paired t-test for the comparison within each group of patients and control subjects and with unpaired t-test for the comparison between the two groups as indicated in the legend for Table 1. Obtained P values were re-estimated with Wilcoxon signed rank test and Mann–Whitney rank sum test. Significance was defined as follows: both t-test and non-parametric method showed P < 0.05. P values < 0.05 obtained with t-test but not with non-parametric analysis were regarded as not significant but showing a tendency. Analysed values were represented as mean ± s.d.
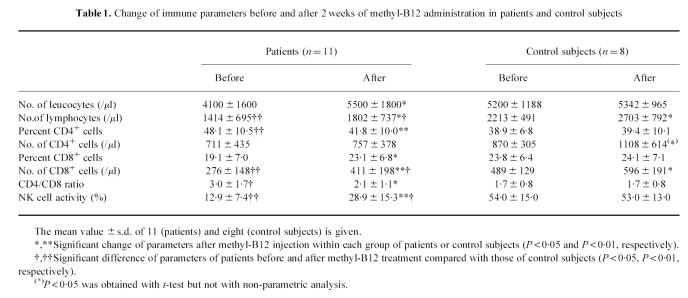
Table 1.
Change of immune parameters before and after 2 weeks of methyl-B12 administration in patients and control subjects
RESULTS
Immunophenotyping and NK cell activity in patients and control subjects before methyl-B12 administration
Although no significant difference in leucocyte counts was noted between patients (n = 11) and control subjects (n = 13) (4100 ± 1600/μl versus 5363 ± 1367/μl; NS), the lymphocyte counts were significantly decreased in patients compared with control subjects (1414 ± 695/μl versus 2110 ± 669/μl; P < 0.01).
The proportion of CD4+ cells was also significantly elevated in patients (48.1 ± 10.5% versus 34.5 ± 8.7%; P < 0.01); however, the absolute number of CD4+ cells was not different from that in controls (711 ± 435/μl versus 714 ± 357/μl; NS). In contrast, while the slight decrease in the proportion of CD8+ cells was not significant (19.9 ± 7.0% versus 24.5 ± 9.6%; NS), the absolute number of CD8+ cells was significantly smaller in patients than in control subjects (276 ± 148/μl versus 481 ± 177/μl; P < 0.01). The CD4/CD8 ratio was significantly elevated in patients (3.0 ± 1.7 versus 1.7 ± 0.8; P < 0.05).
Suppressed NK cell activity was clearly seen in patients compared with control subjects (12.9 ± 7.4% versus 52.5 ± 14.8%; P < 0.01).
Effect of methyl-B12 administration on lymphocyte subsets and NK cell activity in patients and control subjects
As mentioned above, leucocyte counts and lymphocyte counts, CD4+, CD8+, CD56+ cell counts and NK cell activity were measured at the end of the 2-week treatment with methyl-B12. Results of statistical analysis of immunological parameters before and after methyl-B12 administration in both patients and control subjects are summarized in Table 1. The leucocyte counts and lymphocyte counts of patients were increased significantly after methyl-B12 treatment (P < 0.05). After treatment, the lymphocyte counts was still significantly lower in patients than in control subjects (P < 0.05). Interestingly, an increase in the lymphocyte counts was observed even in control subjects (P < 0.05).
As shown in Table 1a significant decrease of percentage CD4+ cells was observed in patients after treatment (P < 0.01), while no significant change was noted in control subjects. No significant change of the absolute number of CD4+ cells was observed in patients after methyl-B12 treatment, but a slight increase was observed in control subjects (NS but tendency).
An increase in percentage CD8+ cells after methyl-B12 treatment was noted in patients (P < 0.05), but not in control subjects. Increases in the absolute number of CD8+ cells were noted in both patients and control subjects (P < 0.01, P < 0.05, respectively); however, the absolute number of CD8+ cells in patients after treatment was still lower than that in control subjects (P < 0.05).
The CD4/CD8 ratio was significantly decreased by methyl-B12 treatment in patients (P < 0.05), but not in control subjects, and the difference between patients and control subjects disappeared after methyl-B12 administration.
In patients, the decreased level of NK cell activity was restored by methyl-B12 administration (P < 0.01); however, the level of NK cell activity was still lower than that of the control group (P < 0.05). In control subjects, NK cell activity was not changed by methyl-B12 treatment. After 1–2 years of follow up, with methyl-B12 administration (1000 μg injection for every 3 months), further restoration of NK cell activity was observed in patients compared with that observed after 2 weeks of methyl-B12 treatment (40.3 ± 11.9% versus 28.9 ± 15.3%; P < 0.01; n = 7, 11, respectively) and the restored NK cell activity was comparable to that of control subjects (40.3 ± 11.9% versus 53.0 ± 13.0%; NS; n = 7, 8, respectively).
Effects of methyl-B12 treatment on NK cell subsets and other immunological parameters
The percentage and absolute number of CD56+ cells were estimated in nine patients before and after methyl-B12 treatment, and compared with those in 10 control subjects. Both proportion and absolute number of CD56+ cells in patients before methyl-B12 administration were lower than those in control subjects (13.9 ± 6.1% versus 23.7 ± 9.8%; P < 0.05; n = 9, 10, respectively; 191.5 ± 64.9/μl versus 461.8 ± 237.3/μl; P < 0.01; n = 9, 10, respectively). After methyl-B12 administration, the proportion of CD56+ cells was not changed (14.3 ± 5.8% versus 15.9 ± 6.3%; NS; n = 9). Although the slight increase in absolute number of CD56+ cells after methyl-B12 treatment in patients was not significant (191.5 ± 64.9/μl versus 333.2 ± 209.1/μl; NS (P = 0.09); n = 9), the difference between patients and control subjects disappeared after methyl-B12 administration.
On the other hand, a slight increase in absolute number of CD3−CD16+ cells was noted (146.7 ± 70.4/μl versus 237.0 ± 120.4/μl; P < 0.05; n = 7); however, both values were significantly (P < 0.01) lower than that of normal subjects (477.2 ± 193.3/μl). Similarly, absolute numbers of CD16+ CD57+ cells in patients before treatment were significantly lower than that in control subjects (129.8 ± 55.0/μl versus 367.9 ± 235.2/μl; P < 0.05; n = 7, 10, respectively). After methyl-B12 treatment, a slight increase in absolute number of CD16+CD57+ cells in patients was noted but was not significant (129.8 ± 55.0/μl versus 201.5 ± 136.9/μl; NS; n = 7); however, the difference between patients and control subjects disappeared after methyl-B12 administration. No other parameters of double-staining analyses of CD3 × CD16, CD8 × CD57, CD16 × CD57 were significantly changed (data not shown).
PHA-, Con A-, and PWM-stimulated blast formation of lymphocytes was measured in five patients before and after methyl-B12 treatment, but no suppression or change was observed after treatment (all results were in the normal range, data not shown). ADCC was evaluated in three patients but no suppression of activity or other significant change was observed before or after treatment with vit.B12 (all results were in the normal range, data not shown). Serum levels of immunoglobulins in patients and control subjects were in the normal range and no change was observed after methyl-B12 treatment (data not shown).
Follow up of patients
In all patients, anaemia was improved within 2–4 weeks and the patients remained well thereafter. No adverse effects were seen in patients or control subjects treated with methyl-B12.
DISCUSSION
In the present study we have demonstrated various immunomodulatory effects of vit.B12. Serum levels of immunoglobulins were not affected by vit.B12 deficiency or supplementation. A decrease in the absolute numbers of lymphocytes, especially CD8+ cells, and an increase in the CD4/CD8 ratio in vit.B12-deficient patients were found. Vit.B12 treatment led to an increase in the number of lymphocytes, including CD8+ cells, not only in patients but also in control subjects, and to a significant increase of NK cell activity in patients.
These results are consistent with others referred to above [3–5] and with clinical observations that we reported previously [6, 7]. In contrast, Soler et al. [8] and Carmel et al. [9] found no significant decrease in CD8+ cells nor a significantly increased CD4/CD8 ratio in PA. Although differences of the races of the subjects can not be ignored, as Carmel et al. discussed [9], the design of the study is also likely to be an important factor explaining the differences. In our study, only newly diagnosed, untreated patients were included, and methyl-B12 was administered to all patients using the same procedure and the same drug.
In order to examine the effects of vit.B12, methyl-B12 was used, because mecobalamin is known to be one of the strongest immunomodulators among the vit.B12 derivatives [1]. Methyl-B12 restored the abnormally high CD4/CD8 ratio and significantly decreased the number of CD8+ cells. In contrast, the absolute number of CD4+ cells was not significantly changed by methyl-B12 in patients. Our observations suggest that a decrease in the absolute number of CD8+ cells is the main abnormality of the lymphocyte phenotype which could be restored by methyl-B12.
Although a basic investigation of the biochemical effects of vit.B12 on DNA synthesis has been reported [10], details of the mechanism of immunomodulation by vit.B12 are still unknown. However, studies on the effects of vit.B12 on cell apoptosis have been reported [11, 12], and we speculated that the prevention of apoptosis of lymphocytes by vit.B12 might be one explanation for immunological abnormalities in megaloblastic anaemia. Decreases in the absolute numbers of lymphocytes and CD8+ cells were observed in our patients and these were partly restored by vit.B12 administration. These reports and the present observations suggest that CD8+ cells are one of the most sensitive subpopulations to apoptosis caused by lack of vit.B12. However, this is only speculation and further investigation is needed.
In addition, suppressed NK cell activity in vit.B12-deficient patients and partial restoration of NK cell activity by 2 weeks of vit.B12 treatment were observed in the present study. After 1–2 years of treatment, complete restoration was observed in patients who showed high serum levels of vit.B12. It is not clear whether the incomplete restoration of NK cell activity is due to too short a period of treatment to prevent apoptosis. Long-term follow up of patients should be useful for further understanding this problem.
Augmentation of the CD3−CD16+ fraction and CD16+CD57+ fraction, which possess strong NK cell activity [13], suggesting anti-tumour activity, was observed after vit.B12 therapy. A high risk of malignancies, especially gastric carcinoma, in PA has been demonstrated [14]. While Rode et al. [15] suggests that the increased risk of gastric tumours is due to the proliferation of endocrine cells induced by hypergastrinaemia in PA, a deficiency of vit.B12 causing low numbers of CD8+ lymphocytes and depressed NK cell activity may be additional risk factors.
On the other hand, the possibility of an anti-tumour effect of methyl-B12 was reported using an experimental model of cancer [16]. In that report, enhancement of PHA-, Con A- and PWM-stimulated lymphocyte blast formation by methyl-B12 was suggested to be one of the mechanisms of anti-tumour immunity. In our study, PHA-, Con A- and PWM-stimulated lymphocyte blast formation and ADCC were measured in some patients, but no suppression or change after methyl-B12 treatment was noted. Although these negative results might be due to the small sample size in this study, it is possible that lymphocyte blast formation and ADCC do not play an important role in anti-tumour immunity compared with the effects of CD8+ and NK cell activity.
In clinical studies of immunological or neurological disorders such as autoimmune diseases and HIV infections, some effects of vit.B12 have been reported. Sandyk et al. reported a relationship between vit.B12 and onset of multiple sclerosis (MS) [17]. They discussed the possibility of involvement of vit.B12 as a cause of MS through effects on immune system regulation. A patient with AIDS dementia complex was apparently successfully treated with vit.B12 [18], and a relationship between vit.B12 deficiency and the development of immune dysfunction in AIDS has been reported [19].
In conclusion, we found a significant decrease in the absolute number of CD8+ cells and suppressed NK cell activity in vit.B12-deficient patients. These abnormalities could be at least partly restored by methyl-B12 treatment. Moreover, augmentation of CD8+ cells by methyl-B12 treatment was observed even in control subjects. These observations may contribute to our understanding of the potential anti-tumour effects of vit.B12, and may partly explain the high risk of gastric carcinoma in PA; our data also provide a rationale for considering the use of vit.B12 for treating a variety of other immunological, neurological, and oncological disorders.
Acknowledgments
This study was supported in part by grants for research from the Ministry of Education, Science and Culture of Japan. We thank Dr Jun Tsuchiya, Dr Takuo Shirakura, Dr Toyoho Morita, Dr Toshimasa Arai, Dr Kimio Morita, Dr Hitoshi Katahira, Dr Hitoshi Take, and Dr Masahiko Shinohara for their help with this study.
References
- 1.Sakane T, Takeda S, Kotani H, et al. Effects of methyl-B12 on the in vitro immune functions of human T lymphocytes. J Clin Immunol. 1982;2:101–9. doi: 10.1007/BF00916893. [DOI] [PubMed] [Google Scholar]
- 2.Vellema P, Rutten VP, Hoek A, et al. The effect of cobalt supplementation on the immune response in vitamin B12 deficient texel lambs. Vet Immunol Immunopathol. 1996;55:151–61. doi: 10.1016/s0165-2427(96)05560-2. [DOI] [PubMed] [Google Scholar]
- 3.Imamura N, Fujimura K, Kuramoto A. Lymphocyte subpopulations in pernicious anemia. N Engl J Med. 1984;311:56. doi: 10.1056/nejm198407053110117. [DOI] [PubMed] [Google Scholar]
- 4.Wodzinski MA, Forrest MJ, Barnett D, et al. Lymphocyte subpopulations in patients with hydroxocobalamin responsive megaloblastic anemia. J Clin Pathol. 1985;38:582–4. doi: 10.1136/jcp.38.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gogos CA, Kapatais-Zoumbos KN, Zoumbos NC. Lymphocyte subpopulations in megaloblastic anaemia due to vitamin B12 deficiency. Scand J Haematol. 1986;37:316–8. doi: 10.1111/j.1600-0609.1986.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 6.Kubota K, Arai T, Tamura J, et al. Restoration of decreased suppresser cells by vitamin B12 therapy in patient with pernicious anemia. Am J Hematol. 1987;24:221–3. doi: 10.1002/ajh.2830240214. [DOI] [PubMed] [Google Scholar]
- 7.Kubota K, Kurabayashi H, Kawada E, et al. Restoration of abnormally high CD4/CD8 ratio and low natural killer cell activity by vitamin B12 therapy in a patient with post-gastrectomy megaloblastic anemia. Int Med. 1992;31:125–6. doi: 10.2169/internalmedicine.31.125. [DOI] [PubMed] [Google Scholar]
- 8.Soler J, Remacha A, Nieto M, Gimferrer E. Lymphocyte subpopulations in patients with untreated pernicious anemia. Scand J Haematol. 1985;35:376. doi: 10.1111/j.1600-0609.1985.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 9.Carmel R, Boone D, Parker JW. Lymphocyte surface phenotypes in pernicious anemia. Dig Dis Sci. 1987;32:846–50. doi: 10.1007/BF01296707. [DOI] [PubMed] [Google Scholar]
- 10.Pfohl-Leszkowicz A, Keith G, Dirheimer G. Effect of cobalamin derivatives on in vitro enzymatic DNA methylation: methylcobalamin can act as a methyl donor. Biochem. 1991;30:8045–51. doi: 10.1021/bi00246a024. [DOI] [PubMed] [Google Scholar]
- 11.Bunting RW, Seling MK, Dickersin GR. Apoptotic cells in peripheral blood from patients with low serum cobalamin. J Submicrosc Cytol Pathol. 1997;29:223–7. [PubMed] [Google Scholar]
- 12.Ingram CF, Davidoff AN, Marais E, et al. Evaluation of DNA analysis for evidence of apoptosis in megaloblastic anaemia. Br J Haematol. 1997;96:576–83. doi: 10.1046/j.1365-2141.1997.d01-2075.x. [DOI] [PubMed] [Google Scholar]
- 13.Poggi A, Sargiacomo M, Biassoni R, et al. Extrathymic differentiation of T lymphocytes and natural killer cells from human embryonic liver precursors. Proc Natl Acad Sci USA. 1993;90:4465–9. doi: 10.1073/pnas.90.10.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer. 1993;71:745–50. doi: 10.1002/1097-0142(19930201)71:3<745::aid-cncr2820710316>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Rode J, Dhillon AP, Papadaki L, et al. Pernicious anaemia and mucosal endocrine cell proliferation of the non-antral stomach. Gut. 1986;27:789–98. doi: 10.1136/gut.27.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu N, Hamazoe R, Kanayama H, et al. Experimental study of antitumor effect of methyl-B12. Oncol. 1987;44:169–73. doi: 10.1159/000226471. [DOI] [PubMed] [Google Scholar]
- 17.Sandyk R, Awerbuch GI. Vitamin B12 and its relationship to age of onset of multiple sclerosis. Int J Neurotics. 1993;71:93–99. doi: 10.3109/00207459309000596. [DOI] [PubMed] [Google Scholar]
- 18.Herzlich BC, Schiano TD. Reversal of apparent AIDS dementia complex following treatment with vitamin B12. J Intern Med. 1993;233:795–7. doi: 10.1111/j.1365-2796.1993.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 19.Liang B, Chung S, Araghiniknam M, et al. Vitamins and immunomodulation in AIDS. Nutrition. 1996;12:1–7. doi: 10.1016/0899-9007(95)00061-5. [DOI] [PubMed] [Google Scholar]