Abstract
To investigate the homing characteristics of T and B lymphocytes which could explain the abnormal partition of IgA-producing cells in tonsils and bone marrow from patients with IgA nephropathy (IgAN), the expression of leucocyte adhesion molecules (CD11a, CD29, CD49d, CD62L, CD31) was assessed using flow cytometry on peripheral blood leucocytes from patients with biopsy-proven IgAN and controls. Higher proportions of T and B lymphocytes expressing higher amounts of l-selectin, as well as higher proportions of B cells expressing more CD31 were evidenced in IgAN patients. Conversely, serum levels of sCD62L were not different from controls, but significantly higher than serum levels in patients suffering from other renal diseases. We hypothesize that this over-expression of CD62L and CD31 may be involved in an enhanced efficiency of lymphoid cells homing to lymphoid tissues in this disease.
Keywords: l-selectin, lymphocytes, peripheral blood, IgA nephropathy, flow cytometry
INTRODUCTION
The pathognomonic presence of IgA in the glomerular mesangium from patients with IgA nephropathy (IgAN) [1] suggests an alteration in the regulation of IgA synthesis in this disease. The involvement of both systemic and mucosal immune systems has been considered in this hyperproduction of IgA. Plasma cells from the bone marrow of patients with IgAN have been shown to have a greater ability to secrete IgA1 in vitro [2] and higher numbers of IgA1-producing plasma cells have been reported in bone marrow biopsies from such patients [3] compared with control subjects. However, many clinical and biological features such as recurrent upper respiratory tract infectious episodes shortly followed by haematuria and proteinuria [4], abnormally high numbers of IgA plasma cells in the tonsils from IgAN patients [5–7], significantly increased levels of tonsillar dimeric IgA-producing plasma cells [5,6,8], also suggest that mesangial IgA may originate from the mucosal immune system. The reasons why an abnormal partition of cells involved in local or systemic humoral immune responses is found in both the tonsils and bone marrow from patients with IgAN are still unknown. These increased levels of IgA-producing cells could result from local modifications in the mechanisms regulating immune responses against environmental antigens in the sites of enhanced production of IgA. They could also be the consequence of upstream abnormalities located in initial sites of activation, giving rise to abnormally activated or phenotypically modified lymphocytes inducing an increased influx of IgA-producing cells into the sites of IgA hyperproduction.
We have previously reported significantly lower levels of IgA-producing and -containing B cells in the peripheral blood of IgAN patients compared with control subjects suffering from other renal diseases and healthy controls, and we proposed that such activated B cells could remain for a shorter time in the peripheral blood from IgAN patients than in other individuals [9]. A possible mechanism would therefore be an enhanced efficiency of cell-trafficking in IgAN patients that would result in lower numbers of circulating activated B cells and higher numbers of IgA-secreting plasma cells relocated in mucosae-associated lymphoid tissue and/or in the bone marrow. Lymphocyte extravasation into lymphoid organs, through specialized microvascular structures, the high endothelial venules (HEV), requires the expression on these cells of adhesion signals complementary to those expressed on the HEV of the lymphoid tissue [10]. This lymphocyte–endothelial interaction is a multi-step process which depends upon the expression of various adhesion molecules, including l-selectin (CD62L), LFA-1 (CD18/CD11a), VLA-4 (CD29/CD49d) and PECAM-1 (CD31) on the membrane of circulating cells' surfaces.
In this study we investigated the hypothesis of more efficient homing mechanisms occurring in IgAN patients by assessing, in flow cytometry, the quantitative expression of the leucocyte adhesion molecules CD11a, CD29, CD49d, CD62L and CD31 on circulating T and B lymphocytes in patients with biopsy-proven IgAN and in control subjects suffering or not from other renal diseases. We also investigated soluble l-selectin levels. The results show higher proportions of lymphocytes which expressed higher amounts of l-selectin in IgAN patients than in the other patients included in this study. These data provide further information in favour of an alteration of the mechanisms of lymphocyte trafficking in IgAN patients.
PATIENTS AND METHODS
Patients
Peripheral blood samples were collected without anti-coagulant and on EDTA-K from 48 patients with renal disease and 20 healthy controls. These samples were forwarded immediately to the laboratory. The serum samples were frozen at −20°C until the ELISA assay was carried out.
Two groups of patients with a biopsy-proven diagnosis were studied. The first group (group 1) included 19 patients with idiopathic IgAN. They were 16 men and three women with a mean age of 39 ± 12 years. At the time of blood sampling, 12 patients had microhaematuria, three had proteinuria > 1 g/24 h, and five had a plasma creatinin level > 1.5 mg/dl. Seven patients had a history of at least one episode of macrohaematuria.
The second group (group 2) was composed of 29 patients (16 men and 13 women) with renal diseases other than IgAN. Their mean age was 49 ± 12 years. At the time of blood sampling, seven patients had microhaematuria and 20 had proteinuria > 1 g/24 h. Eighteen patients had a plasma creatinin level > 1.5 mg/dl.
At the time of blood sampling, none of the patients studied suffered from a mucosal infection and no macrohaematuria was detected. Twenty healthy adults (nine men and 11 women) with a mean age of 34 ± 9 years and with normal urine analysis composed the control group (group 3).
Methods
Lymphocyte purification
Lymphocytes were isolated from peripheral blood samples by density gradient centrifugation on Ficoll (Lymphoprep; Nycomed, Oslo, Norway). The cells were washed twice in RPMI 1640 (Sigma, St Louis, MO). From these cells, enriched suspensions of T or B cells were, respectively, obtained after negative selection of CD19+ or CD2+ cells, using metallic beads (Dynabeads M-450; Dynal, Oslo, Norway). These beads coated with a MoAb specific for the CD19 or CD2 antigens were washed four times in PBS containing 0.3% bovine serum albumin (BSA), then incubated with 2 × 106 lymphocytes at a ratio of 40 beads for one estimated cell and gently mixed for 45 min at 4°C by tilt rotation. The cell suspensions depleted of rosetted cells were then collected, washed in PBS–BSA and immediately used for flow cytometric analysis.
Flow cytometric analysis
For each sample, the efficiency of negative selection was evaluated. Enriched cell suspension (50 μl) was incubated 30 min at 4°C with anti-CD2 or anti-CD19 MoAbs (Immunotech, Coulter Co., Marseille, France), respectively, and washed in PBS–0.5% BSA. After 30 min of incubation at 4°C with FITC–sheep antiserum to mouse immunoglobulin, the cells were washed in cold PBS–0.5% BSA, resuspended in PBS–1% paraformaldehyde and maintained at 4°C until they were analysed on a flow cytometer (EPICS-XL; Coultronics Co., Miami, FL).
The quantitative expression of adhesion molecules was studied on enriched T and B lymphocyte suspensions using FITC-conjugated MoAbs directed to CD11a, CD29, CD49d, CD62L, CD31 (Immunotech, Coulter Co.). Fifty microlitres of each cell suspension were incubated with 3 μl of the appropriate antibody for 30 min at 4°C. After a wash in cold PBS–0.5% BSA, the cells were fixed with 300 μl of 1% paraformaldehyde in PBS and stored at 4°C until they were analysed on a flow cytometer (EPICS-XL).
Analysis on the same protocol of calibrated fluorescent particles (Immunobrite; Coulter, Hialeah, FL) allowed the setting up of a reference curve allowing quantification of the numbers of fluorescent molecules bound to the cells tested. The results were expressed as percentages of positive cells among the cell suspensions tested using the level of T and B cell enrichment to correct fluorescent cell proportions as well as equivalent numbers of fluorescent molecules bound on these cells.
Soluble CD62L
Serum levels of soluble CD62L were measured using a sandwich ELISA (human soluble l-selectin ELISA; R&D Systems Europe, Abingdon, UK) according to the manufacturer's instructions. Briefly, the serum samples diluted 1:100 in buffer solution were incubated for 1 h at room temperature in the wells of a 96-well ELISA plate containing an immobilized mouse MoAb directed to human CD62L. In parallel, a calibration curve was established using six standard solutions of recombinant soluble l-selectin. An anti-selectin-horseradish peroxidase (HRP) conjugate (100 μl) directed to another epitope of the CD62L molecule was incubated for 30 min at room temperature. After a series of thorough washes, 100 μl of an HRP substrate/chromogen mixture were added into each well and incubated for 30 min at room temperature. The colour reaction was stopped with an acid solution. The absorbance in each well was measured at 450 nm using a Titerskan spectrophotometer (Multiwell; Flow Labs, Helsinki, Finland) within 30 min after the reaction had been stopped. Individual values were calculated using the calibration curve and the dilution factor.
Statistical analysis
All data were fed into a personal computer using the MYOSOTIS database and statistics software (Coultronics, Margency, France). Results were expressed for each group of patients as mean values ± s.e.m. Comparisons were performed using anova first, followed by Student's t-test whenever significance appeared in the anova. Linear regression tests were used where appropriate.
RESULTS
Lymphocyte subset enrichment efficiency
The efficiency of the enrichment of T or B cell suspensions evaluated using anti-CD2 and anti-CD19, respectively, showed mean proportions of 94.2 ± 0.5% of CD2+ cells among enriched T cell suspensions and 72.4 ± 2.1% of CD19+ cells among enriched B cell suspensions.
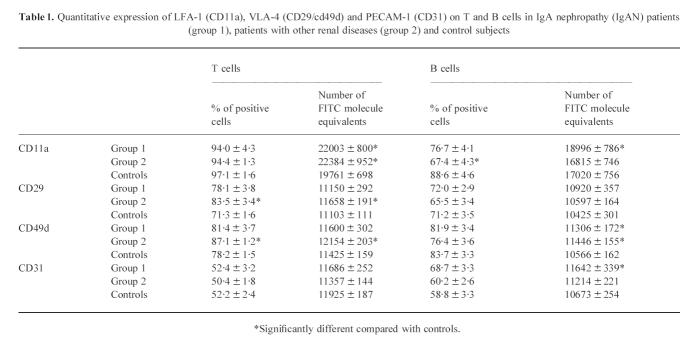
Adhesion molecules on T cells (Table 1 and Fig. 1)
Table 1.
Quantitative expression of LFA-1 (CD11a), VLA-4 (CD29/cd49d) and PECAM-1 (CD31) on T and B cells in IgA nephropathy (IgAN) patients (group 1), patients with other renal diseases (group 2) and control subjects
Fig. 1.
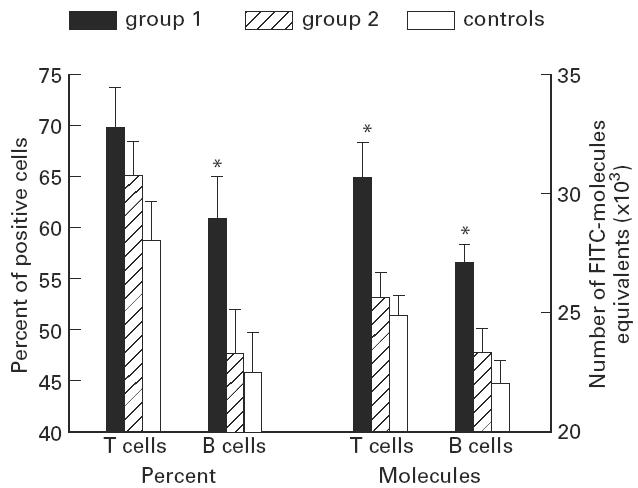
Comparative levels of the quantitative expression of l-selectin (CD62L) on T and B cells between patients with IgA nephropathy (IgAN) (group 1), patients with other renal diseases (group 2) and healthy controls. Data are expressed as percentages of positive cells expressing l-selectin and as numbers of FITC molecule equivalents fixed on these cells (means + s.e.m.). *Significant P values (< 0.05) between patients with IgAN and both other groups.
CD11a
No significant difference was observed in the proportions of CD11a+ T lymphocytes between the three groups of subjects. However, CD11a was expressed with a significantly lower intensity on T cells from healthy controls than from renal patients suffering from IgAN (P = 0.04) or not (P < 0.05).
CD29
Significantly lower levels of CD29+ T cells were found in healthy controls, compared with patients with other renal diseases than IgAN (P < 0.0001). The density of CD29 antigen expression also was significantly lower on T cells from healthy controls compared with group 2 patients (P = 0.03).
CD49d
The proportions of CD49d+ T cells were significantly lower in the control group than in group 2 patients (P < 0.0001). Similar results were obtained regarding the density of CD49d expression, significantly higher in group 2 patients (P = 0.01) compared with controls.
CD62L
The proportions of CD62L+ T cells were higher in IgAN patients (group 1) than in other patient groups without reaching statistical significance (group 2, P = 0.2; controls, P = 0.053). CD62L antigen density on T lymphocytes was, however, significantly higher in IgAN patients than in group 2 patients (P < 0.01) and healthy controls (P < 0.01).
CD31
The expression of CD31 on T cells was similar in the three groups of subjects tested.
Adhesion molecules on B cells (Table 1 and Fig. 1)
The analysis of adhesion molecules on circulating B cells showed predominant proportions of B lymphocytes with CD49d on their surface (79 ± 2%) followed by CD11a (79 ± 2%), CD29+ (70 ± 2%), and CD31 (61 ± 2%). CD62L+ B cells (46 ± 2%) constituted a subpopulation of B lymphocytes equivalent to about half of all peripheral blood B cells.
CD11a
The proportions of CD11a+ B cells were significantly higher in healthy controls than in group 2 patients (P < 0.01). CD11a was expressed with a significantly lower density on B lymphocytes from healthy controls than group 1 patients (P < 0.01).
CD29
No significant difference in the percentages of CD29+ B cells or in the density of this molecule was observed between the various groups included in this study.
CD49d
Similarly, no significant difference was noted regarding the expression of CD49d between the various groups of subjects. However, the density of CD49d expression on B cells was significantly lower in healthy controls than in patients with renal disease (group 1, P < 0.01; group 2, P < 0.001).
CD62L
The levels of CD62L+ B cells were significantly higher in IgAN patients than in the two other groups (group 2, P = 0.04; controls, P = 0.03). Moreover, B lymphocytes expressed CD62L with a significantly higher intensity in IgAN patients (group 2, P = 0.01; controls, P < 0.001).
CD31
The expression of CD31 was more frequently observed among B cells from IgAN patients than from other subjects, and this difference was significant between IgAN patients and healthy controls (P = 0.03). The intensity of CD31 expression was also significantly higher in IgAN patients than in healthy controls (P = 0.03).
Serum levels of soluble CD62L
Similar levels of soluble CD62L were observed in serum samples from IgAN patients and controls. However, group 2 patients showed significantly lower levels (9.5 ± 1.1 ng/ml) of this parameter compared with both group 1 patients (14.2 ± 1.1 ng/ml; P = 0.006) and healthy controls (15.4 ± 1.4 ng/ml; P = 0.002). No correlation was found between the serum levels of soluble CD62L, the proportions of CD62L+ T or B or the density of CD62L expression.
Influence of renal function
No significant difference from the results described above was observed in patients with microhaematuria and/or chronic renal failure, defined as having creatinin levels > 1.5 mg/dl and/or proteinuria > 1 g/24 h.
Serum levels of soluble CD62L also seemed not to be affected either by creatinin levels or by proteinuria or microhaematuria.
DISCUSSION
This study reports higher proportions of peripheral blood l-selectin (CD62L)-positive T and B lymphocytes in IgAN patients compared both with other renal patients and with healthy controls. A significantly higher density of this molecule on the surface of T and B cells was also observed in IgAN patients compared with other subjects. Significantly higher proportions of B cells expressing significantly higher levels of CD31 were likewise observed in IgAN patients compared with healthy controls. The other differences noted appeared more related to renal dysfunction than specifically associated with IgAN. CD62L over-expression, especially on B cells, therefore appears as a novel feature, validated by the fact that all samples were handled in similar conditions, and by the otherwise similar expression of adhesion molecules on lymphocytes from IgAN patients and other groups of subjects.
Few studies have investigated the expression of adhesion molecules on peripheral blood mononuclear cells in IgAN patients. Namie et al. [11] have shown a similar expression of β1-integrins (VLA-1 to VLA-5) on lymphocytes from IgAN patients and healthy controls. However, the functional capacity of VLA-4 and VLA-5 to bind fibronectin was higher in IgAN patients than in controls. We also found no significant difference in the expression of CD29 and CD49d (VLA-4) on T and B cells from IgAN patients compared with patients with other renal diseases and healthy controls.
CD62L (l-selectin) plays a critical role in the attachment of leucocytes to activated endothelium [12]. This adhesion molecule is constitutively expressed on leucocytes, including granulocytes, monocytes and most lymphocytes [13], and is lost at the end of cell activation [14]. Picker et al. [15] have shown that during CD45RA/RO conversion, l-selectin was initially up-regulated, concomitantly with blastogenesis, and then heterogeneously down-regulated, leading to the production of both CD62L+ and CD62L− memory/effector T cells. The degree to which l-selectin expression is down-regulated during this process appears to be dependent of the site of activation. These authors reported that lymphocytes from peripheral lymph nodes at the late stage of CD45RA/RO transition were CD62L+. In contrast, the majority of late transition T cells in the appendix were CD62L−. This site-specific regulation of l-selectin expression was not observed for other adhesion molecules such as LFA-3, LFA-1 and intercellular adhesion molecule-1 (ICAM-1), which are similarly up-regulated in both mucosal and peripheral secondary lymphoid tissues. The over-expression of CD62L on circulating lymphocytes from IgAN patients observed in this study may correspond either to an enhanced production of virgin cells in primary lymphoid organs or to an abnormal regulation of lymphocyte activation in peripheral sites. This would result in production of higher numbers of memory/effector cells expressing CD62L or in a default of the shedding of this molecule from the surface of lymphocytes.
Farstad et al. [16] have reported that l-selectin seemed to be expressed more often on memory T and B cells present in the mesenteric lymph than on such cells in the microlymphatics of Peyer's patches. These authors suggested that l-selectin might be up-regulated on memory cells after they have left the mucosa. The higher proportions of CD62L+ lymphocytes and especially B lymphocytes that we observed in IgAN patients might therefore also originate from the Peyer's patches or from mucosa-associated lymphoid tissues. Assuming that this molecule is fully functional, the over-expression of l-selectin on lymphocytes from IgAN patients would then be likely to favour an enhanced traffic of these cells through the HEV of secondary lymphoid organs, especially in tonsils. Indeed, previously reported data [17,18] have shown an increased expression of adhesion molecules on more numerous HEV in tonsils from IgAN patients. Moreover, this lymphoid tissue expresses with a relatively high intensity the sialomucin CD34 [19], which constitutes the major l-selectin ligand in human tonsil HEV [20]. The over-expression of CD31 on B cells from IgAN patients could therefore also contribute to the enhanced efficiency of homing mechanisms of these cells, especially into tonsil HEV which express CD31 at significantly higher levels in patients with IgAN [17]. The hypothesis of an enhanced efficiency of homing mechanisms in IgAN patients via the over-expression of CD62L and/or CD31 on lymphocytes could then explain the lower numbers of circulating activated B cells and the higher numbers of IgA-secreting plasma cells relocated in tonsils from these patients that we previously reported [5,7].
Acknowledgments
This work was supported in part by PHRC93 Nephropathie à IgA from Ministère de la Santé.
References
- 1.Berger J, Hinglais N. Les dépôts intercapillaires d'IgA -IgG. J Urol Nephrol (Paris) 1968;74:694–5. [PubMed] [Google Scholar]
- 2.Van den Wall Bake AWL, Daha MR, Radl J, et al. Elevated production of polymeric and monomeric IgA1 by the bone marrow in patients with IgA nephropathy. Kidney Int. 1989;35:1400–4. doi: 10.1038/ki.1989.139. [DOI] [PubMed] [Google Scholar]
- 3.Harper SJ, Allen AC, Layward L, Hattersley J, Veitch PS, Feehally J. Increased immunoglobulin A and immunoglobulin A1 cells in bone marrow trephine biopsy specimens in immunoglobulin A nephropathy. Am J Kidney Dis. 1994;24:888–92. doi: 10.1016/s0272-6386(12)81056-0. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G. Idiopathic IgA nephropathy. Nephron. 1985;41:1–13. doi: 10.1159/000183538. [DOI] [PubMed] [Google Scholar]
- 5.Béné MC, Faure G, de Hurault Ligny B, Kessler M, Duheille J. IgA nephropathy: quantitative immunohistomorphometry of the tonsillar plasma cells evidences an inversion of the IgA versus IgG plasma secreting cell balance. J Clin Invest. 1983;71:1342–7. doi: 10.1172/JCI110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egido J, Blasco R, Lozano L, Sancho J, Garcia-Hoyo R. Immunological abnormalities in the tonsils of patients with IgA nephropathy: inversion in the ratio of IgA:IgG bearing lymphocytes and increased polymeric IgA synthesis. Clin Exp Immunol. 1984;57:101–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Béné MC, de Hurault Ligny B, Kessler M, Faure GC. Confirmation of tonsillar anomalies in IgA nephropathy: a multicenter study. Nephron. 1991;58:425–8. doi: 10.1159/000186474. [DOI] [PubMed] [Google Scholar]
- 8.Harper SJ, Allen AC, Béné MC, Pringle JH, Faure G, Lauder I. Increased dimeric IgA-producing B cells in tonsils in IgA nephropathy determined by in situ hybridization for J chain mRNA. Clin Exp Immunol. 1995;101:442–8. doi: 10.1111/j.1365-2249.1995.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennel-De March A, Béné MC, Renoult E, Kessler M, Faure GC. Low levels of spontaneously activated peripheral IgA-secreting cells in nontransplanted IgA nephropathy patients. Am J Kidney Dis. 1997;30:64–70. doi: 10.1016/s0272-6386(97)90566-7. [DOI] [PubMed] [Google Scholar]
- 10.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 11.Namie S, Ozono Y, Harada T, Hara K. Expression and function of fibronectin receptors on peripheral mononuclear cell in IgA nephropathy. Nephrol Dial Transplant. 1995;10:1342. [PubMed] [Google Scholar]
- 12.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62:3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- 13.Gallatin WM, Weissman IL, Butcher EC. A cell surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 14.Kanof ME, James GA. Leu-8 antigen is diminished during cell activation but does not correlate with effector function of activated T lymphocytes. J Immunol. 1988;114:3701–6. [PubMed] [Google Scholar]
- 15.Picker JL, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LWMM. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor l-selectin on T cells during virgin to memory cell transition. J Immunol. 1993;150:1105–21. [PubMed] [Google Scholar]
- 16.Farstad IN, Norstein J, Brandtzaeg P. Phenotypes of B and T cells in human intestinal and mesenteric lymph. Gastroenterology. 1997;112:163–73. doi: 10.1016/s0016-5085(97)70231-2. [DOI] [PubMed] [Google Scholar]
- 17.Kennel-De March A, Béné MC, de Hurault Ligny B, Kessler M, Faure GC. Enhanced expression of CD31 and CD54 on tonsillar high endothelial venules in IgA nephropathy. Clin Immunol Immunopathol. 1997;84:158–65. doi: 10.1006/clin.1997.4389. [DOI] [PubMed] [Google Scholar]
- 18.Béné MC, de Hurault Ligny B, Kessler M, Foliguet B, Faure GC. Tonsils in IgA nephropathy. In: Béné MC, Faure GC, Kessler M, editors. IgA nephropathy: the 25th year. Basel: Karger; 1993. pp. 153–61. [Google Scholar]
- 19.Girard JP, Springer TA. Expression of sialomucin CD34 by high endothelial venules (HEV) in human tonsils. In: Boumsell L, Gilles W, Harlan JM, editors. Leukocyte typing V. New York: Oxford University Press; 1995. pp. 1801–3. [Google Scholar]
- 20.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major l-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–70. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]