Abstract
We assessed the roles of proinflammatory cytokines IFN-γ and TNF-α, and immunoregulatory cytokines IL-10 and TGF-β in the modulation of the anti-microbial activity of murine peritoneal macrophages against Mycobacterium avium-intracellulare complex (MAIC). First, both IFN-γ and TNF-α significantly reduced the bacterial growth in macrophages, indicating that these cytokines participate in up-regulation of macrophage anti-MAIC function. Second, although MAIC-infected macrophages produced substantial amounts of IL-10 and TGF-β, neutralization of endogenous IL-10 and TGF-β with anti-IL-10 and anti-TGF-β antibodies, respectively, did not affect the intracellular growth of MAIC in macrophages from mice with BcgS (MAIC-susceptible) or Bcgr (MAIC-resistant) genotype, regardless of the virulence of test MAIC strains. The same result was also obtained for macrophages stimulated with IFN-γ or TNF-α. Third, in MAIC-infected mice, the growth of organisms at the sites of infection (lungs and spleens) was not affected by administration of anti-IL-10 or anti-TGF-β antibodies. These findings indicate that, in the case of mice, endogenous IL-10 and TGF-β are essentially ineffective in down-regulating macrophage anti-MAIC functions not only in vitro but also in vivo.
Keywords: interferon-gamma, tumour necrosis factor-alpha, IL-10, transforming growth factor-beta, macrophage, Mycobacterium avium-intracellulare complex
INTRODUCTION
Mycobacterium avium-intracellulare complex (MAIC) frequently causes disseminated and fatal infections in AIDS patients [1,2]. MAIC infections are intractable because of intrinsic resistance of the pathogens to most anti-tuberculosis drugs. Host resistance against mycobacterial organisms including MAIC is principally dependent on the bactericidal and/or bacteriostatic functions of macrophages activated by proinflammatory cytokines such as IFN-γ, TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) produced by Th1 cells, natural killer (NK) cells, and macrophages themselves [3–6].
Although IFN-γ is regarded as a crucial activator of macrophage cell functions, its ability to up-regulate macrophage anti-microbial activity against mycobacterial organisms still remains somewhat controversial, as follows. First, in the case of murine macrophages, IFN-γ is generally known to up-regulate their anti-microbial activity against M. tuberculosis, M. bovis bacille Calmette–Guérin (BCG), and MAIC [5–9]. However, some investigators reported that IFN-γ conversely suppressed the anti-MAIC activity of mouse peritoneal macrophages [10]. The same situation is also known for human macrophages. Some investigators reported IFN-γ-mediated increase in the anti-MAIC activity of human monocyte-derived macrophages [11], while these investigators demonstrated IFN-γ-mediated down-regulation of anti-MAIC activity of human monocytes or monocyte-derived macrophages depending on the phase of cultivation [10,11].
Second, TNF-α is widely known to up-regulate macrophage microbicidal activity against mycobacteria [3,5,6,10,12]. TNF-α-mediated potentiation of macrophage anti-MAIC activity has been observed for mouse peritoneal macrophages [10,13,14], human monocytes [15], and human monocyte-derived macrophages [13,16]. However, a recent study by Bermudez et al. [17] revealed that entry of MAIC organisms into macrophages by alternate receptors other than complement and mannose receptors was associated with resistance to TNF-α-induced bactericidal mechanisms of human monocyte-derived macrophages.
Third, IL-10 and TGF-β, immunoregulatory cytokines possessing macrophage-deactivating functions [18–24], have been reported by some investigators to down-regulate the anti-microbial activity of murine and human macrophages against MAIC [25–27]. Previously, we found a long-lasting increase in the tissue levels of TGF-β in the spleens of mice during weeks 2–8 of infection and this was concurrent with the accelerated growth of infected organisms [28], suggesting TGF-β-mediated suppression of macrophage anti-MAIC functions in MAIC-infected mice. However, other investigators reported that IL-10 and TGF-β did not modulate the anti-microbial activity of human monocytes against MAIC [29] and M. tuberculosis [30].
In the present study, we assessed the roles of proinflammatory cytokines IFN-γ and TNF-α, and immunosuppressive cytokines IL-10 and TGF-β, on the anti-microbial activity of mouse peritoneal macrophages against high- or low-virulence MAIC strains, using mouse strains with BcgS (MAIC-susceptible) or Bcgr (MAIC-resistant) genotypes [31]. We have confirmed that both IFN-γ and TNF-α are efficacious in up-regulating the anti-MAIC functions of murine macrophages, based on statistical analysis of the data obtained from a number of repeated experiments. On the other hand, neither IL-10 nor TGF-β was found to modulate the anti-MAIC activities of Bcgr and BcgS macrophages, although Bcgr macrophages displayed more potent microbicidal activity than did BcgS macrophages.
MATERIALS AND METHODS
Organisms
MAIC N-254 (serovar 9) and MAIC N-260 (serovar 16) isolated from patients with MAIC infection were used. These MAIC strains were identified as M. avium and M. intracellulare by DNA probe test, respectively. They produced smooth, flat, and transparent colonies. MAIC N-254 is weakly virulent in mice, showing slow growth in the visceral organs (lungs and spleen), and this strain did not kill infected mice within 1 year [32]. In contrast, MAIC N-260 is highly virulent in mice, showing much more rapid growth at the sites of infection, and this strain caused the death of all mice within 260 days after infection [32]. The organisms were grown in Middlebrook 7H9 medium (Difco Labs, Detroit, MI), collected by centrifugation at 1700 g for 15 min, and suspended in 0.1% bovine serum albumin (BSA) in PBS. The bacterial suspension was then sonicated using Handy Sonic (Model UR-20P; Tomy Seiko Co., Tokyo, Japan) at maximum power for 20 s and stored at −80°C until use.
Mice
Seven to 12-week-old female mice of BALB/c (BcgS genotype), C57Bl/6 (BcgS), and CBA/JN (Bcgr) strains [31] were purchased from Japan Clea Co. (Osaka, Japan) (BALB/c and C57Bl/6) and Charles River Co. (Kanagawa, Japan).
Special agents
Murine recombinant IFN-γ and TNF-α were obtained from Genzyme Co. (Cambridge, MA). One unit of IFN-γ is defined as the amount required to protect 50% of the indicator cell population (L929 cells) from viral (VSV) destruction. One unit of TNF-α is defined as the amount required to mediate half-maximal cytotoxicity of L929 cells. Rat anti-mouse IL-10 MoAb, mouse anti-human TGF-β MoAb (also specific to mouse TGF-β) were obtained from Genzyme. Rat IgG and mouse IgG were purchased from ICN Pharmaceuticals Inc. (Costa Mesa, CA) and Chemicon International Inc. (Temecula, CA), respectively. These cytokine and antibody preparations were essentially free from lipopolysaccharide (LPS) contamination in the Limulus J Single Test (Wako Pure Chemical Industry Co., Osaka, Japan).
In separate experiments using bioassay of IL-10 (costimulation assay using MC/9 cells) and TGF-β (Mv1Lu growth inhibition assay), the presently used anti-IL-10 MoAb and anti-TGF-β MoAb exerted sufficient levels of neutralizing activity of IL-10 and TGF-β, respectively. Anti-IL-10 MoAb and anti-TGF-β MoAb (1 μg/ml) neutralized ≥ 5.0 and 0.4 ng/ml of IL-10 and TGF-β, respectively.
Medium
RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) containing 5% or 10% (v/v) fetal bovine serum (FBS) (BioWhittaker Co., Walkersville, MD) was used for cell culture.
Intracellular growth of MAIC in macrophages
Peritoneal cells suspended (PCs) in 5% FBS–RPMI 1640 medium were seeded into 96-well round-bottomed microculture wells (Becton Dickinson, Lincoln Park, NJ) at a density of 1 × 105cells/well. After 1 h incubation at 37°C in a CO2 incubator (5% CO2–95% humidified air), the wells were gently rinsed with Hanks balanced salt solution (HBSS) containing 2% FBS to remove non-adherent cells. The resultant macrophages were cultured in 0.1 ml of the medium with the addition of 1 × 107 colony-forming units (CFU)/ml of MAIC at 37°C in a CO2incubator for 1 h. After washing with 2% FBS–HBSS, the macrophage culture was cultivated in the medium (0.2 ml) with or without the addition of IFN-γ (300 U/ml), TNF-α (1000 U/ml), anti-IL-10 MoAb (20 or 30 μg/ml), anti-TGF-β MoAb (20 or 30 μg/ml), or combinations of them at 37°C in a CO2 incubator for up to 7 days, unless otherwise specified. At intervals, culture fluid was withdrawn and 0.15 ml of lysis buffer (0.07% SDS in 7H9 broth) was added to the macrophage culture. The macrophage lysate was mixed with 50 μl of 20% BSA in PBS, and the numbers of bacterial CFU in the macrophage lysate were counted on 7H11 agar plates (Difco).
The value ‘relative growth rate’ of MAIC in macrophages treated with test agents, such as cytokines and anti-cytokine antibodies, was calculated as: relative growth rate = (bacterial growth rate in macrophages treated with test agents)/(bacterial growth rate in untreated macrophages).
Cytokine measurement
Macrophage monolayer cultures prepared on 16-mm culture wells (Corning Glass Works, Corning, NY) by seeding 5 × 106 PCs were cultivated in 1.0 ml of 10% FBS–RPMI medium in the presence or absence of 1 × 107 CFU/ml of MAIC at 37°C in a CO2incubator for up to 14 days. At intervals, cytokine concentrations in culture fluids were measured by an ELISA as reported previously [28]. Briefly, Immulon 4 plates (Dynatech Labs, Chantilly, VA) were coated with a capture antibody for each cytokine using rat anti-mouse IL-10 and mouse anti-human TGF-β (specific to mouse TGF-β) (R&D Systems Inc., Minneapolis, MN) MoAbs. Biotinylated rat anti-mouse IL-10 MoAb (Pharmingen Co., San Diego, CA) and chicken anti-human TGF-β MoAb (R&D Systems) were used as the detecting antibodies. Alkaline phosphatase-conjugated streptavidin (Life Technologies Co., Gaithersburg, MD) and alkaline phosphatase-conjugated rabbit anti-chicken IgG MoAb (Zymed Labs Inc., San Francisco, CA) were further bound to biotinylated antibody and chicken anti-TGF-β antibody, respectively. Colour development was achieved by using p-nitrophenyl phosphate tablets (Sigma Chemical Co., St Louis, MO) as substrate.
Experimental MAIC infection
Seven to 8-week-old female BALB/c mice were infected with 1 × 107 CFU of MAIC N-260 via the i.v. route. Infected mice were given or were not given either i.p. injections of goat anti-IL-10 antibody (Sigma) or i.v. injections of mouse anti-TGF-β antibody (Genzyme) at the dose of 50–100 μg/mouse weekly or biweekly from week 1 to week 5 after infection. Goat IgG (Zymed) was used as a control antibody for goat anti-IL-10 antibody. In some cases, mice were given KRM-1648 (Kaneka Corp., Hyogo, Japan) finely emulsified in a 2.5% gum arabic–0.2% Tween 80 solution at a dose of 8 mg/kg by gavage, once daily, 6 times per week, from day 1 for up to 6 weeks after infection. Mice were killed at week 6, and the bacterial loads in the lungs and spleens of mice were measured by counting the number of CFU in the homogenates of individual organs with distilled water on Middlebrook 7H11 agar plates, as previously described [28].
Statistical analysis
Statistical analysis was performed by Mann–Whitney test unless otherwise specified.
RESULTS
Roles of IFN-γ and TNF-α in modulation of macrophage anti-MAIC activity
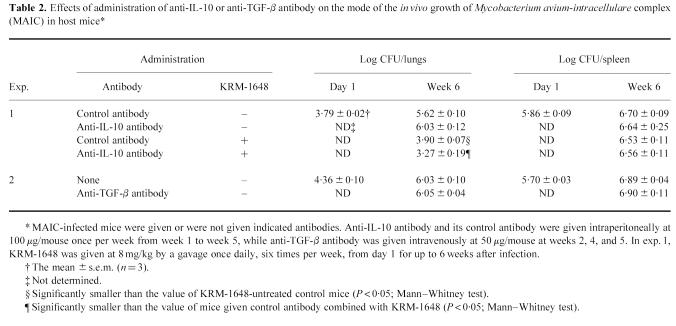
In order to assess the effects of IFN-γ and TNF-α on macrophage anti-MAIC functions, we measured the intracellular growth of high-virulence MAIC N-260 in peritoneal macrophages from BALB/c (BcgS) mice during cultivation in the presence of IFN-γ or TNF-α. Figure 1 shows the result of the representative experiment which demonstrated IFN-γ- and TNF-α-mediated potentiation of macrophage anti-MAIC activity. In this experiment, both cytokines did not endow the macrophages with microbicidal activity against MAIC. However, significant inhibition of bacterial growth was observed when MAIC-infected macrophages were cultured in the presence of these cytokines: P < 0.05 by Mann–Whitney test (IFN-γ) or Student's t-test (TNF-α).
Fig. 1.
Potentiating effects of IFN-γ and TNF-α on macrophage anti-Mycobacterium avium-intracellulare complex(MAIC) activity. The result shown is representative of six experiments separately carried out. Peritoneal macrophages of BALB/c (BcgS) mice were infected with high-virulence MAIC N-260 strain and cultured in the absence (□) or presence of IFN-γ (▪) or TNF-α (hatched bar). IFN-γ and TNF-α were added at concentrations of 300 and 1000 U/ml, respectively. Each bar indicates the number of bacterial colony-forming units (CFU) per macrophage culture (mean ± s.e.m.; n = 3). †Significantly smaller than the value of the control macrophages (− cytokine) (P < 0.05; Mann–Whitney test); *smaller than the value of the control macrophages at P < 0.05 by Student's t-test. The recovery of bacterial CFU in culture fluids was < 10% of that recovered from macrophages.
We repeated the same experiments six times or more by using different lots of peritoneal macrophages from BALB/c (BcgS) strain mice. In each individual experiment we estimated the mean value of ‘relative growth rate’ of MAIC N-260 in IFN-γ- or TNF-α-treated macrophages by performing triplicate incubations per regimen. In most experiments, the values of ‘relative growth rate’ of MAIC in IFN-γ- or TNF-α-treated macrophages distributed below 1.0, i.e. the inter-experimental averages of the parameter (six to seven experiments (18–21 incubations)) were 0.69 ± 0.06 and 0.82 ± 0.07 for IFN-γ- and TNF-α-treated macrophages, respectively. The observed reductions in ‘relative growth rate’ were statistically significant (IFN-γ, P < 0.01; TNF-α, P < 0.05; Mann–Whitney test), confirming both IFN-γ and TNF-α are efficacious in up-regulating macrophage anti-MAIC activity.
Roles of IL-10 and TGF-β in modulation of macrophage anti-MAIC activity
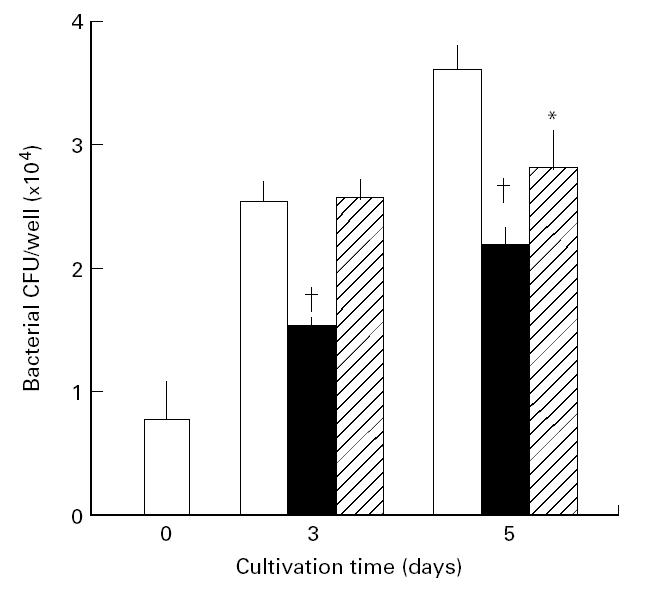
In the next series of experiments we attempted to assess the roles of IL-10 and TGF-β in down-regulation of the anti-MAIC activity of mouse peritoneal macrophages. For this purpose, we used anti-IL-10 and anti-TGF-β antibodies to neutralize the activity of IL-10 and TGF-β which were endogenously produced by MAIC-infected macrophages [25,27]. Figure 2 shows results of the representative experiment in which BALB/c (BcgS) macrophages were infected with high-virulence MAIC N-260 and cultured in the presence or absence of anti-IL-10 or anti-TGF-β antibody. Rat and mouse IgGs were used as a control antibody for the anti-IL-10 and anti-TGF-β antibodies, respectively. No significant reduction of the MAIC growth was observed in macrophages treated with anti-IL-10 antibody or anti-TGF-β antibody, compared with the case where macrophages were treated with corresponding control antibodies. Although some degree of reduction of MAIC growth rate was noted in macrophages treated with anti-TGF-β antibody compared with untreated control macrophages (P < 0.05 at day 5; Mann–Whitney test), the control antibody (mouse IgG) also caused similar levels of growth inhibition. Although the precise reason why the control antibody reduced the bacterial growth is unknown, it is possible that the IgG molecules exerted some opsonin-like activity or caused non-specific potentiation of macrophage anti-MAIC activity.
Fig. 2.
Failure of anti-IL-10 and anti-TGF-β antibodies to modulate macrophage anti-Mycobacterium avium-intracellulare complex (MAIC) activity. The result shown is representative of six experiments separately carried out. Peritoneal macrophages of BALB/c (BcgS) mice were infected with high-virulence MAIC N-260 strain and cultured in the absence (□) or presence of 30 μg/ml each of anti-IL-10 antibody, anti-TGF-β antibody, or control antibody (rat IgG for anti-IL-10 antibody and mouse IgG for anti-TGF-β antibody). *Smaller than the value of control macrophages (P < 0.05; Mann–Whitney test). There was no significant difference in the bacterial loads between macrophages treated with each anti-cytokine antibody and those treated with a control antibody. The other details are the same as in Fig. 1.
We repeated the same experiments six times using different lots of macrophages from BALB/c (BcgS) strain mice. In each individual experiment we estimated the mean value of ‘relative growth rate’ of MAIC N-260 in anti-IL-10 or anti-TGF-β antibody-treated macrophages in triplicate incubations per regimen. Inter-experimental averages of ‘relative growth rate’ (six experiments (18 incubations)) were estimated as 0.89 ± 0.08 and 0.88 ± 0.07 for macrophages treated with anti-IL-10 antibody and its control antibody, respectively; and 0.81 ± 0.07 and 0.84 ± 0.09 for macrophages treated with anti-TGF-β antibody and control antibody, respectively. Thus, it is concluded that the anti-MAIC activity of BALB/c (BcgS) macrophages was not significantly potentiated by blocking endogenous IL-10 or TGF-β using anti-IL-10 and anti-TGF-β antibodies.
Next, we examined the effects of anti-IL-10 and anti-TGF-β antibodies on the mode of growth of high-virulence MAIC N-260 in BALB/c (BcgS) macrophages stimulated with either IFN-γ or TNF-α and the following results were obtained. First, in the case of IFN-γ-stimulated macrophages, the inter-experimental averages of ‘relative growth rate’ of six to seven experiments (18–21 incubations) were estimated as 0.74 ± 0.06 and 0.69 ± 0.05 for macrophages treated with anti-IL-10 antibody and its control antibody, respectively; and 0.89 ± 0.09 and 0.77 ± 0.11 for macrophages treated with anti-TGF-β antibody and its control antibody, respectively. Second, in the case of TNF-α-stimulated macrophages, the inter-experimental averages of ‘relative growth rate’ of six experiments (18 incubations) were estimated as 0.96 ± 0.06 and 0.72 ± 0.05 for macrophages treated with anti-IL-10 antibody and its control antibody, respectively; and 0.96 ± 0.10 and 0.88 ± 0.11 for macrophages treated with anti-TGF-β antibody and its control antibody, respectively. These findings indicate that endogenous IL-10 and TGF-β lack modulating effect on anti-MAIC N-260 activity of BALB/c macrophages, even when the macrophages are activated by IFN-γ or TNF-α.
Effects of anti-IL-10 and anti-TGF-β antibodies on the anti-microbial functions of BcgS and Bcgr macrophages against high- and low-virulence MAIC
Next, we examined the effects of anti-IL-10 and anti-TGF-β antibodies on the anti-microbial activities of peritoneal macrophages from C57Bl/6 (BcgS; MAIC-susceptible) and CBA/JN (Bcgr; MAIC-resistant) strain mice [31] against high-and low-virulence MAIC.
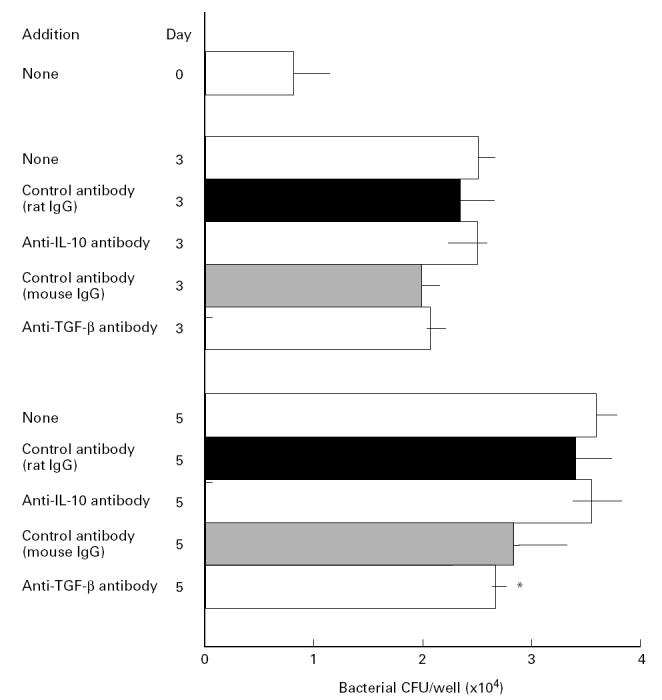
Figure 3 shows the representative results of three experiments separately repeated. When macrophages were infected with high-virulence MAIC N-260, C57Bl/6 (BcgS) macrophages allowed progressive growth of infected organisms during whole cultivation periods for up to 5 days (Fig. 3a). In the case of CBA/JN (Bcgr) macrophages, infected organisms showed transient growth during the first 3 days but thereafter they were gradually eliminated during day 3 to day 5 (Fig. 3b). On the other hand, when macrophages were infected with low-virulence MAIC N-254, the organisms residing in C57Bl/6 (BcgS) macrophages were temporarily eliminated during the first 3 days, followed by subsequent regrowth during day 3 to day 5 (Fig. 3c). In the case of CBA/JN (Bcgr) macrophages, progressive killing of the infected organisms was observed during whole cultivation periods for up to 5 days (Fig. 3d). In these cases, anti-IL-10 and anti-TGF-β antibodies did not significantly affect the growth of MAIC N-254 or N-260 strain residing in C57Bl/6 (BcgS) and CBA/JN (Bcgr) macrophages. The same result was also obtained for the effects of anti-IL-10 and anti-TGF-β antibodies on the anti-microbial activity of BALB/c (BcgS) macrophages against MAIC N-254 strain (data not shown). These findings indicate that IL-10 and TGF-β are essentially lacking in modulation effects on macrophage anti-MAIC functions regardless of virulence of test MAIC strains and regardless of Bcg genotypes of macrophage-donor mice.
Fig. 3.
Effects of anti-IL-10 and anti-TGF-β antibodies on the anti-Mycobacterium avium-intracellulare complex (MAIC) activity of peritoneal macrophages of C57Bl/6 (BcgS) (a,c) and CBA/JN (Bcgr) (b,d) mice. The result shown is representative of three experiments separately carried out. Macrophages were infected with either high-virulence MAIC N-260 strain (a,b) or low-virulence MAIC N-254 strain (c,d) and cultured in the absence (○) or presence of 20 μg/ml each of anti-IL-10 antibody (•) or anti-TGF-β antibody (▴). ‘Growth index’ was calculated as: growth index = (intracellular colony-forming units (CFU) at day 3 or day 5)/(intracellular CFU at day 0). Each plot indicates the mean ± s.e.m. (n = 3) of the ‘Growth index’ value. The day 0 values for intracellular CFU residing in macrophages were: (a) 5.78 × 103/well; (b) 5.76 × 103/well; (c) 1.07 × 103/well; (d) 1.42 × 103/well. There was no significant difference in the bacterial loads between macrophages treated with each anti-cytokine antibody and untreated control macrophages. The recovery of bacterial CFU in culture fluids was < 10% of that recovered from macrophages.
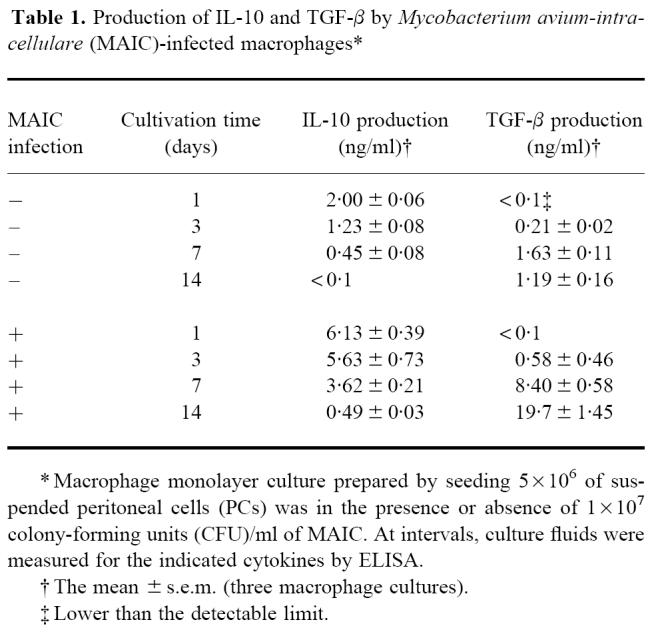
Production of IL-10 and TGF-β by MAIC-infected macrophages
As shown in Table 1, peritoneal macrophages from BALB/c strain mice produced substantial amounts of IL-10 and TGF-β in response to MAIC N-260 infection during the course of chase cultivation. IL-10 production was observed in the early phase of cultivation around days 1–3, while TGF-β production was initiated from day 3 and continuously increased during subsequent cultivation up to day 14. In experiments in Figs 2 and 3, anti-IL-10 and anti-TGF-β antibodies were added to macrophage cultures at concentrations of 20 or 30 μg/ml, which can neutralize ≥ 100 or ≥ 150 ng/ml of IL-10 and 8 or 12 ng/ml of TGF-β. Maximum production of IL-10 and TGF-β by MAIC-infected macrophages during cultivation during the first 7 days was < 6.1 and 8.4 ng/ml, respectively. These values are therefore smaller than or comparable to the amounts of IL-10 and TGF-β which can be neutralized with 20 or 30 μg/ml of the anti-IL-10 and anti-TGF-β antibodies. Thus, the present dosing of the anti-IL-10 and anti-TGF-β antibodies in Figs 2 and 3 seems to be sufficient for neutralization of endogenous IL-10 and TGF-β produced by MAIC-infected macrophages.
Table 1.
Production of IL-10 and TGF-β by Mycobacterium avium-intracellulare (MAIC)-infected macrophages*
Profiles of the bacterial growth in MAIC-infected mice with or without administrations of anti-IL-10 or anti-TGF-β antibody
Since the above findings strongly suggest that IL-10 and TGF-β were essentially ineffective in down-regulating anti-MAIC activity of murine macrophages, we examined the effects of in vivo neutralization of IL-10 and TGF-β with anti-IL-10 and anti-TGF-β antibodies on the mode of bacterial growth in mice infected with MAIC N-260. As shown in Table 2, multiple injections of either anti-IL-10 (exp. 1) or anti-TGF-β antibody (exp. 2) did not significantly affect the growth of the organisms in the lungs and spleen. Therefore, in vivo neutralization of endogenous IL-10 or TGF-β appears not to change the mode of growth of MAIC N-260 at the sites of infection, indicating that these immunoregulatory cytokines do not play important roles in suppression of macrophage anti-MAIC functions, which allows progressive growth of the organisms at the sites of infection. However, the result obtained by exp. 1 suggests the possibility that IL-10 still plays some minor roles in down-regulation of the expression of host resistance to MAIC infection. That is, injection of anti-IL-10 antibody to infected mice receiving oral administrations of a rifamycin derivative KRM-1648, a potent anti-mycobacterial drug [33], increased the in vivo anti-MAIC activity of KRM-1648 in MAIC-infected mice (Table 2; exp. 1). In this experiment, the reduction of bacterial loads at week 6 in the lungs of KRM-treated mice from those of untreated control mice was 1.72 ± 0.07 log-units. When anti-IL-10 antibody was given to mice in combination with KRM-1648 treatment, the reduction of the bacterial loads from those of control mice was increased to 2.35 ± 0.19 log-units. On the other hand, the growth of MAIC in the spleens of KRM-1648-treated mice was not affected by anti-IL-10 administration.
Table 2.
Effects of administration of anti-IL-10 or anti-TGF-β antibody on the mode of the in vivo growth of Mycobacterium avium-intracellulare complex (MAIC) in host mice*
DISCUSSION
In the present study, we examined the roles of proinflammatory cytokines IFN-γ and TNF-α, and immunoregulatory cytokines IL-10 and TGF-β, in modulating anti-MAIC activity of murine peritoneal macrophages, and the following results were obtained. First, both IFN-γ and TNF-α caused significant inhibition of the growth of MAIC organisms in macrophages (Figs 1 and 2). This finding is consistent with the majority of previous observations, although, the effectiveness of IFN-γ and TNF-α in promoting macrophage anti-MAIC functions, observed here, were not so great as reported [7,8,10,14–16]. The present study also showed that the efficacy of IFN-γ in promoting the macrophage activity was greater than that of TNF-α. This is consistent with our previous finding that IFN-γ was much more efficacious than TNF-α in potentiating macrophage anti-M. tuberculosis functions [34].
Second, in cases of both human and murine macrophages, IL-10- or TGF-β-mediated modulation of macrophage anti-mycobacterial activity is controversial, as follows. Bermudez et al. [25,27] and Denis & Ghardirian [26] reported IL-10- or TGF-β-mediated down-regulation of the anti-MAIC activity of murine peritoneal macrophages [25,26] and human monocytes [27]. On the other hand, Shiratsuchi et al. [29] and Warwick-Davies et al. [30] reported that neither IL-10 nor TGF-β affected the anti-microbial activity of human monocytes against MAIC [29] or M. tuberculosis[30]. In the present study, we could not obtain any evidence which supported the concept that IL-10 and TGF-β had the effect of modulating the anti-MAIC anti-microbial activity of murine peritoneal macrophages. That is, anti-IL-10 and anti-TGF-β antibodies failed to enhance macrophage anti-MAIC activity, even when the macrophages were treated with sufficient doses of antibody to neutralize macrophage-derived endogenous IL-10 and TGF-β. The same results were obtained regardless of Bcg genotypes of strains of macrophage-donor mice, i.e. CBA/JN (Bcgr), BALB/c (BcgS), and C57Bl/6 (BcgS) macrophages, although Bcgr macrophages displayed greater anti-MAIC activity than did BcgS macrophages (Fig. 3). Moreover, the same results were obtained regardless of virulence of test MAIC strains. That is, anti-IL-10 and anti-TGF-β antibodies did not affect macrophage anti-microbial activity against low-virulence N-254 and high-virulence N-260 strains, although the N-254 strain was more easily killed or inhibited by macrophages than the N-260 strain (Fig. 3). Furthermore, neither anti-IL-10 antibody nor anti-TGF-β antibody affected the expression of the anti-MAIC activity of macrophages stimulated with either IFN-γ or TNF-α.
Therefore, it is concluded that IL-10 and TGF-β endogenously produced by macrophages are themselves substantially ineffective in modulating macrophage anti-MAIC activity, regardless of the virulence of MAIC organisms and regardless of Bcg (Nramp1) genotypes of macrophages, which determine host natural resistance to MAIC infection by regulating macrophage anti-microbial activity [35,36]. In addition, this conclusion is also applicable to strains of macrophage-donor mice with different levels of IL-12 responsiveness to mycobacterial and leishmanial infections, which is decisive for development of a Th1 response [37], since neither anti-IL-10 nor anti-TGF-β antibody affected the anti-MAIC activity of not only IL-12 high-responder (C57Bl/6 and CBA/JN) macrophages but also IL-12 low-responder (BALB/c) macrophages [37–40]. Therefore, although the precise reason for the inconsistency of our findings and those of Shiratsuchi et al. [29] and Warwick-Davies et al. [30] from those reported by Bermudez et al. [25,27] and Denis & Ghardirian [26] is unknown, the difference in experimental conditions, other than virulence of test MAIC organisms or Bcg genotype and IL-12 responsiveness of test macrophages, may be one reason for such a discrepancy.
Concerning the failure of IL-10 and TGF-β to down-regulate macrophage anti-MAIC activity, an alternative explanation is also possible. That is, the effects induced by IFN-γ and TNF-α are rather small, and so probably represent bacteriostasis rather than bacterial killing. This raises the possibility that the role of IL-10 and TGF-β is to inhibit bacterial killing by macrophages but not to inhibit macrophage-mediated bacteriostasis. Since, in the present study, macrophage anti-MAIC activity was principally expressed as a bacteriostatic action, the effects of IL-10 and TGF-β on killing might not be observed.
Third, multiple injections of anti-IL-10 and anti-TGF-β into MAIC-infected mice did not affect the growth of organisms at the sites of infection (Table 2) and this in vivo result is consistent with the above findings obtained by in vitro experiments. However, in this in vivo experiment, the neutralization of IL-10 and TGF-β seems to be incomplete, since mice were given anti-IL-10 or anti-TGF-β antibody at 1- or 2-week intervals and these time scales are much longer than the half-life of murine IgG (about 3 days). Thus, the possibility cannot be excluded that anti-IL-10 or anti-TGF-β antibody could inhibit the in vivo growth of MAIC, when infected mice are given large doses of these antibodies at a high frequency of dosing. Moreover, it appears that IL-10 and TGF-β play some restricted roles in modulating the expression of host resistance to MAIC infection, since administration of anti-IL-10 antibody potentiated to some extent the in vivo anti-MAIC anti-microbial activity of a rifamycin derivative, KRM-1648, and moreover, bacterial growth at sites of infection was accompanied by increased expression of TGF-β in infected tissues [28]. On these points, further studies are currently underway.
Acknowledgments
This study was partly supported by the grant from the Ministry of Education, Science and Culture of Japan (07670310 and 07307004), the Ministry of Public Welfare of Japan (Project for Emerging and Re-emerging Infectious Diseases), the U.S.-Japan Cooperative Medical Science Program (Tuberculosis and Leprosy Section).
References
- 1.Falkinham JO. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young LS. Mycobacterial infections in immunocompromised patients. Curr Opin Infect Dis. 1996;9:240–5. [Google Scholar]
- 3.Bermudez LE, Young LS. Killing of Mycobacterium avium: insights provided by the use of recombinant cytokines. Res Microbiol. 1990;141:241–3. doi: 10.1016/0923-2508(90)90037-q. [DOI] [PubMed] [Google Scholar]
- 4.Denis M. Modulation of Mycobacterium avium growth in vivo by cytokines: involvement of tumour necrosis factor in resistance to atypical mycobacteria. Clin Exp Immunol. 1991;83:466–71. doi: 10.1111/j.1365-2249.1991.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangadharam PRJ, Reddy MV. Contributions of animal and macrophage models to the understanding of host parasite interaction of Mycobacterium avium complex (MAC) disease. Res Microbiol. 1994;145:214–24. doi: 10.1016/0923-2508(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez LE, Kaplan G. Recombinant cytokines for controlling mycobacterial infections. Trend Microbiol. 1995;3:22–27. doi: 10.1016/s0966-842x(00)88864-2. [DOI] [PubMed] [Google Scholar]
- 7.Flesch I, Kaufmann SHE. Mycobacterial growth inhibition by interferon-γ-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987;138:4406–13. [PubMed] [Google Scholar]
- 8.Flesch I, Kaufmann SHE. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–8. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermudez LE, Young LS. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-γ, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–13. [PubMed] [Google Scholar]
- 11.Blanchard DK, Michelini-Norris MB, Djeu JY. Interferon decreases the growth inhibition of Mycobacterium avium-intracellulare complex by fresh human monocytes but not by culture-derived macrophages. J Infect Dis. 1991;164:152–7. doi: 10.1093/infdis/164.1.152. [DOI] [PubMed] [Google Scholar]
- 12.Appelberg R, Sarmento A, Castro Ag. Tumour necrosis factor-alpha (TNF-α) in the host resistance to mycobacteria of distinct virulence. Clin Exp Immunol. 1995;101:308–13. doi: 10.1111/j.1365-2249.1995.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermudez LE. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–81. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarmento A, Appelberg R. Involvement of reactive oxygen intermediates in tumor necrosis factor alpha-dependent bacteriostasis of Mycobacterium avium. Infect Immun. 1996;64:3224–30. doi: 10.1128/iai.64.8.3224-3230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis M, Gregg EO. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990;71:139–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Bermudez LE, Petrofsky MW, Young LS. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992;60:4245–52. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermudez LE, Parker A, Goodman JR. Growth within macrophages increases the efficacy of Mycobacterium avium in invading other macrophages by complement receptor-independent pathway. Infect Immun. 1997;65:1916–25. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 20.De Waal Malefyt R, Yssel H, Roncarolo M-G, Spits H, De Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 21.Espevik T, Figari IS, Shalaby MR, Lackides GA, Lewis GD, Shepard HM, Paliadino MA. Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J Exp Med. 1987;166:571–6. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding A, Nathan CF, Graycar J, Derynck R, Stuehr DJ, Srimal S. Macrophage deactivating factor and transforming growth factors-β1, β2, and β3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-γ. J Immunol. 1990;145:940–4. [PubMed] [Google Scholar]
- 23.Wahl SM. Transforming growth factor beta (TGF-β) in inflammation: a cause and a cure. J Clin Immunol. 1992;12:61–73. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 24.Vodovotz Y, Bogdan C, Paik J, Xie Q, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor β. J Exp Med. 1993;178:605–13. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denis M, Ghardirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–30. [PubMed] [Google Scholar]
- 27.Bermudez LE. Production of transforming growth factor-β by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-γ. J Immunol. 1993;150:1838–45. [PubMed] [Google Scholar]
- 28.Tomioka H, Sato K, Shimizu T, Sano C, Akaki T, Saito H, Fujii K, Hidaka T. Effects of benzoxazinorifamycin KRM-1648 on cytokine production at sites of Mycobacterium avium complex infection induced in mice. Antimicrob Agents Chemother. 1997;41:357–62. doi: 10.1128/aac.41.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiratsuchi H, Hamilton B, Toossi Z, Ellner JJ. Evidence against a role for interleukin-10 in the regulation of growth of Mycobacterium avium in human monocytes. J Infect Dis. 1996;173:410–7. doi: 10.1093/infdis/173.2.410. [DOI] [PubMed] [Google Scholar]
- 30.Warwick-Davies J, Lowrie DB, Cole PJ. Selective deactivation of human monocyte functions by TGF-β. J Immunol. 1995;155:3186–93. [PubMed] [Google Scholar]
- 31.Goto Y, Nakamura R, Takahashi H, Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984;46:135–40. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomioka H, Saito H, Sato K, Dawson DJ. Comparison of the virulence for mice of Mycobacterium avium and Mycobacterium intracellulare identified by DNA probe test. Microbiol Immunol. 1993;37:259–64. doi: 10.1111/j.1348-0421.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 33.Tomioka H, Saito H, Sato K, Yamane T, Yamashita K, Hosoe K, Fujii K, Hidaka T. Chemotherapeutic efficacy of a newly synthesized benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex infection induced in mice. Antimicrob Agents Chemother. 1992;36:387–93. doi: 10.1128/aac.36.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Akaki T, Tomioka H. Differential potentiation of antimycobacterial activity and reactive nitrogen intermediate producing ability of murine peritoneal macrophages activated by interferon-γ and tumor necrosis factor-α. Clin Exp Immunol. 1998;112:63–68. doi: 10.1046/j.1365-2249.1998.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–85. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 36.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 37.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 38.Viera LQ, Hondowicz BD, Afonso LCC, Wysocka M, Trinchieri G, Scott P. Infection with Leishmania major induced interleukin-12 production in vivo. Immunol Letters. 1994;40:157–61. doi: 10.1016/0165-2478(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida A, Koide Y, Uchijima M, Yoshida TO. Dissection of strain difference in acquired protective immunity against Mycobacterium bovis Calmette–Guerin Bacillus (BCG). Macrophages regulate the susceptibility through cytokine network and the induction of nitric oxide synthase. J Immunol. 1995;155:2057–66. [PubMed] [Google Scholar]
- 40.Launois P, Swihart KG, Milon G, Louis JA. Early production of IL-4 in susceptible mice infected with Leishmania major induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–24. [PubMed] [Google Scholar]