Abstract
Cardiovascular manifestations are common in systemic lupus erythematosus (SLE). Oxidized low-density lipoprotein (oxLDL) is implicated in cardiovascular disease, especially atherosclerosis, and cross-reacts with antibodies to cardiolipin (aCL). β2-GPI is a plasma protein participating in the coagulating cascade, and is also cofactor for aCL, and some aCL have been shown to be directed against β2-GPI and/or complexes between β2-GPI and phospholipids. Lysophosphatidylcholine (LPC) is a phospholipid present both in oxLDL and in damaged endothelium, and we recently showed that LPC is involved in the antigenicity of oxLDL. Antibodies to endothelial cells (aEC) correlate with disease activity in SLE and vasculitis, and we recently showed that aEC are enhanced in cardiovascular diseases such as borderline hypertension and early atherosclerosis. aEC were determined using EC from adult V. Saphena Magna. Antibody levels were determined by ELISA. aEC of IgG type were enhanced in 184 patients with SLE compared with 85 healthy controls. There was a close correlation between aoxLDL, aCL, aLPC, aβ2-GPI and aEC. Binding of sera to EC was competitively inhibited by β2-GPI, LPC and oxLDL. Taken together, the data indicate that EC share antigenic epitopes with β2-GPI and with oxLDL, especially LPC. Phospholipids in EC membranes may thus be antigenic epitopes. β2-GPI may bind to these phospholipids, and become an autoantigen. LPC is formed by oxidation of phospholipids and/or proinflammatory factors leading to activation of phospholipase A2, and the findings indicate the potential role of both lipid oxidation and phospholipase A2 in SLE.
Keywords: anti-phospholipid antibodies, endothelial cells, lysophosphatidylcholine, oxidized low-density lipoprotein, systemic lupus erythematosus
INTRODUCTION
Patients with systemic lupus erythematosus (SLE) have a decreased life expectancy, and enhanced risk of cardiovascular disease is a major factor behind this [1]. Cardiovascular disease in SLE includes thrombo-embolic complications, where haemostatic factors, often related to enhanced antibody levels to phospholipids such as cardiolipin (CL), are important, but also premature atherosclerosis and vasculitis may play a role [2–8]. These findings indicate that damage to or activation of the endothelium may be an important underlying feature in SLE. Recently, a cofactor for antibody binding to CL, β2-GPI, has been defined [9,10]. However, the precise relation between antibodies to CL (aCL) and aβ2-GPI is not clear, though recent studies show that aCL recognize oxidized phospholipids and some aCL may bind to adducts of oxidized phospholipid and β2-GPI [10,11].
In line with this are recent findings indicating that antibodies to endothelial cells (aEC) from human umbilical veins are enhanced in SLE [12–14] and may be related to disease activity [13]. We recently showed that aEC were associated with early atherosclerosis, and with borderline hypertension, and that some of the aEC in this patient group bound to β2-GPI [15]. A directly pathogenic role of aEC is suggested by animal experiments where aEC caused vasculitis [16], though little is known about the role in disease development of aEC in human disease.
Atherosclerosis is a chronic inflammation in the artery wall, and the major underlying cause of cardiovascular disease [17]. According to a leading hypothesis, oxidized low-density lipoprotein (oxLDL) may play a pivotal role in atherosclerosis [18]. Antibodies against oxLDL (aoxLDL) are present in the atherosclerotic plaque and are related to progression of atherosclerosis [19]. Furthermore, aoxLDL antibodies cross-react with CL in SLE patients [20]. Lysophosphatidylcholine (LPC) is formed during oxidation of LDL [21] or enzymatically from phosphatidylcholine by enzymes with phospholipase A2-activity [22]. Recently we showed that LPC is an important antigen in oxLDL [23]. Furthermore, we identified secretory phospholipase A2 type II (sPLA2-II, non-pancreatic type) expression and activity in both normal and atherosclerotic arterial wall [24]. LPC may thus be generated in the arterial wall by extracellular hydrolysis of phospholipids in retained LDL, as well as in platelets, smooth muscle cells and EC [25]. We here present evidence that β2-GPI, oxLDL and LPC share antigenic epitopes with EC. The potential implications for cardiovascular disease in SLE are discussed.
PATIENTS AND METHODS
Study group
The study group consisted of 184 unselected patients with SLE from the Rheumatology Clinic, Karolinska Hospital, Stockholm. All patients fulfilled the 1982 revised criteria of the American Rheumatism Association for classification of SLE [26]. The study was approved by the local Ethics Committee of Karolinska Hospital and conducted in accordance with the Helsinki Declaration. The control group consisted of 85 age-matched healthy volunteers (blood donors). The SLE group consisted of 160 females and 24 males aged 46.6 ± 11.5 years (mean ± s.d.) There were 61 females and 24 males (46.2 ± 13.5 years) in the control group.
Lipids and reagents
l-a-lysophosphatidylcholine (LPC; from egg yolk type 1, produced by PLA2 treatment) and CL (from bovine brain) were from Sigma (St Louis, MO).
LDL was isolated from plasma of healthy donors by sequential preparative ultra-centrifugation in a 50.3 Ti Beckman fixed angle rotor (Beckman L8-80 ultracentrifuge) for 48 h at 1°C and collected in the density interval 1.025–1.050 kg/l. The protein content was determined according to Lowry and adjusted to 200 μg/ml [27,28]. The LDL was dialysed against PBS pH 7.4 for 24 h and then oxidized by exposure to 5 μm CuSO4 for 18 h at 37°C. This procedure has previously been shown to result in extensive oxidation of LDL [29].
MDA-LDL was prepared by incubating LDL for 3 h at 37°C with 0.5 m MDA at a constant ratio of 100 μl/ml of LDL. MDA (0.5 m), freshly generated from malonaldehyde bis dimethylacetal, was incubated with 12 μl 4 n HCL and 400 μl H2O at 37°C for 10 min. The reaction was then stopped by adjusting the pH to 7.4 by the addition of 1 n NaOH and the volume was brought to 1 ml with water. After conjugation, MDA-LDL was extensively dialysed against PBS to remove any unreacted MDA. The degree of MDA modification was determined by trinitrobenzenesulphonic acid assay (TNBS) and averaged 77% of the lysine residues for a typical preparation. In addition, the electrophoretic mobility of the MDA-LDL was compared with that of native LDL by electrophoresis using 1% agarose gels in borate buffer pH 8.6.
Purified protein derivative (PPD; 2.5 mg/ml) was from Statens Seruminstitut (Copenhagen, Denmark), and EBNA was from Biotest (Dreieich, Germany).
Cell culture
EC were isolated and cultured from 3 to 5 cm long segments of the saphenous vein, derived from patients undergoing coronary by-pass surgery, as described in detail previously [30]. Briefly, the vein was rinsed and then filled with a collagenase solution (0.1%; Worthington, Freehold, NJ). Harvested cells were routinely cultured in minimum essential medium (MEM; GIBCO BRL, Paisly, UK) with the addition of 40% pooled heat-inactivated (56°C, 30 min) human serum (HS), antibiotics and cyclic AMP elevating compounds. Two days prior to the experiments, the EC were gently detached using a 0.1% trypsin–0.02% EDTA 1:1 solution. The cells were seeded on gelatin-coated plastic wells (24-well plates; Costar, Cambridge, MA) at a density corresponding to 100 000 cells/cm2 in MEM containing only 30% HS and antibiotics. The EC were characterized as endothelial using immunohistochemical staining of von Willebrand factor-related antigen, PGI2 production and by their typical cobblestone appearance. In each experiment, cells from a single donor from the passages 4–7 were used. The use of human great saphenous veins was approved by the ethics committee at the Karolinska Hospital.
Detection of antibody levels to EC
Antibodies to EC were detected essentially as described earlier [31]. The EC were suspended in the RPMI 1640 medium containing 20% heat-inactivated fetal calf serum (FCS) and seeded on the 96-well flat-bottomed tissue culture plates at a density of 1 × 104 cells/well. After the EC were incubated for 2 days, the plates were washed three times with PBS pH 7.4. The EC were fixed for 15 min at room temperature with 0.2% glutaraldehyde. The fixed cells were washed four times in the washing buffer (PBS–0.2% bovine serum albumin (BSA)). The plates were blocked by 200 μl of blocking buffer (PBS–1% BSA and 0.1 n glycine) for 1 h at room temperature. The serum samples were diluted 1:50 in PBS and 100 μl of this dilution were added to each well and incubated at 37°C for 2 h.
Determination of antibody levels to lipids and EBNA
IgG and IgM antibodies against oxLDL, MDA-LDL and native LDL were determined by an ELISA essentially as described [29]. OxLDL and LDL were diluted to 2 μg/ml in coating buffer (carbonate-bicarbonate buffer 50 mm pH 9.7), and 100 μl/well were used to coat ELISA plates (Costar 2581). The plates were kept at 4°C overnight, washed three times with PBS containing 0.05% Tween-20, and then blocked with 20% adult bovine serum in PBS (20% ABS–PBS) for 2 h at room temperature. They were then incubated with 100 μl serum diluted 1:30 in 20% ABS–PBS at 4°C overnight.
Antibodies against CL and LPC were analysed essentially as described [23,32]. Briefly, Titertek 96-well polyvinylchoride microplates (Flow Labs, Costa Mesa, CA) were coated with 50 μl/well of 50 μg/ml lipid dissolved in ethanol and allowed to dry overnight at 4°C. Blocking was accomplished with 20% ABS–PBS for 2 h. Serum samples (50 μl) diluted 1:30 in 20% ABS–PBS were added to each well.
Antibody reactivity to EBNA in Epstein–Barr virus (EBV) was detected with ELISA according to the manufacturer's description (Blotest). Briefly, 100 μl/well of 50 ng/ml EBNA was used to coat ELISA plates (Costar 2581). Blocking was accomplished with 20% ABS–PBS for 2 h. They were then incubated with 100 μl serum diluted 1:30 in 20% ABS–PBS at 4°C overnight.
Antibody reactivity to β2-GPI was detected by coating irradiated Titertek 96-well polyvinylchoride microplates (Flow Labs) with 50 μl/well of (30 μg/ml) β2-GPI (Calbiochem B18287) dissolved in 10 mm HEPES, 150 mm NaCl pH 7.4 (HEPES buffer) at 4°C overnight. The plates were blocked with 0.3% gelatin for 1 h. After washing, the wells were incubated with 50 μl of 50 × diluted samples for 1 h at room temperature (2 mm of EDTA was included in buffer). Control assay were performed in the absence of β2-GPI.
After four washings with PBS the plates were incubated with 50 (or 100) μl/ml of alkaline phosphatase-conjugated goat anti-human IgG (Sigma A-3150) diluted 1:9000, or IgM (Sigma A-3275) diluted 1:7000 with PBS at 37°C for 2 h. After four washings, 100 μl of substrate (phosphatase substrate tablets; Sigma 104) 5 mg in 5 ml diethanolamine buffer pH 9.8 were added. The plates were incubated at room temperature for 30 min and read in an ELISA Multiskan Plus spectrophotometer at 405 nm. Each determination was done in triplicate. The coefficient of variation between triplicate tests was < 5%.
Analysis of total serum immunoglobulin levels
Serum immunoglobulins, IgG and IgM were determined by immunoturbidimetry. Specific anti-IgG and anti-IgM reagents and calibrators were obtained from Dako (Copenhagen, Denmark). The turbidimetric reaction was quantified in a Hitachi 911 analyser by measuring light transmission at 340 nm wavelength.
Cross-reactivity between antibodies to LPC, oxLDL and CL
In order to investigate if there was an immunological cross-reactivity between aEC and LPC, oxLDL, LDL, MDA-LDL, β2-GPI, CL or PPD, competition assays were performed. Sera, at a dilution giving 50% of maximal binding to EC, were preincubated with EC or with the different competitors at 4°C, and inhibition of binding to EC was tested. The percentage of inhibition was calculated as follows:
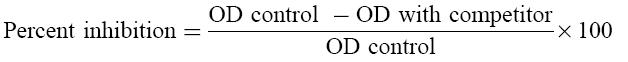 |
Statistical analysis
Conventional methods were used for calculation of means and s.d. Simple regression was used to analyse the correlations between the antibodies, and between age and antibodies. Paired Student's t-test was used to compare inhibition of binding to EC with control and between compounds tested.
RESULTS
Antibody levels
The levels of aEC of IgG type were enhanced in SLE (OD405 ± s.d. was 0.249 ± 0.106 in SLE patients versus 0.204 ± 0.073 in controls; P = 0.0011) but not IgM type (0.478 ± 0.263 in SLE patients versus 0.458 ± 0.204 in controls; P = 0.8272). In females and males separately, aEC levels of IgG were significantly enhanced in SLE patients compared with controls (P < 0.01), but aEC of IgM type showed no significant difference.
Antibody levels against CL, oxLDL, β2-GPI and LPC were significantly higher (P < 0.05) in the patient group compared with the control group (data not shown).
A box plot where aEC, aCL, aoxLDL, aβ2-GPI and aLPC of IgG type are compared in SLE and control groups is shown in Fig. 1.
Fig. 1.
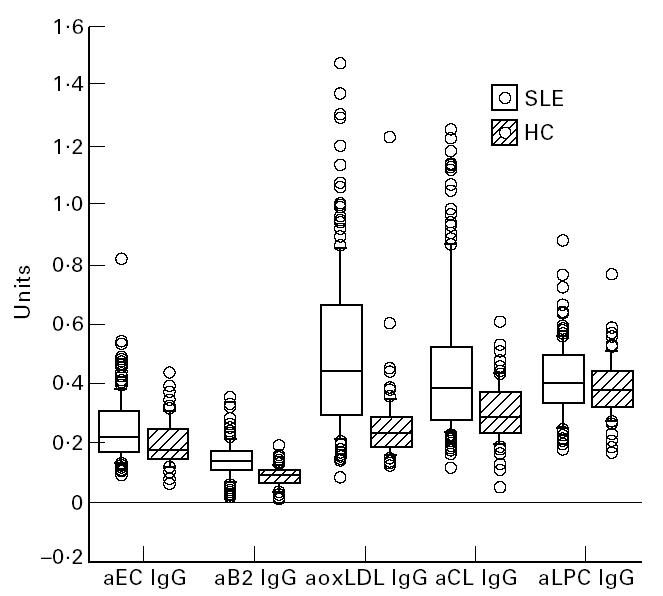
Box plots of antibody levels (OD405) of IgG to endothelial cells (EC), cardiolipin (CL), oxidized low-density lipoprotein (oxLDL), β2-GPI and lysophosphatidylcholine (LPC) of IgG type in systemic lupus erythematosus (SLE) patients (n = 184) and controls (n = 85).
Total IgG and IgM levels were significantly higher in the patient group compared with the control group (IgG 21.1 ± 8.2 g/l versus 12.6 ± 5.2 g/l and IgM 2.1 ± 3.4 g/l versus 1.3 ± 0.8 g/l, respectively).
Analysis of antibodies against EC
The correlation between aEC and aLDL, aoxLDL, aLPC, aCL, aβ2-GPI and aEBNA, and also correlations with total immunoglobulin are shown in Table 1. There was a significant correlation (P < 0.001) between antibody levels against EC on the one hand and LPC, β2-GPI, oxLDL, LDL, and CL both of IgG and IgM isotype in the patient group. In the control group, only aβ2-GPI correlated with aEC. There was no correlation between antibodies to an unrelated antigen, EBNA, and antibodies to EC and there was no correlation between total IgG or IgM levels and aEC.
Table 1.
Association of antibodies to endothelial cells (EC) with antibodies against oxidized low-density lipoprotein (oxLDL), LDL, cardiolipin (CL), lysophosphatidylcholine (LPC) or β2-GPI
Cross-reactivity of aEC with different antigens
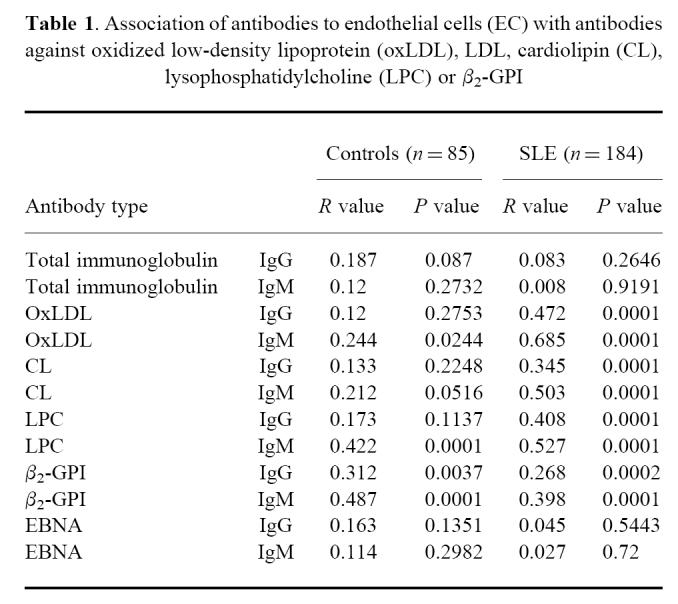
To study possible cross-reactivity between the antibodies, we performed competition experiments in eight randomly chosen high aEC patient sera. As a control an unrelated antigen, PPD, was used. The sera were tested at a dilution giving 50% of maximal binding to EC. To test if antibodies to EC could be competed out by EC themselves, serum was added to wells for 24 h and then the serum was moved to another plate coated with EC. When oxLDL, LPC, β2-GPI and EC were compared with controls in the eight samples tested, they showed a significant inhibition. CL was a weak and non-significant competitor for binding of antibodies to EC. An unrelated antigen, PPD, did not inhibit binding to EC (Table 2). Figure 2 shows a representative experiment where oxLDL and LPC at different concentrations inhibited serum binding to EC to various degrees in three different individuals.
Table 2.
Percentage inhibition of antibody binding to endothelial cells (EC) by different antigens (100 μg/ml) in eight high-titre sera compared with control values without antigen
Fig. 2.
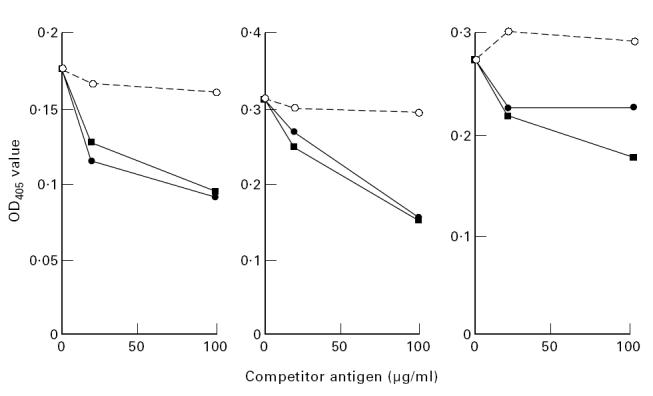
Effect of oxidized low-density lipoprotein (oxLDL) (•), lysophosphatidylcholine (LPC) (▪) and purified protein derivative (PPD) (○) on serum binding to endothelial cells (EC) in a representative experiment with three individuals. Sera were incubated with the antigens of different concentrations and as indicated, at 4°C overnight. After this, the binding to ELISA plates coated with EC was investigated. Results are presented as mean of duplicate determinations.
DISCUSSION
SLE is characterized by elevated antibody production against a large variety of autoantigens. Enhanced antibody levels to endothelial cells (aEC) have been reported in SLE [12–14] and also in several other supposedly autoimmune diseases, including rheumatoid arthritis with systemic manifestations, Wegener's granulomatosis and vasculitis [12]. Furthermore, we recently showed that aEC are enhanced in borderline hypertension, and correlated with endothelin, a very potent vasoconstrictor, but it is not known if these antibodies actually induce hypertension, though this possibility has not been excluded [15]. Enhanced aEC have been suggested to be the disease activity marker in SLE [13]. EC from the long saphenous vein from adults were used here, as opposed to these previous investigators, who use EC from umbilical veins (HUVEC). Functional characteristics and antigenic properties may show differences between HUVEC and the EC from adults used here, and we believe that endothelial cells from the great saphenous vein (VSMEC) represent an advantage in studies of autoantibodies in adults, as here [15,33].
We here demonstrate that antibodies to EC of IgG type are common in SLE, with significantly higher antibody levels compared with controls. The total IgG levels were significantly higher in SLE patients compared with controls, but did not correlate with aEC. Furthermore, antibodies to an unrelated and common antigen of EBV, EBNA, did not correlate with aEC. The enhanced levels of aEC are thus not likely merely to reflect the total immunoglobulin levels.
OxLDL is generally believed to be pivotal in the development of atherosclerosis, which is a chronic inflammatory disease in arteries, mainly confined to the intima [17,18]. A main focus of our research recently has been to define the proinflammatory effects and antigenic properties of oxLDL, and we and others have suggested that oxLDL may be an important factor causing atherosclerotic inflammation, since oxLDL can induce enhanced endothelial adhesiveness [33–35] and monocyte and lymphocyte activation [36–39]. These proinflammatory effects are largely mediated by platelet-activating factor (PAF)-like lipids in oxLDL [40], and we recently found that LPC is one such PAF-like lipid either in itself or as a precursor (unpublished observation), and also a major antigen in oxLDL [23].
We here show that both oxLDL and LPC inhibited antibody binding to EC, indicating that similar epitopes are present in LPC, oxLDL and EC.
SLE patients have enhanced risk of early death due to cardiovascular disease [1] and several studies indicate accelerated atherosclerosis may contribute to this. SLE patients also have enhanced risk of thrombosis, especially when aCL or aβ2-GPI are elevated [2–8].
Recently, an important cofactor for antibody binding to CL, β2-GPI, was reported to be implicated in antibody binding to EC [41]. We confirm and extend these findings, demonstrating that β2-GPI is involved in the binding of aEC from SLE patients. The precise role β2-GPI in the binding of aEC is not clear. Recent studies show that some phospholipid antibodies may bind to adducts of oxidized phospholipid and β2-GPI [10], which may in principle be the case also for phospholipids in EC.
Lysophospholipids in general are formed during oxidation of phospholipid membranes, but they are also produced by a heterogeneous group of enzymes with PLA2 activity [25]. Phosphatidylcholine is the dominant membrane phospholipid, so LPC is likely to be formed. In inflammatory diseases like rheumatoid arthritis and SLE, PLA2 activity in serum is enhanced [42,43]. In most cell types, PLA2s are induced by proinflammatory cytokines, including tumour necrosis factor-alpha (TNF-α) and IL-6, which are present in increased levels in sera from SLE patients [44]. It is therefore possible that these cytokines and also other proinflammatory factors induce PLA2 in the vessel wall, generating enhanced formation of LPC, and a subsequent immune reaction to LPC in the endothelium, which theoretically may lead to vasculitis, thrombosis and aggravated atherosclerosis.
It is also possible that inflammatory factors such as superoxide induce oxidation in LDL, and phospholipid-containing membranes such as those in EC, generating LPC. Antibodies to oxLDL are related to atherosclerosis [19] and an immune reaction to oxLDL or oxidized membrane phospholipids may predispose to aggravated atherosclerosis in SLE. Furthermore, antibodies to EC may react with LPC or other oxidized phospholipids present also in other phospholipid-containing membranes, e.g. in erythrocytes and platelets, and may thus be involved both in thrombosis and other complications seen in SLE, such as haemolysis, nephritis and thrombocytopenia.
Low levels of aEC were present in most individuals, both patients and controls. The role of these normally occurring antibodies is not known. One possibility is that they are involved in removal of obnoxious compounds with potentially damaging effect, such as those produced during oxidation of phospholipids in cell membranes. This may be the case also in SLE patients. If these oxidized antigens reach a certain level, and the antigens remain in the vessels, the antibodies may become pathogenic.
Taking all data together, aEC may recognize LPC and oxLDL in vessels of different sizes, leading to an inflammatory reaction due to immune complex deposition, complement activation, and possibly uptake by macrophages, foam cell formation, and increased risk of thrombosis and vasculitis and other manifestations of SLE.
Acknowledgments
This work was supported by King Gustaf V80th Birthday Fund, the Swedish Society of Medicine, The Swedish Rheumatism Association, and the Swedish Heart Lung Foundation. We thank Jill Gustafsson for valuable help in conducting this study.
References
- 1.Rubin LA, Urowitz MB, Gladman DD. Mortality in systemic lupus erythematosus: the bimodal pattern revisited. Q J Med, New Series. 1985;55:87–9. [PubMed] [Google Scholar]
- 2.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–9. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 3.Drenkard C, Villa AR, Alarcón-Segovia D, Pérés-Vazquez ME. Influence of the antiphospholipid syndrome in the survival of patients with systemic lupus erythematosus. J Rheumatol. 1994;21:1067–72. [PubMed] [Google Scholar]
- 4.Ginsburg KS, Liang MH, Newcomer L, Goldhaber SZ, Schur PH, Hennekens CH, Stampfer MJ. Anticardiolipin antibodies and the risk for ischemic stroke and venous thrombosis. Ann Intern Med. 1992;117:997–1002. doi: 10.7326/0003-4819-117-12-997. [DOI] [PubMed] [Google Scholar]
- 5.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidem. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 6.Sturfelt G, Eskilsson J, Nived O, Truedsson L, Valand S. Cardiovascular disease in systemic lupus erythematosus, a study of 75 patients from a defined population. Medicine. 1992;71:216–23. doi: 10.1097/00005792-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Belmont M, Abramson B, Lie JT. Pathology and pathogenesis of vascular injury in systemic lupus erythematosus. Arthritis Rheum. 1996;39:9–22. doi: 10.1002/art.1780390103. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro S. The lupus anticoagulant/antiphospholipid syndrome. Ann Rev Med. 1996;47:533–53. doi: 10.1146/annurev.med.47.1.533. [DOI] [PubMed] [Google Scholar]
- 9.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hörkkö S, Miller E, Branch DW, Palinski W, Witztum JL. The epitopes for some antiphospholipid antibodies are adducts of oxidized phospholipid and β2 glycoprotein 1 (and other proteins) Proc Natl Acad Sci USA. 1997;94:10356–61. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hörkkö S, Miller E, Dudl E, et al. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids. J Clin Invest. 1996;98:815–25. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro M, Cervera R, Font J, et al. Anti-endothelial cell antibodies in systemic autoimmune diseases: prevalence and clinical significance. Lupus. 1997;6:521–6. doi: 10.1177/096120339700600608. [DOI] [PubMed] [Google Scholar]
- 13.Li JS, Liu MF, Lei HY. Characterization of anti-endothelial cell antibodies in the patients with systemic lupus erythematosus: a potential marker for disease activity. Clin Immunol Immunopathol. 1996;79:211–6. doi: 10.1006/clin.1996.0070. [DOI] [PubMed] [Google Scholar]
- 14.Hill MB, Phipps JL, Milford-Ward A, Greaves M, Hughes P. Further characterization of anti-endothelial cell antibodies in lupus erythematosus by controlled immunoblotting. Br J Rheumatol. 1996;35:1231–8. doi: 10.1093/rheumatology/35.12.1231. [DOI] [PubMed] [Google Scholar]
- 15.Frostegård J, Wu R, Haegerstrand C, Lemne C, de Faire U. Serum antibodies to endothelial cells in borderline hypertension. Circulation. 1998;98:1092–8. doi: 10.1161/01.cir.98.11.1092. [DOI] [PubMed] [Google Scholar]
- 16.Damianovich M, Gilburd B, George J, et al. Pathogenic role of anti-endothelial cell antibodies in vasculitis. An idiotypic experimental model. J Immunol. 1996;156:4946–51. [PubMed] [Google Scholar]
- 17.Ross R. The pathogenesis of atherosclerosis—a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Eng J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 19.Salonen JT, Yla-Herttuala S, Yamamoto R, et al. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 20.Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341:923–5. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 21.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85:2805–9. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Subbaiah PV. Hydrolysis and transesterification of platelet-activating factor by lecithin-cholesterol acyltransferase. Proc Natl Acad Sci USA. 1994;91:6035–9. doi: 10.1073/pnas.91.13.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu R, Huang Y, Schäfer-Elinder L, Frostegård J. Lysophosphatidylcholine is involved in the antigenicity of oxLDL. Arterioscler Thromb Vasc Biol. 1998;18:626–30. doi: 10.1161/01.atv.18.4.626. [DOI] [PubMed] [Google Scholar]
- 24.Schäfer-Elinder L, Hedin U, Dumitrescu A, Larsson P, Frostegård J, Claesson HE. Presence of different isoforms of phospholipase A2 in tissue slices from atherosclerotic carotid plaque. Arterioscler Thromb Vasc Biol. 1997;17:2257–62. doi: 10.1161/01.atv.17.10.2257. [DOI] [PubMed] [Google Scholar]
- 25.Dennis EA. Diversity of group types, regulation and function of phospholpiase A2. J Biol Chem. 1994;269:13057–60. [PubMed] [Google Scholar]
- 26.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 27.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry O, Rosebrough NJ, Farr AL, Randall AJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 29.Wu R, Lefvert AK. Autoantibodies against oxidized low density lipoprotein: characterization of antibody isotype, subclass, affinity and effect on the macrophage uptake of oxLDL. Clin Exp Immunol. 1995;102:174–80. doi: 10.1111/j.1365-2249.1995.tb06652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haegerstrand A, Gillis C, Bengtsson L. Serial cultivation of adult human endothelium from the great saphenous vein. J Vasc Surg. 1992;16:280–5. [PubMed] [Google Scholar]
- 31.Quadros NP, Roberts-Thomson PJ, Gallus AS. IgG and IgM anti-endothelial cell antibodies in patients with collagen vascular disorders. Rheumatol Int. 1990;10:113–9. doi: 10.1007/BF02274825. [DOI] [PubMed] [Google Scholar]
- 32.Harris EN, Gharavi AE, Patel SP, Hughes GR. Evaluation of the anti-cardiolipin antibody test: report of an international workshop held 4 April 1986. Clin Exp Immunol. 1986;68:215–22. [PMC free article] [PubMed] [Google Scholar]
- 33.Frostegård J, Haegerstrand A, Gidlund M, Nilsson J. Biologically modified low density lipoprotein increases the adhesive properties of vascular endothelial cells. Atherosclerosis. 1991;90:119–26. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- 34.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–6. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frostegård J, Wu R, Haegerstrand A, Patarroyo M, Lefvert AK, Nilsson J. Mononuclear leucocytes exposed to oxidized low density lipoprotein secrete a factor that stimulates endothelial expression of adhesion molecules. Atherosclerosis. 1993;103:213–9. doi: 10.1016/0021-9150(93)90264-u. [DOI] [PubMed] [Google Scholar]
- 36.Frostegård J, Nilsson J, Haegerstrand A, Hamsten A, Wigzell H, Gidlund M. Oxidized low-density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:904–8. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frostegård J, Wu R, Giscombe R, Holm G, Lefvert AK, Nilsson J. Induction of T cell activation by oxidized low density lipoprotein. Arterioscler Thromb. 1992;12:461–7. doi: 10.1161/01.atv.12.4.461. [DOI] [PubMed] [Google Scholar]
- 39.Huang YH, Rönnelid J, Frostegård J. Oxidized LDL induces enhanced antibody formation and MHC class II-dependent IFN-gamma production in lymphocytes from healthy individuals. Arterioscler Thromb Vasc Biol. 1995;15:1577–83. doi: 10.1161/01.atv.15.10.1577. [DOI] [PubMed] [Google Scholar]
- 40.Frostegård J, Huang Y, Rönnelid J, Schäfer-Elinder L. PAF and oxidized LDL induce immune activation by a common mechanism. Arterioscler Thromb Vasc Biol. 1997;17:963–8. doi: 10.1161/01.atv.17.5.963. [DOI] [PubMed] [Google Scholar]
- 41.Del Papa N, Guidali L, Spatola L, Bonara B, Borghi MO, Tincani A, Balestriera G, Meroni PL. Relationship between anti-phospholipid and anti-endothelial cell antibodies III. beta 2 glycoprotein I mediates the antibody binding to endothelial membranes and induces the expression of adhesion molecules. Clin Exp Rheumatol. 1995;13:179–85. [PubMed] [Google Scholar]
- 42.Pruzanski W, Goulding N, Flower RJ, Gladman DD, Urowitz MB, Goodman PJ, Scott KF, Vadas P. Circulating Group II phospholipase A2 activity and antilipocortin antibodies in systemic lupus erythematosus. Correlative study with disease activity. J Rheumatol. 1994;21:252–7. [PubMed] [Google Scholar]
- 43.Pruzanski W, Keystone EC, Sternby B, Bombardier C, Snow KM, Vadas P. Serum phospholipase A2 correlates with disease activity in rheumatoid arthritis. J Rheumatol. 1988;15:1351–5. [PubMed] [Google Scholar]
- 44.Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Brit J Rheumatol. 1996;35:1067–74. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]