Abstract
Dendritic cells (DC) are professional antigen-presenting cells, capable of priming naive T cell responses. Glucocorticoids (GC) are frequently used in asthmatic patients. In this study we describe the effects of GC on the development and function of monocyte-derived DC (MoDC) in vitro and in vivo. Monocytes from healthy individuals were isolated and incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 for 6 days, to induce maturation into MoDC. To study the role of GC on DC differentiation in vitro cells were incubated with dexamethasone at different stages of MoDC development. At day 6 cells were characterized phenotypically by flow cytometry and functionally in an allogeneic mixed leucocyte reaction. To study the effect of GC in vivo patients with mild/moderate atopic asthma were selected. In one group no GC were used, whereas the other group used inhalation GC. MoDC from these patients were generated as described above and tested functionally. Incubation of MoDC or its peripheral blood precursors with dexamethasone decreased the accessory potency dose-dependently. The functional differences could not be explained by the changes in the expression of MHC II and the costimulatory molecules CD40 and CD86. The relevance of this mechanism was confirmed for the in vivo situation as well. MoDC from patients using inhalation GC showed a decreased accessory potency. These data suggest a modulatory effect of GC therapy at the level of the peripheral blood monocyte. The results indicate that GC influence DC development and function in vitro as well as in vivo.
Keywords: dendritic cell, asthma, glucocorticoids, human, mixed leucocyte reaction
INTRODUCTION
Dendritic cells (DC) are very potent antigen-presenting cells (APC). In addition to their unique potency in antigen presentation to naive T cells, functionally mature DC are also most effective in reactivating memory T cells in comparison with monocytes and B cells [1]. Monocytes can develop into immature DC in the presence of different cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 [2]. These monocyte-derived DC (MoDC) express high levels of CD1a and MHC II. MoDC will further mature in the presence of IL-1, CD40L, tumour necrosis factor-alpha (TNF-α) or lipopolysaccharide (LPS). Mature DC show an increased accessory potency, whereas their antigen-processing capacity decreases 10–100-fold [3]. Mature DC can be recognized by the expression of CD83, and the costimulatory molecules CD40 and CD86 are markedly up-regulated [4].
In asthma, the number of DC is increased in the airway epithelium, suggesting an important role in the pathophysiological mechanism of airway inflammation [5,6]. Recently, we showed also qualitative differences between DC from asthmatics and healthy controls. The differences could already be found at the level of blood DC precursors. MoDC from asthmatics not using glucocorticoid (GC) inhalation therapy showed an increased accessory potency compared with healthy non-allergic volunteers [7]. This increased accessory function in addition to the increased numbers might induce an exaggerated immune response in reaction to air-borne antigens, thereby possibly initiating and/or sustaining the chronic inflammation.
GC form the mainstay of therapy in asthma and induce a marked reduction in the number of macrophages, mast cells, T lymphocytes, and eosinophils in the bronchial epithelium and submucosa [8]. Besides, it has been shown that GC therapy leads to a rapid decrease in the number of DC in the bronchial epithelium of asthmatics [9,10]. The decrease has been explained by influences on the chemo-attraction of the DC or its precursors [11]. However, it can also be hypothesized that GC modulate the DC development and maturation from their blood precursors, thereby reducing the numbers and potency of DC in the lung.
In this study we examine the influence of GC on DC development and maturation in vitro and investigate whether GC inhalation therapy could affect the DC functionally by an influence at the level of their blood precursors.
PATIENTS AND METHODS
Patients
Patients were selected on the basis of their asthmatic and allergic history. Allergy was confirmed by skin test and radioallergosorbent test (RAST). Bronchial hyperreactivity was evaluated by means of the histamine threshold. The relevant data of selected patients are shown in Table 1. For the experiments on the in vitro influence of dexamethasone (DEX) on MoDC development and maturation, blood from healthy volunteers was used.
Table 1.
Patient characteristics
Cells
Preparation of T cells
Mononuclear cells were isolated from a buffy coat with a Lymphoprep gradient (Nycomed, Oslo, Norway). T lymphocytes were isolated by rosetting to sheep erythrocytes (Biotrading, Mijdrecht The Netherlands). Buffy coat T cells were further purified by an adherence step of 2 h on Petri dishes in RPMI 1640 supplemented with 2 mml-glutamine, 10% heat-inactivated fetal calf serum (FCS; Gibco, Bio-Cult, Irvine, UK), 50 mm mercaptoethanol, 50 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml gentamycin. Non-adherent cells were layered on hypertonic 12.5% metrizamide, dissolved in medium (Nycomed) and sedimented at 600 g for 10 min at room temperature. T cells were recovered from the pellet and washed twice, aliquoted and stored in liquid nitrogen until further use. FACS analyses showed a purity of > 95%.
Differentiation of MoDC
In all experiments monocytes were isolated from the mononuclear fraction of a Lymphoprep gradient by adherence of the non-rosetting (T cell-depleted) cell fraction for 2 h on Petri dishes (Becton Dickinson, Franklin Lakes, NY). Non-adherent cells were removed by gently flushing with medium. The monocytes were cultured in RPMI/10% FCS (as above) containing 1000 U/ml rGM-CSF (Schering-Plough, Madison, NY) and 500 U/ml rIL-4 (Genzyme, lot no. B5665; Cambridge, MA). On day 6 the cells had become non-adherent and were collected. Viability was always > 95%. The morphology of the cells was determined using May–Grünwald–Giemsa staining. These MoDC were used for functional assays as well as for phenotypic analysis.
In the experiments on the in vitro influence of the synthetic glucocorticoid DEX on DC development monocytes from healthy controls were used. For the experiments on the influence of inhalation GC on MoDC function monocytes were isolated from 30 ml heparinized blood (as described above).
Cell adherence on plastic
To determine the influence of DEX on the adherence of monocytes, non-rosetting (T cell-depleted) cells were plated on 96-well flat-bottomed tissue culture plates (0.2 ml of medium/well) in the presence of graded doses of DEX. After 2 h the non-adherent cells were removed and the adherent cells were cultured for another 3–24 h. Adherent cells were cultured in RPMI/FCS with GM-CSF and IL-4. Cells were then fixed with formaldehyde, plates were rinsed thoroughly with PBS, and a 0.5% crystal violet solution in 25% methanol in water was added. After 15 min plates were rinsed again with water. The protein-bound crystal violet was then dissolved in 25% methanol in water and optical density (OD) was measured at 550 nm, to quantify the adherent cells.
Cell loss due to GC-induced apoptosis
Regulated cell death was measured with the propidium iodide (PI) staining method [12]. In short, cells were incubated with graded doses of DEX during the adherence step (as mentioned above). After culture for 3 h and 24 h, in the presence of GM-CSF and IL-4, the cells were harvested, washed in PBS with 0.1% glucose, spun down, and the supernatant was discarded. Cells were then resuspended, cold ethanol (70%, 4°C) was added slowly, and the cell suspension was stored overnight at 4°C. Cells were incubated 30 min in a PI solution (5 mg/ml PI, 1000 U RNase A, 0.1% glucose in PBS) at room temperature. Based on PI contents cells can be divided into G1 and G2 cells (undergoing S phase) by FACS analysis. Cells undergoing apoptosis show reduced fluorescence due to fragmented DNA resulting in impaired PI uptake. Apoptotic cells were quantified as percentage of total cells in the samples.
Mixed leucocyte reaction assay
In all allogeneic mixed leucocyte reaction (MLR) assays buffy coat T cells from the same donor were used. On the day of use T cells were thawed. Viability was always > 90%. Stimulator cells were irradiated (30 Gy) and added in graded doses to 1.0 × 105 T lymphocytes in 96-well round-bottomed tissue culture plates (0.2 ml of medium per well). T cell proliferation was quantified by incubating with 1 μCi (37 kBq) of 3H-thymidine deoxyriboside (Amersham Life Sciences, Aylesbury, UK) during the last 6 h of the 4-day cultures. Cells were harvested on a filter and radioactivity was measured in a liquid scintillation counter.
Flow cytometry
Cells were resuspended in PBS with 0.1% bovine serum albumin (BSA) and incubated with a panel of mouse anti-human MoAbs for 45 min at 4°C. Besides the panel of antibodies, isotype controls were used for each patient. Cells were washed twice and incubated for 45 min with FITC-labelled F(ab′)2 fragments of rabbit anti-mouse antibody (Dako, Glostrup, Denmark) at 4°C. Cells were washed and the fluorescence was measured on a FACScan flow cytometer (Becton Dickinson, San José, CA) The minimal positivity was used to determine the percentage of positive cells [13]. The intensity was calculated by dividing the mean fluorescence of the marker by the mean fluorescence of its isotype control on the same cells.
RESULTS
In vitro experiments with DEX
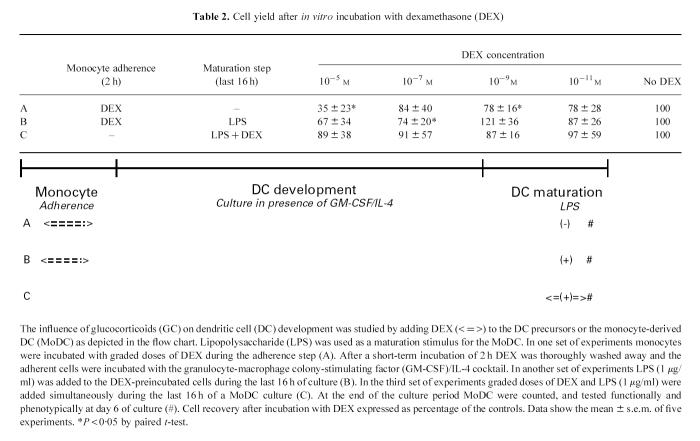
In order to mimic the influence of GC on DC development in vivo, DEX was added to cell cultures either during the first 2 h adherence step or during the final 16 h maturation step at the end of the 6-day differentiation (Table 2). First, to study the influence of GC on DC development from its peripheral blood monocytes, DEX was added to the mononuclear cells during the adherence step (during the first 2 h of culture) and then washed away. The preincubated DC precursors were then cultured in the presence of GM-CSF and IL-4 and the development of immature MoDC was evaluated phenotypically and functionally at day 6 (A). Subsequently the influence of this preincubation on the last stage of DC development, the maturation, was investigated. This was done by adding LPS to cultures of these DEX preincubated cells during the last 16 h of the 6-day culture (B). Finally, the influence of GC on the final maturation of the MoDC was investigated by adding both LPS and DEX simultaneously during the last 16 h of culture (C).
Table 2.
Cell yield after in vitro incubation with dexamethasone (DEX)
GC incubation influenced the number of recovered cells, as is shown in Table 2. Especially the highest concentration of 10−5m reduced the number of cells by 20–60% compared with the experiments in which no GC was added. Also, lower doses influenced cell recovery, but an important experimental variation existed. The viability of the adherent cells was always > 90% and was not related to DEX concentration.
DEX did not influence the adherence, as was determined by measurement of the total number of adherent cells by total protein amount (Fig. 1). Another reason for the decreased recovery could be the enhanced induction of apoptosis. Indeed, a short incubation of DC precursors with DEX induced apoptosis, which was dose-dependent and increased with time and the concentration of DEX (Fig. 2).
Fig. 1.

The influence of dexamethasone (DEX) on the adherence of dendritic cell precursors (monocytes). T cell-depleted mononuclear cells were incubated with graded doses of DEX during the 2 h of adherence to plastic. The non-adherent cells were then washed away and the adherent cells were cultured for 0, 3 or 24 h in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF)/IL-4. The protein content of the adherent fraction, which is a measure of the number of adherent cells, was determined by crystal violet staining and read out at 540 nm. Cell numbers were varied as additional control for the experimental condition. Shown are the results of a representative donor.
Fig. 2.
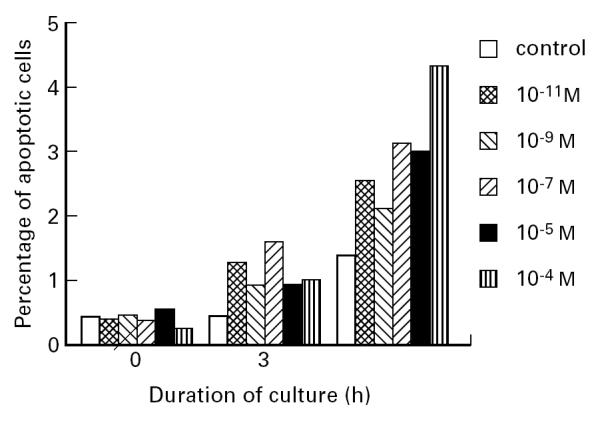
Dexamethasone (DEX)-induced apoptosis of dendritic cell precursors (monocytes). T cell-depleted mononuclear cells were incubated with graded doses of DEX during the 2 h of adherence to plastic. The non-adherent cells were then washed away and the adherent cells were cultured for 0, 3 or 24 h in the presence of IL-4/granulocyte-macrophage colony-stimulating factor (GM-CSF). The percentage of apoptotic cells was measured using the propidium iodide (PI) method. Shown are the results of a representative donor. Each bar represents a percentage based on a sample of 10 000 total cells measured.
Phenotypic studies
To study the influence of GC on the development and maturation of MoDC, FACS analysis was performed using MoAbs to MHC class II molecules, costimulatory molecules (CD40, CD86), and maturation markers (CD1a, CD83). Incubation with the cytokine cocktail GM-CSF and IL-4 induced expression of CD1a and CD40 whereas expression of CD83 was nearly absent. LPS induced the expression of CD83, and costimulatory molecules CD40 and CD86 were further up-regulated (data not shown) [14].
DEX was added at the different time points before culture and during the final maturation of DC. The in vitro results showed no statistically significant influence of GC on the expression of the tested markers (Table 3,Fig. 3), but the effects varied markedly between separate experiments. In some experiments DEX had a clear influence on the expression of maturation markers CD1a, CD83 and molecules relevant for the antigen presentation (CD40, CD86 and MHC II), whereas in other experiments it did not.
Table 3.
FACS analysis of monocyte-derived dendritic cells (MoDC) after incubation with graded doses of dexamethasone (DEX)
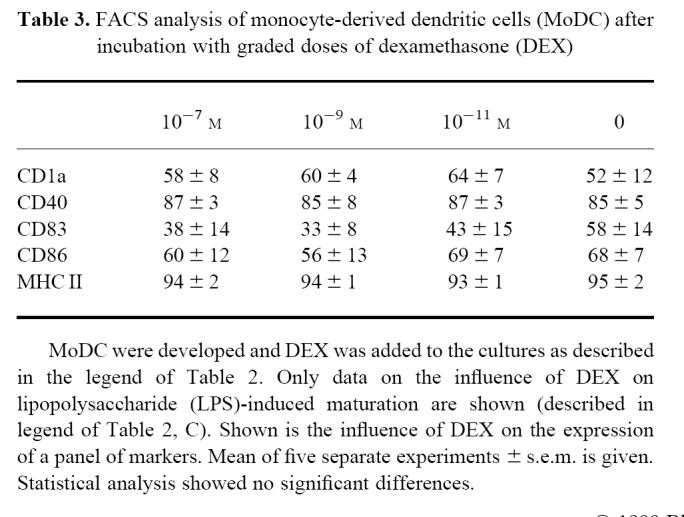
Fig. 3.
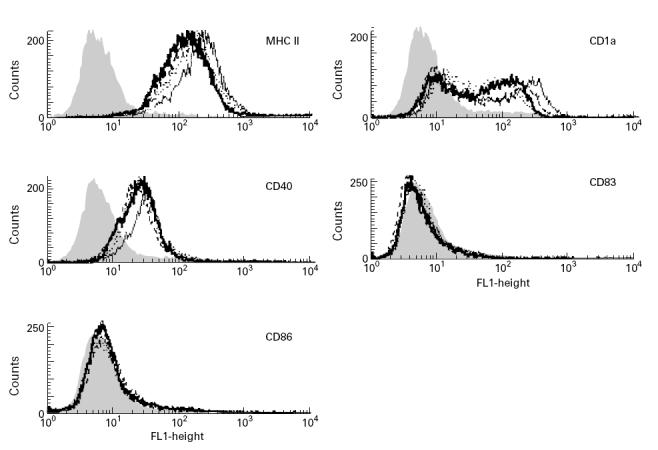
Influence of dexamethasone (DEX) on the phenotype of monocyte-derived dendritic cell (MoDC). Monocytes were cultured for 6 days in the presence of IL-4/granulocyte-macrophage colony-stimulating factor (GM-CSF) to develop into MoDC and DEX was added during the last 16 h of culture (see also legend of Table 2, A). The expression was analysed using FACS analysis. Data from one of five experiments are shown; isotype control (grey filled curve), MoDC without (dotted line), DEX 10−7m (solid thick line), DEX 10−9m (dashed line), DEX 10−11m (solid thin line).
Functional activity of MoDC
In contrast to the phenotypic studies no donor variation was seen in the functional studies of MoDC incubated with DEX, as is shown in Fig. 4. The accessory potency was influenced in a dose-dependent way. The short incubation of the DC precursors as well as the incubation during the last 16 h of the MoDC culture decreased the accessory potency. The highest concentration of DEX (10−5m) inhibited the accessory capacity by 20–72%, but the lowest concentrations also showed a moderate effect.
Fig. 4.

The influence of in vitro dexamethasone (DEX) on the accessory potency of monocyte-derived dendritic cells (MoDC). MoDC were developed and DEX was added to the cultures as described in the legend of Table 2 (A = preincubation with DEX during adherence, B = preincubation with DEX during adherence and lipopolysaccharide (LPS) on day 5, C = incubation with DEX and LPS on day 5). The legend shows the different concentrations of DEX. The accessory potency of MoDC was measured in a 4-day mixed leucocyte reaction, tritium thymidine was added during the last 6 h of culture. The MoDC/T cell ratio was 1/10. Mean of three separate experiments ± s.e.m. is given. *P < 0.05; **P < 0.01 (paired t-test).
The influence of inhalation GC on the function of MoDC from asthmatics
To investigate whether these in vitro results could be related to effects of in vivo administration of GC, we cultured MoDC from asthmatic patients that were on GC inhalation therapy and compared the results with a group of asthmatic patients not using GC therapy. The number of recovered MoDC was similar in groups with and without GC inhalation treatment (2.1 ± 1.4 × 106 and 3.3 ± 1.9 × 106, respectively) and also no morphological differences were detected (data not shown). However, MoDC from asthmatics using GC inhalation therapy showed a decreased accessory potency in the allogeneic MLR, as is shown in Fig. 5.
Fig. 5.

The influence of inhalation glucocorticoid (GC) in vivo on the accessory capacity of monocyte-derived dendritic cells (MoDC) from asthmatics (n = 4 in both patient groups). Peripheral blood monocytes were isolated from asthmatic patients and cultured in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). At day 6 the MoDC were harvested. The accessory potency of MoDC was measured in a 4-day mixed leucocyte reaction, tritium thymidine was added during the last 6 h of culture. Mean ± s.e.m. is given. Statistical analysis by paired t-test. □, No GC; ▪, inhalation GC.
DISCUSSION
Most studies on the influence of GC on myeloid cells have been performed using macrophages. A role for GC was shown in the regulation of cytokine production as well as in the expression of surface molecules involved in antigen uptake and interactions with T cells [15–22]. Recently, effects of GC on DC have also been shown. GC negatively influence antigen presentation function, cytokine production, and inhibit the maturation of a mouse DC cell line [23,24]. Moreover, all in vivo studies on inflammation show increased numbers of macrophages, whereas the numbers of DC decrease after GC exposure [25,26]. These observations suggest that GC skew the macrophage/DC balance towards the macrophage side. In the light of the recently accepted idea that macrophages and DC have a common precursor, it can be hypothesized that GC influence the precursor at an early stage of differentiation, and thus direct the development more towards the macrophage lineage. The findings presented in this study that GC inhibit the accessory function of MoDC both quantitatively and qualitatively fit into this model.
The mechanism by which GC inhalation therapy influences DC blood precursors in vivo is currently unknown. Our data show that GC influence MoDC in two different ways. First, GC can impair cell survival, thereby decreasing the total number of DC. For the low concentrations (10−9m or less) the situation is less clear. Although the cell yield was less, no dose dependency could be shown. The decreased cell recovery can partly be explained by the shown induction of apoptosis. When GC are applied locally, relatively high concentrations might be achieved that can explain part of the observed reduction in the number of DC in the airway mucosa of allergic asthmatics after GC inhalation therapy [10]. Second, GC influence the function of MoDC that have been incubated at different time points during maturation. The mechanism behind the decreased accessory function as shown in our system is not clear yet. Moser showed in an animal model, in vivo as well as in vitro, that high concentrations of DEX have a marked effect on the expression of costimulatory molecules [24]. This correlated with a decreased accessory potency. In our model the decreased accessory function could not be explained by the expression of costimulatory and MHC class II molecules. Donor variability in our model might explain the different results, although differences in GC concentration may play a role as well.
Our study shows that low dose GC inhalation therapy indeed influences the circulating DC precursors. Inhalation therapy is known to have systemic effects too (e.g. suppression of the hypothalamic–pituitary–adrenal axis function and bone metabolism) [8]. However, most studies on low dose inhalation therapy do not show any influence. It can be speculated that inhaled GC, that becomes available systemically, affects the DC precursor directly. Such a systemic effect can be assumed, as 80–90% of the inhaled dose is deposited in the oropharynx and swallowed [8]. Besides, GC have been shown to function at very low concentrations (10−16m) [18]. Our DEX results show a decrease in the accessory function at concentrations as low as 10−11m.
An alternative mechanism for the systemic influence of inhalation GC might be that it influences cells in the periphery indirectly, by the modulation of locally produced factors that are secreted into the blood. The relevance of this mechanism has been shown in a canine model of an allergen-induced airway inflammation [27].
In conclusion, several cells have been described to be important in the pathogenesis of asthma. GC influences many of them. Our in vitro studies shows that the DC is also one of the target cells. GC is capable of influencing the development and function of DC. This modulatory effect, also shown in vivo, is likely to be of importance in the therapeutic action of inhalation GC during allergic asthma.
Acknowledgments
This study was supported by the Dutch Asthma Foundation (NAF 32.94.37).
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Peters JH, Ruhl S, Friedrichs D. Veiled accessory cells deduced from monocytes. Immunobiology. 1987;176:154–66. doi: 10.1016/s0171-2985(87)80107-9. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from peripheral blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 5.Bellini A, Vittori E, Marini M, Ackerman V, Mattoli S. Intraepithelial D.C. and the selective activation of Th2-like lymphocytes in patients with atopic asthma. Chest. 1993;102:997–1005. doi: 10.1378/chest.103.4.997. [DOI] [PubMed] [Google Scholar]
- 6.Poulter LW, Burke C. Macrophages and allergic lung disease. Immunobiology. 1996;195:574–87. doi: 10.1016/S0171-2985(96)80023-4. [DOI] [PubMed] [Google Scholar]
- 7.Van den Heuvel MM, Vanhee DDC, Postmus PE, Hoefsmit ECM, Beelen RHJ. Functional and phenotypical differences between monocyte derived dendritic cells (MoDC) from allergic and non-allergic patients. J Allergy Clin Immunol. 1998;101:90–95. doi: 10.1016/S0091-6749(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med. 1995;332:868–75. doi: 10.1056/NEJM199503303321307. [DOI] [PubMed] [Google Scholar]
- 9.Nelson DJ, McWilliam AS, Haining S, Holt PG. Modulation of airway intraepithelial dendritic cells following exposure to steroids. Am J Respir Crit Care Med. 1995;151:475–81. doi: 10.1164/ajrccm.151.2.7842209. [DOI] [PubMed] [Google Scholar]
- 10.Möller GM, Overbeek SE, Van-Helden-Meeuwsen CG, Van Haarst JMW, Prens EP, Mulder PG, Postma DS, Hoogsteden HC. Increased numbers of dendritic cells in the bronchial mucosa of atopic asthmatic patients: downregulation by inhaled corticosteroids. Clin Exp Allergy. 1996;26:517–24. [PubMed] [Google Scholar]
- 11.McWilliam AS, Napoli N, Marsh AM, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–32. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JJ. Apoptosis: physiologic cell death. J Lab Clin Med. 1994;124:761–5. [PubMed] [Google Scholar]
- 13.13 Coligan J, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. New York: John Wiley & Sons, Inc; 1995. Analysis of flow cytometric data; p. 5.2. [Google Scholar]
- 14.Hart DNJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 15.Hoff T, Spencker T, Emmendoerffer A, Goppelt-Struebe M. Effects of glucocorticoids on the TPH-induced monocytic differentiation. J Leukoc Biol. 1992;52:173–82. doi: 10.1002/jlb.52.2.173. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton J. Coordinate and noncoordinate colony stimulating factor formation by human monocytes. J Leukoc Biol. 1994;55:355–61. doi: 10.1002/jlb.55.3.355. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg JB, Wortham TS, Misukonis MA, Patton KL, Chitneni SR. Synovial mononuclear phagocytes in rheumatoid arthritis and osteoarthritis: quantitative and functional aspects. Immunol Invest. 1993;22:365–74. doi: 10.3109/08820139309063415. [DOI] [PubMed] [Google Scholar]
- 18.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;376:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 19.Cowan HB, Vick S, Conary JT, Shepherd VL. Dexamethasone up-regulates mannose receptor activity by increasing mRNA levels. Arch Biochem Biophys. 1992;296:314–20. doi: 10.1016/0003-9861(92)90578-k. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berg T, Van Die I, Renardel de Lavalette C, et al. Regulation of sialoadhesin expression on rat macrophages. J Immunol. 1996;157:3130–8. [PubMed] [Google Scholar]
- 21.Masferrer JL, Seibert K, Zweifel B, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci USA. 1992;89:3917–21. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo-Marie F. Mcrophages and the glucocorticoids. J Neuroimmunol. 1992;40:281–6. doi: 10.1016/0165-5728(92)90144-a. [DOI] [PubMed] [Google Scholar]
- 23.Kitayima T, Ariizumi K, Bergstresser PR, Takashima A. A novel mechanism of glucocorticoid-induced immune suppression: the inhibition of T cell-mediated terminal maturation of a murine dendritic cell line. J Clin Invest. 1996;98:142–7. doi: 10.1172/JCI118759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser M, De Smedt T, Sornasse T, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–24. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 25.Leszczynski D, Ferry B, Schellekens, van der Meide PH, Häyra P. Antagonistic effects of γ interferon and steroids on tissue antigenicity. J Exp Med. 1986;164:1470–7. doi: 10.1084/jem.164.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kizaki T, Oh-Ishi S, Ookawara T, Yamamoto M, Izawa T, Ohno H. Glucocorticoid-mediated generation of suppressor macrophages with high density FcγRII during acute cold stress. Endocrinology. 1996;137:4260–7. doi: 10.1210/endo.137.10.8828485. [DOI] [PubMed] [Google Scholar]
- 27.Inman MD, Denburg JA, Ellis R, Dahlback M, O'Byrne PM. Allergen-induced increase in the bone marrow progenitors in the airway hyperresponsive dogs—regulation by a serum hemopoietic factor. Am J Respir Cell Mol Biol. 1996;15:305–11. doi: 10.1165/ajrcmb.15.3.8924277. [DOI] [PubMed] [Google Scholar]