Abstract
Vitamin B2 and flavin cofactors are transported tightly bound to immunoglobulin in human serum. We reasoned that anti-mitochondrial flavoprotein autoantibodies (αFp-AB) present in the serum of patients with myocarditis and cardiomyopathy of unknown aetiology may form immunoglobulin aggregates with these serum proteins. However, immunodiffusion and Western blot assays demonstrated that the flavin-carrying proteins were not recognized by αFp-AB. Apparently the flavin moiety in the native protein conformation was inaccessible to αFp-AB. This conclusion was supported by the absence of an immunoreaction between the riboflavin-binding protein from egg white and αFP-AB. Intravenous application of vitamin B2 to rabbits immunized with 6-hydroxy-d-nicotine oxidase, a bacterial protein carrying covalently attached FAD, did not neutralize αFp-AB which had been raised in the serum of the animals. FAD-carrying peptides generated from 6-hydroxy-d-nicotine oxidase by trypsin and chymotrypsin treatment were not recognized by the αFp-AB, but those generated by endopeptidase Lys were. This demonstrates that the epitope recognized by αFp-AB comprises, besides the flavin moiety, protein secondary structure elements.
Keywords: anti-flavoprotein autoantibodies, vitamin B2, myocarditis, dilated cardiomyopathy
INTRODUCTION
Patients with myocarditis of unknown aetiology and with dilated cardiomyopathy show a high incidence of serum autoantibodies (M7) [1] directed against mitochondrial flavoproteins (αFp-AB) [2]. In vitro these antibodies bind flavins (riboflavin, FMN or FAD) in the nanomolar range, with an apparent kD of 10 nmol. Transport of flavins in the blood takes place predominantly in a protein-bound form, and, besides albumin [3], immunoglobulins represent the predominant class of flavin-binding proteins in the serum [4]. Riboflavin and FAD are the major flavin forms detected in serum, with FMN occurring only in trace amounts. Seventy-two percent of flavins are precipitated with plasma globulins [3], and immunoglobulin subclasses IgG, IgM and IgA have been isolated from serum of normal humans by flavinyl affinity chromatography. The main flavin-binding IgG subclass is IgG2 with an apparent kD for riboflavin of 0.23 μm [5]. Fab fragments generated by papain digestion of the immunoglobulins also bind riboflavin, indicating that part of the antigenic binding site may be involved [5]. Blood cells contain several times more flavin than serum [4] due to the presence of flavoenzymes in reticulocytes and leucocytes.
As shown previously [2], it is possible to isolate αFp-AB from the serum of patients with myocarditis and dilated cardiomyopathy using affinity chromatography on immobilized FAD-enzyme. No such fraction was obtained from the sera of control individuals. Thus the αFp-AB fraction was not identical to the flavin-binding fraction of immunoglobulins. Its occurrence must reflect the development of an immune response in the patients. In this study we analysed the interaction of αFp-AB with flavin-carrying proteins and investigated the possibility of neutralizing these antibodies by i.v. administration of vitamin B2. Epitope mapping results and the cellular site of αFp-AB-binding determined on both neonatal rat cardiomyocytes and histological section of human heart are discussed.
PATIENTS AND METHODS
Patients
Patients selected for this study showed high titres of αFp-AB. They presented dilated hearts with systolic dysfunction and unexplained heart failure of variable duration in the absence of coronary artery or valvular heart disease as documented by heart catheterization, echocardiography, myocardial scintigraphy, and coronarography. Sera from healthy individuals were included in the study as controls.
Chemicals
Immunochemicals, sarcosine oxidase, riboflavin binding protein from egg white and protein weight markers were obtained from Sigma (Deisenhofen, Germany), 5-bromo-4-chloro-3-indolyl-phosphate, nitrotetrazolium and o-phenylenediamine (OPD) were purchased from Boehringer-Mannheim (Mannheim, Germany), vitamin B2 (10-mg inject) was from Jenapharm (Jena, Germany).
Antiserum
Polyclonal antisera against sarcosine oxidase (SaO) from Sigma and against 6-hydroxy-d-nicotine oxidase (6HDNO) were raised in our laboratory in rabbits according to standard protocols [6]. The specificity of the antisera was tested on Western blots with 6HDNO, SaO and mitochondrial membrane and matrix fraction.
Affinity purification of antibodies
Affinity purification of the αFp-AB fraction of human sera was performed with the aid of 6HDNO immobilized to nitrocellulose membrane as described [7]. 6HDNO was prepared as described [8].
Western blotting
Purified proteins (1 μg) and digested peptides (100 μg) were resolved by SDS–PAGE and transferred onto nitrocellulose (Optipran BA-S85; Schleicher & Schuell, Dassel, Germany) using a semidry horizontal apparatus (BioRad, München, Germany). Individual lanes of the membrane were incubated with affinity-purified αFp-AB, with human sera at a dilution of 1:300 and with rabbit sera at a dilution of 1:1000. Second antibodies conjugated to alkaline phosphatase were used at a dilution of 1:3500 and 5-bromo-4-chloro-3-indolyl-phosphate/nitrotetrazolium blue as substrate.
ELISA
An ELISA was performed according to standard methods [9]. Maxisorb microtitre plates (Nunc, Roskilde, Denmark) were coated with 500 ng purified 6HDNO per well. Sera were employed in dilutions of 1:500. Bound antibodies were visualized using peroxidase-conjugated goat anti-rabbit IgG antibodies at a dilution of 1:3500 and OPD as substrate.
Immunoelectrophoresis and immunodiffusion assay
Immunoelectrophoresis and immunodiffusion assays were performed according to [10].
High performance liquid chromatography analysis of serum flavins
Human and rabbit sera were separated by centrifugation at 5000 g through a Centricon 10 (Amicon Inc., Beverly, MA) into a protein-free filtrate and a serum protein fraction. The flavin content in the fractions was determined according to [11].
Protein digestion
6HDNO (100 μg) was digested with trypsin, chymotrypsin and endopeptidase Lys for 4 h at room temperature and then subjected to SDS–PAGE and to Western blotting.
Immunofluorescence microscopy
Frozen sections of human, left-ventricular heart tissue and primary cultures of neonatal rat cardiomyocytes [12] were treated with purified αFp-AB from patients, followed by incubation with FITC-labelled rabbit anti-human antibodies and microscopic analysis.
RESULTS
Interaction of αFp-AB with flavin-carrying proteins
The presence of αFp-AB in the serum of patients with heart disease raises the possibility of an immunological reaction between the flavin-carrying proteins and these antibodies. The subsequent formation of immune complexes in the serum could be detrimental to the patient's health.
We tested the ability of the affinity-purified αFp-AB from patients' serum to interact with flavin-carrying serum proteins using immunoelectrophoresis. As shown in Fig. 1, there was no formation of precipitation lines with the αFp-AB. The control with anti-human immunoglobulins, however, gave the expected precipitation lines (Fig. 1). The absence of an immunoreaction between αFp-AB and flavin-carrying serum proteins could reflect the fact that the hydrophobic riboflavin moiety was hidden inside the carrier protein, as it is in the cofactor-binding pocket of flavoenzymes [2], and was therefore inaccessible to the antibodies.
Fig. 1.
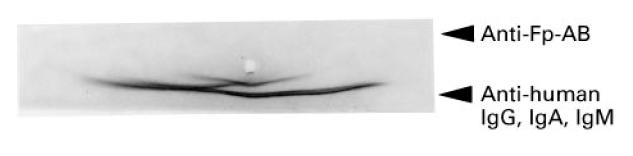
Interaction of αFp-AB with serum proteins. Serum proteins were separated electrophoretically under native conditions on agarose; affinity-purified human αFp-AB and as control anti-human IgG, IgA, IgM were then applied to the agarose as indicated and the formation of precipitation lines was monitored.
To test this assumption we chose riboflavin binding protein (RBP) from egg white as a model for a flavin-carrying protein. Western blots with RBP revealed no immunoreaction with αFp-AB-containing serum from patients or with serum from rabbits immunized with 6HDNO. This bacterial enzyme contains covalently linked FAD and when injected into rabbits gives rise to αFp-AB with the same characteristics as those present in the serum of patients with heart disease [2] (Fig. 2a). The absence of an immune reaction indicated that RBP had lost the bound flavin during electrophoresis under denaturing conditions. To test the ability of the αFp-AB to interact with native RBP, an immunodiffusion assay was performed (Fig. 2b). Both αFp-AB-containing sera gave negative results. Apparently the bound riboflavin was shielded in the protein and was inaccessible to the antibodies.
Fig. 2.
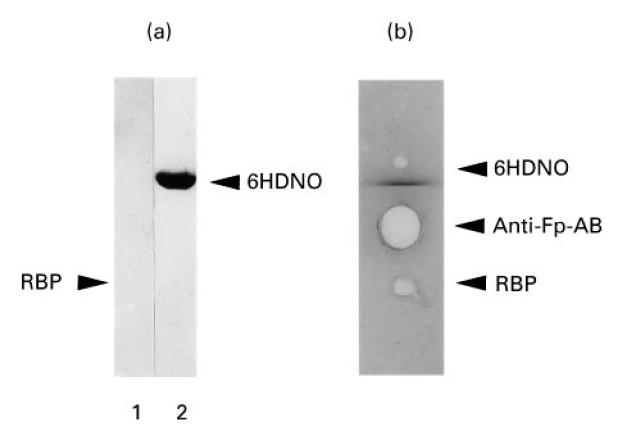
Immunoreaction of flavin-carrying proteins with αFp-AB. (a) Riboflavin binding protein (RBP) (lane 1) and 6-hydroxy-d-nicotine oxidase (6HDNO) (lane 2) were analysed on Western blots decorated with αFp-AB+ human serum. The position of RBP on the Western blot is indicated by an arrow. (b) The same proteins were tested under native conditions by an immunodiffusion assay on 1% agarose. The affinity-purified αFp-AB fraction of human serum was used for precipitation.
Effect of i.v. vitamin B2 applications on the reactivity of αFP-AB in rabbit serum
αFp-AB can be neutralized in vitro by the addition of riboflavin to the antiserum [2]. We asked whether the i.v. application of vitamin B2 has the same effect. A rabbit with serum containing αFP-AB elicited by immunization with bacterial sarcosine oxidase, an enzyme with covalently attached FAD, was injected on 3 consecutive days with 5 mg vitamin B2 of a clinically used preparation (10-mg inject from Jenapharm). Each day, 5 h after the application of the vitamin, a blood sample was taken from the rabbit and the serum tested for αFp-AB activity against 6HDNO. No inhibition of the immune reaction was observed (Fig. 3). Determination by high performance liquid chromatography (HPLC) of the flavin content of the sera taken on each day revealed that the total concentration of flavins (riboflavin, FMN and FAD) did not change and remained at 15 nm. Determination of the flavin content in human sera gave a similar value of 15–16 nm, which is close to the approximate concentration of 30 nm flavin reported previously for human serum [4]. The affinity of the αFp-AB for flavins is within this range. However, filtration of the human serum revealed that approx. 95% of the flavin content determined by HPLC was protein-bound. Taking into account that some flavin may be released from the protein during handling of the samples and centrifugation, one may conclude that virtually all flavin in the serum was protein-bound. When the same analysis was performed with the rabbit serum obtained during the 3 days of vitamin B2 application, the content of non-protein-bound flavin increased from 2 nm to 4 nm and to 5.5 nm on day 3, with a constant amount of 12 nm protein-bound flavin (average of three independent determinations). The capacity of the serum proteins to bind flavins was about 150 nm when tested in vitro by the addition of riboflavin to human serum followed by filtration and determination of the flavin content in the filtrate. Since the kD of the αFp-AB is at about 10 nm, the free flavin concentration obtained in the serum appears to be too low to neutralize these antibodies.
Fig. 3.
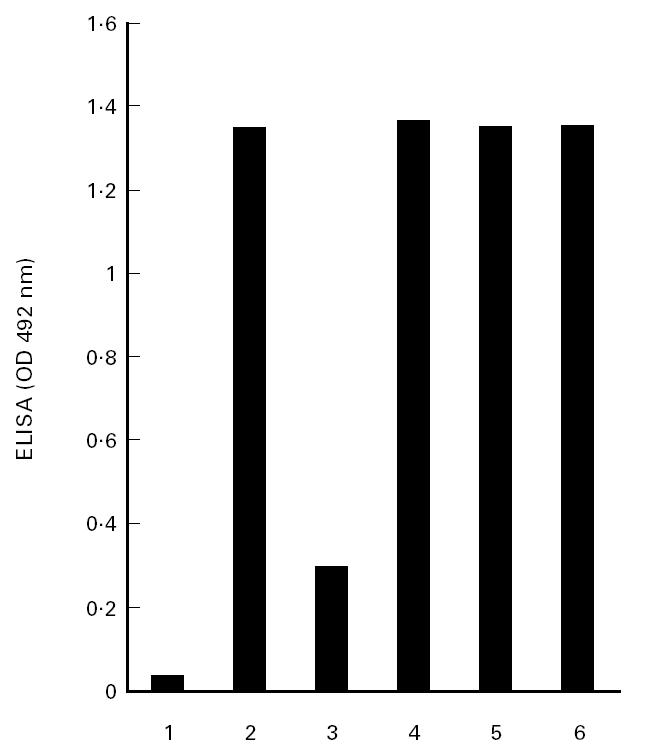
αFp-AB activity in rabbit sera after vitamin B2 application. An ELISA was performed with 6-hydroxy-d-nicotine oxidase (6HDNO) as antigen and sera from a rabbit immunized with sarcosine oxidase (SaO). The assay shows the αFp-AB-activity of the sera before (lane 1) and after immunization (lane 2), after in vitro preincubation of the antiserum with vitamin B2 (lane 3) and after i.v. application of vitamin B2 to the rabbit on 3 consecutive days (lanes 4, 5 and 6).
Characterization of the epitope of flavoenzymes recognized by αFp-AB
The ability to neutralize in vitroαFp-AB by incubation with flavins indicated that the covalently attached FAD moiety of flavoenzymes represents a major antigenic determinant. Conformational elements of the polypeptide, however, may contribute to the antigen-αFp-AB recognition. To test this possibility we employed proteolytic digests of 6HDNO and tested the antigenic reactivity against αFp-AB using Western blotting. A schematic representation of the 6HDNO FAD-binding site and the cleavage sites of endopeptidase is represented in Fig. 4a. Trypsin cleaves three amino acid residues N-terminally to the FAD-histidine, chymotrypsin five residues C-terminally from the FAD-histidine and endopeptidase Lys cleaves 18 amino acid residues N-terminally and 44 amino acid residues C-terminally from the FAD-histidine, respectively. Treatment with trypsin and chymotrypsin abolished the antigenicity of 6HDNO, but treatment with endopeptidase Lys (Fig. 4b) did not. This observation may indicate that a minimal amino acid sequence N-terminally and C-terminally from the FAD-binding histidine is required for αFp-AB recognition. Secondary structure prediction revealed that the FAD-histidine is situated in a turn flanked on both sides by a β-sheet. Cleavage by trypsin and by chymotrypsin removes the N-terminally or the C-terminally located β-sheet, respectively. Severing of the polypeptide chain at these sites may lead to a change in conformation of the released peptides, leading to loss of recognition by the αFp-AB.
Fig. 4.

Characterization of the epitope recognized by the αFp-AB. (a) Shown are the amino acid sequences of the 6-hydroxy-d-nicotine oxidase (6HDNO) peptides generated by digestion with trypsin, chymotrypsin and endopeptidase Lys and a schematic representation of the predicted secondary structure of the peptides. (b) 6HDNO (100 μg per assay) was subjected to treatment with trypsin (lane 2), chymotrypsin (lane 3) and endopeptidase Lys (lane 4). The peptides were separated by SDS–PAGE (15%), blotted onto nitrocellulose membrane and decorated overnight with purified human αFp-AB. Undigested 6HDNO was applied as control (lane 1); 96 kD and 48 kD indicate the dimeric and monomeric form of 6HDNO, respectively.
Cellular antigenes recognized by αFp-AB on neonatal rat cardiomyocytes and on cryosections through human heart
A harmful effect of αFp-AB on the heart of patients with myocarditis and dilated cardiomyopathy could be induced by the interaction of these antibodies with cell surface antigens of cardiomyocytes. When neonatal rat cardiomyocytes in primary culture were treated with αFp-AB and analysed by immunofluorescence, no reaction with cellular surface antigens could be detected (results not shown). On sections through an undiseased human heart, the antibodies recognized mitochondrial antigens within the cardiomyocytes, as expected, but again no immunoreaction at the cell surface was detected (results not shown).
DISCUSSION
The presence of αFp-AB in myocarditis and dilated cardiomyopathy may have negative consequences on the general health status of these patients. Especially intriguing is the fact that flavins are carried in the serum in protein-bound form, mainly complexed with immunoglobulins. Were the flavin moiety accessible on the surface of the carrier protein, this could lead to recognition by the αFp-AB with the formation of immunocomplexes. As shown previously [2], the αFp-AB are not identical to the flavin-carrying immunoglobulin fraction. They are quite specific for this group of patients and can be isolated using antigen in affinity chromatography from patient's serum but not from the serum of non-diseased individuals. From the results presented in this study one may conclude that the bound flavin on the carrier proteins is inaccessible to the αFp-AB. This conclusion is valid for the flavin-carrying serum proteins as well as for the egg white riboflavin-carrying protein. There is direct support for this assumption from the recently solved crystal structure of hen egg white riboflavin-binding protein [13]. The isoalloxazine ring of the riboflavin molecule lays hidden in a cleft of the protein, stacked between the aromatic rings of a tyrosine and tryptophan residue.
A second aspect relates to the question: why are αFp-AB in the serum active at all and not neutralized by free flavin in the serum? Our results suggest that under physiological conditions there is essentially no free flavin in the serum. Less then 10% of the total flavin present in the serum filtrate is liberated from the flavin-carrying proteins during the handling and centrifugation of samples. Following i.v. injections of vitamin B2, the increase in the flavin concentration recovered as free flavin in the filtrate is small, and the amount of protein-bound flavin does not change, although the capacity for flavin-binding of the serum proteins is far from saturated. We interpret this observation as a rapid uptake of the administered vitamin B2 into blood cells and into hepatocytes and possibly filtration by the kidneys. These cells apparently possess an efficient uptake system for the vitamin and flavin cofactors [4]. Even if there is a fraction of free flavin in the serum, its concentration is too low to neutralize the αFpAB induced in myocarditis and dilated cardiomyopathy patients. Application of vitamin B2 at doses commonly used clinically does not lead to the neutralization of these antibodies.
Not unexpectedly, αFp-AB were shown to recognize conformational elements of the flavoenzyme, besides the FAD moiety. 6HDNO was used as a model enzyme. The secondary structure elements of the covalent FAD-binding site are similar among proteins of this flavin family [14]. The recognition of conformational elements of the protein is a common feature of antibodies raised against protein–hapten complexes. The specificity of the αFp-AB compared with the flavin-binding immunoglobulins of the serum may be explained by this fact.
The origin of the immune reaction leading to the formation of αFp-AB in patients suffering from myocarditis and dilated cardiomyopathy and the consequences of the presence of these antibodies for the health status of these patients are not yet clear. Cardiomyocytes with their intense respiration are especially rich in mitochondria. Lesions of cardiomyocytes following a viral infection or apoptotic events in the course of the establishment of a cardiomyopathy may lead to the liberation of the mitochondrial Fp subunit of SDH, the quantitatively predominant flavoprotein with covalently bound FAD in the cell. Macrophages phagocytosing these cell remnants may present the FAD-peptide on MHC complexes and stimulate autoreactive T cells. In the context of an inflammatory reaction of the heart, T and B lymphocytes sensitized by bacterial SDH Fp antigen may be also activated. Activated autoreactive T helper cells and αFp autoantibodies generated as a consequence of these immune processes could recognize cell surface antigens of cardiomyocytes by molecular mimicry, leading to their damage. As shown here for cardiomyocytes of normal human heart, this was not the case. Tissue from the hearts of myocarditis or dilated cardiomyopathy patients was not available. Therefore, it remains to be established whether there is a change in cell surface antigen expression in diseased hearts, e.g. following a viral infection [15], which could lead to a specific recognition of such newly expressed antigens by the αFp-AB. Further investigations will be conducted to evaluate the possible pathogenic and diagnostic relevance of the αFp-AB.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to R.B.
References
- 1.Klein R, Maisch B, Kochisek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1990;82:283–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Otto A, Stähle I, Klein R, Berg PA, Pankuweit S, Brandsch R. Anti-mitochondrial antibodies in patients with dilated cardiomyopathy (anti-M7) are directed against flavoenzymes with covalently bound FAD. Clin Exp Immunol. 1998;111:541–7. doi: 10.1046/j.1365-2249.1998.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker H, Frank O, Feingold S, Leevy CM. Vitamin distribution in human plasma. Nature. 1967;215:84–85. doi: 10.1038/215084a0. [DOI] [PubMed] [Google Scholar]
- 4.McCormick DB. Two interconnected B vitamins: riboflavin and pyridoxine. Physiol Rev. 1989;69:1170–98. doi: 10.1152/physrev.1989.69.4.1170. [DOI] [PubMed] [Google Scholar]
- 5.Merill AH, Innis-Whitehouse WSA, McCormick DB. Characterization of human riboflavin-binding immunoglobulins. Flavins and Flavoproteins. 1987;9:445–8. [Google Scholar]
- 6.Harlow E, Lane D. A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. Antibodies; pp. 92–135. [Google Scholar]
- 7.Sambrook J, Fritsch EF, Maniatis T. A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. Molecular cloning. [Google Scholar]
- 8.Berthold H, Scanarini M, Abney CC, Frorath B, Northemann W. Purification of recombinant antigenic epitopes of the human 68-kDa (U1) ribonucleoprotein antigen using the expression system pH6EX3 followed by metal chelating affinity chromatography. Prot Exp Purif. 1992;3:50–56. doi: 10.1016/1046-5928(92)90055-2. [DOI] [PubMed] [Google Scholar]
- 9.Engvall E. Enzyme Immunoassay ELISA and EMIT. Methods Enzymol. 1980;70:419–39. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- 10.Rose NR, Milisauskas V, Sampson HA. Species-specific tissue antigens. III. Immunological relationships of enzymic antigens in various species. Clin Exp Immunol. 1975;20:359–70. [PMC free article] [PubMed] [Google Scholar]
- 11.Zempleni J, Link G, Kübler W. The transport of thiamine, riboflavin and pyridoxal 5′-phosphate by human placenta. Int J Vit Nutr Res. 1992;62:165–72. [PubMed] [Google Scholar]
- 12.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Circ Res. 1982;50:101–16. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Monaco HL. Crystal structure of chicken riboflavin-binding protein. EMBO J. 1997;16:1475–83. doi: 10.1093/emboj/16.7.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraaije M, van Berkel WJH, Benen JAE, Visser J, Mattevi A. A novel oxidoreductase family sharing a conserved FAD-binding domain. TIBS. 1998;23:206–7. doi: 10.1016/s0968-0004(98)01210-9. [DOI] [PubMed] [Google Scholar]
- 15.Neumann DA, Rose NR, Ansari AA, Herskowitz A. Induction of multiple heart autoantibodies in mice with Coxsackievirus B3- and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994;152:343–50. [PubMed] [Google Scholar]


