Abstract
Dysfunction of cytotoxic activity of T and natural killer (NK) lymphocytes is a main immunological feature in patients with AIDS, but its basis are not well understood. It has been recently described that T and NK cell-mediated cytotoxicity can be regulated by HLA killer inhibitory receptors (KIR). In this work, we have determined on cytotoxic T cells and NK cells from HIV-1-infected individuals the expression of the following KIR molecules: p58, p70, and ILT2 (immunoglobulin-like family KIR) as well as CD94 and NKG2A (C-lectin-type family KIR). With some exceptions, no significant changes were found on the expression of immunoglobulin-like KIR in either CD8+ or CD56+ cells. Interestingly, the percentages of CD8+ and CD56+ cells expressing CD94 were significantly increased in these individuals. We also show that, in vitro, IL-10 up-regulates CD94 expression on CD8+ and CD56+ cells obtained from normal individuals, suggesting that the augmented expression observed in HIV-infected individuals could be related to the high levels of IL-10 previously described in HIV-1-infected individuals.
Keywords: NK, KIR, CD94, cytotoxic T lymphocytes, HIV-1 and NKG2
INTRODUCTION
Alterations in the cytotoxicity mediated by T lymphocytes (CD3+CD8+) and natural killer (NK) cells (CD3−CD56+) are a common feature in HIV-1 infection. [1–7]. The underlying causes of these cellular defects have been extensively studied. Abnormalities in the mechanisms of anergy and/or apoptosis of HIV-1-specific T cell clones [1], as well as CD3-mediated stimulation defects [8], have been described in HIV-1-infected individuals. NK cell alterations include a defective triggering of NK cytotoxicity after binding target cells, as well as inability to release NK cytotoxic factors [9]. Nevertheless, these data cannot explain the full extent of alterations in the cytotoxic mechanisms observed in HIV-1 patients.
Recently, HLA-specific killer inhibitory receptors (KIR) have been proposed as regulators of cytotoxic events in HIV-1-infected individuals [10,11]. KIR participate in the regulation of the cytotoxic function of NK and some Tc cells by recognizing specific motifs of HLA molecules [12–15]. These receptors belong to two different structural families. The first group comprises immunoglobulin-superfamily member molecules p58, p70, p70/p140 [16] and the immunoglobulin-like transcript 2 (ILT2) [17], which interact with classical HLA class I antigens expressed on target cells. The second group, which recognizes the non-classical HLA-E class I antigen [18–20], is conformed by heterodimers sharing a common C-type lectin glycoprotein chain, namely CD94, and a second chain encoded for by a member of the NKG2 gene family [21,22]. Thus, CD94/NKG2A heterodimer is considered to be functionally inhibitory, while CD94/NKG2C heterodimer triggers NK cytotoxicity [18,23].
Since cytotoxic cells are involved in protection against viral infections including HIV-1 [24,25], it could be speculated that the cytotoxic dysfunctions reported in HIV-1-infected individuals may be related to the modulation of KIR molecules on Tc and NK cells. Therefore, we have analysed the expression of KIR (p58.1, p58.2, p70 and ILT2) as well as CD94 and NKG2A molecules on peripheral blood T and NK cells of patients with HIV-1 infection.
PATIENTS AND METHODS
Patients and controls
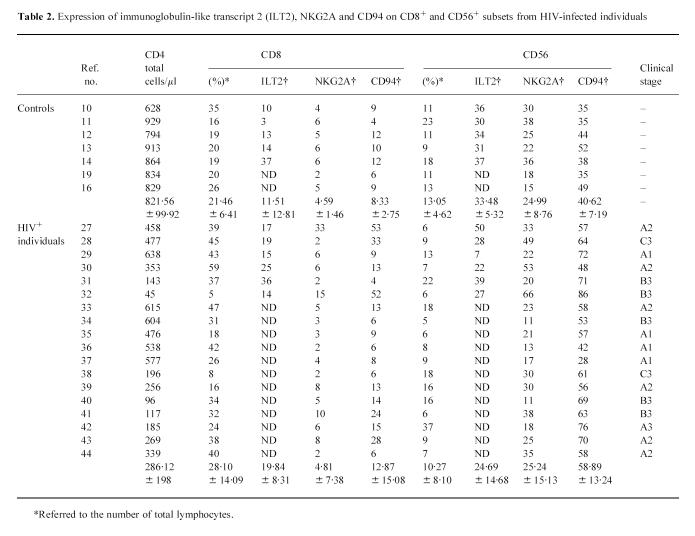
HIV-1+ infected individuals (n = 44) were studied for expression of HLA-specific killer inhibitory receptors on CD8+ and CD56+ peripheral blood cells. Blood was obtained during routine follow-up visits for the monitoring of disease progression. All subjects gave informed consent under the auspices of the appropriate Research and Ethics Committees. Peripheral blood mononuclear cells (PBMC) were obtained by density gradient centrifugation (Lymphoprep, Nycomed Pharma, Oslo, Norway). Patient classification was according to their total CD4+ cell numbers and clinical stage. Cells were analysed using a FACScount cell counter (Becton Dickinson, Mountain View, CA). At the time of the study all patients were receiving anti-retroviral therapy and were not suffering from other infectious diseases. Healthy HIV-1− donors (n = 16) were included as controls.
Fluorescence analysis
KIR expression was studied by double immunofluorescence on CD8+ and on CD56+ PBMC as previously described [26]. Cells were incubated for 30 min at 4°C in the presence of 1% bovine serum albumin (BSA) with the following MoAbs: GL183 (anti-p58.2), EB6 (anti-p58.1), HP-3B1 (anti-CD94) from Immunotech (Marseille, France), HP-F1 specific for the immunoglobulin-like transcript 2 (ILT2) (a gift from Dr M. Lopez-Botet, Hospital de la Princesa, Madrid, Spain) and MoAb Z199 specific for NKG2A (a gift from Dr A. Moretta, University of Genoa, Italy). After extensive washing, cells were incubated with FITC-conjugated goat anti-mouse IgG antibodies (Becton Dickinson). FITC-conjugated anti-NKB1 (p70), from Becton Dickinson, was also used. Subsequently cells were incubated for 30 min at 4°C with PE-conjugated anti-CD56 (Leu-19) or anti-CD8 (Leu-2A). Fluorescence analysis was performed on a FACSort cytometer (Becton Dickinson) after acquisition of 104 events. Viable cells were selected using forward and side scatter characteristics. Resulting profiles were analysed using the Lysis II software.
T cell enrichment and culture
PBMC from healthy individuals were obtained by density gradient centrifugation and adherent cells removed by incubating the PBMC in 100-mm plastic Petri dishes (Costar, Cambridge, MA) at 37°C for 60 min. Non-adherent cells were passed over a nylon-wool column to deplete residual B cells and macrophages [27]. This resulted in a population of T cells (NWT) that was CD3+ > 90%, CD56+ < 10%, CD19+ < 1%, CD14+ < 1% cells as determined by flow cytometry. Cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 1% l-glutamine, 1% penicillin/streptomycin (Bio-Whittaker, Verviers, Belgium). Anti-CD3 MoAb (OKT3) was used to coat 24-well culture plates. Cells were cultured at a concentration of 106cells/ml in the presence of IL-10 (10 ng/ml) (Pharmingen, San Diego, CA) and IL-2 (25 U/ml) (Hoffmann-La Roche, Nutley, NJ). OKT3-mediated stimulation was repeated every 5 days.
Statistical analysis
Statistical significance between two means was determined by Student's t-test. P < 0.05 was considered significant.
RESULTS
Expression of KIR on CD8+ T cells
The expression of CD94, measured by the MoAb HP3B1, on CD8+ cells was significantly increased (P < 0.0001) in HIV-1-infected individuals (31.6 ± 25.1) when compared with normal controls (4.6 ± 4.9) (Table 1). A representative flow cytometry analysis from an HIV-1-infected individual is shown in Fig. 1. In order to analyse further whether this CD94 augmentation was associated with the inhibitory or activatory fractions, the expression of the inhibitory NKG2A molecule was analysed. NKG2A was increased on CD8+ T cells in some patients. On the other hand, no significant changes were found in the expression of immunoglobulin-like KIR (p58, p70 and ILT2) on CD8+ cells (Tables 1 and 2).
Table 1.
Expression of killer inhibitory receptors (KIR) and CD94 on CD8+ and CD56+ subpopulations of lymphocytes from HIV+ individuals
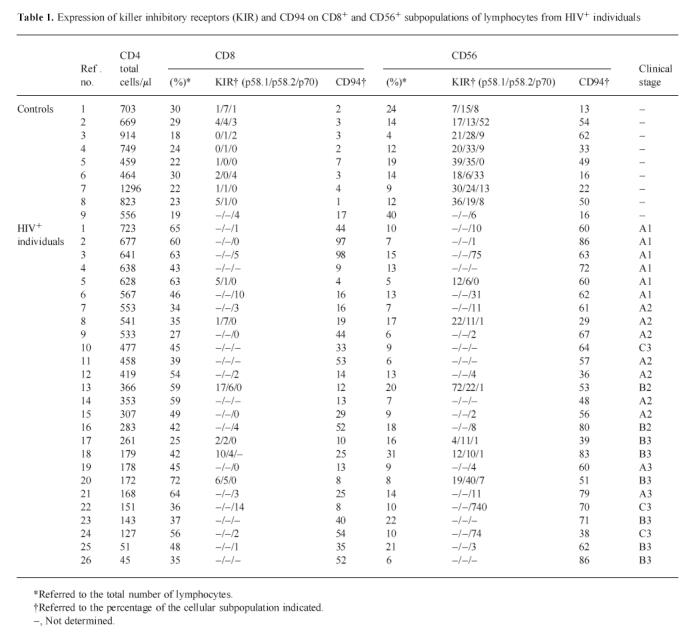
Fig. 1.
A representative flow cytometry diagram showing an elevated expression of CD94 on CD8+ cells from HIV-1-infected individuals.
Table 2.
Expression of immunoglobulin-like transcript 2 (ILT2), NKG2A and CD94 on CD8+ and CD56+ subsets from HIV-infected individuals
Expression of KIR on CD56+ cells
The expression of CD94 on CD56+ cells was significantly increased (P < 0.001) in HIV-1-infected individuals (61.2 ± 15.2) compared with normal controls (35.0 ± 19.0) (Table 1 and Fig. 2). No changes in the expression of NKG2A were found on CD56+ cells. No significant changes were found in the expression of immunoglobulin-like KIR in CD56+ cells (Tables 1 and 2). No relationship between the expression of CD94 on T or NK cytotoxic cells and the number of CD4+ cells or the clinical stage was found.
Fig. 2.
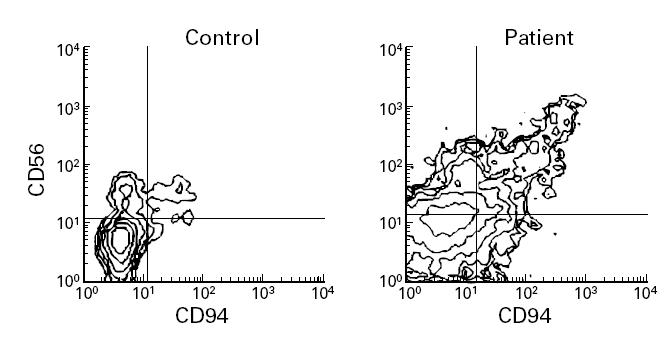
A representative flow cytometry diagram showing an elevated expression of CD94 on CD56+ cells from HIV-1-infected individuals.
IL-10 mediates CD94 expression on activated T cells
Enriched T cell populations from healthy individuals stimulated by immobilized anti-CD3 MoAb were incubated in the absence or presence of IL-10. The expression of CD94 was determined every 5 days. CD94 was widely expressed on CD8+ T cells (Fig. 3) after 20 days in the presence of IL-10. An increased expression of CD94 on CD56+ cells after IL-10 treatment was also found, although to a lesser extent (data not shown).
Fig. 3.
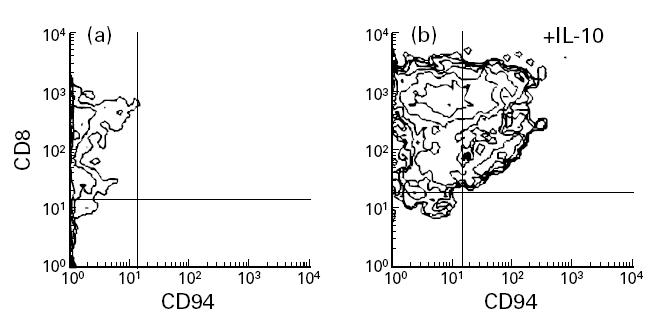
Induction of CD94 expression on activated T cells treated with IL-10. T cells were stimulated with immobilized anti-CD3 MoAb and cultured during 20 days (a) in the absence or (b) in the presence of exogenous IL-10 (10 ng/ml). A representative experiment is shown.
DISCUSSION
The cause(s) underlying the dysfunction of the cytotoxic activity of Tc and NK cells described in HIV-1+ individuals are not completely understood despite previous reports which suggest a variety of causes [1–7]. It has been recently proposed that changes in the expression of HLA-specific KIR on cytotoxic T lymphocytes and NK cells would contribute, at least in part, in these deficiencies [10,11]
We have found that the number of CD8+ and CD56+ cells expressing CD94 was significantly increased in HIV-1-infected individuals. The relevance of this observation is the fact that CD94 is the invariant component of the C-lectin-type KIR, broadly expressed on cytotoxic cells [28], although the functional significance of these findings is controversial. Thus, CD94 may be found associated to the NKG2A molecule giving rise to the functionally differentiated HLA-specific inhibitory receptor termed CD94/NKG2A. CD94 can also be associated with NKG2C, a molecule defined in humans by molecular means, constituting the NK cell-triggering receptor designated as CD94/NKG2C heterodimer. In addition, CD94 could bind to other molecules of the NKG2 family whose expression and function on the cell membrane have not yet been described, and the possibility that CD94 may be expressed as a homodimer can not be excluded.
The number of cells expressing KIR belonging to the immunoglobulin-like superfamily is also restricted to a minor subpopulation of CD8+ cells. These results are in agreement with data previously reported [10,11], which have shown that this expression is limited to a subset of specific T cells reactive against HIV-infected target cells.
Understanding the regulation of the expression of these receptors is relevant for both the biology of cytotoxic cells and HIV-1 infection progression. Expression of most leucocyte surface antigens is regulated by cytokines. Whereas very little is known about the regulation of immunoglobulin-like KIR on NK or T cells, it has been recently described that IL-15 up-regulates the expression of CD94 on NK cells [29] and cytotoxic T cells [30]. It might be speculated that during HIV-1 infection, where a number of immune alterations occur as the disease progresses, several factors may account for the increased KIR expression observed. Among them, the influence of elevated levels of some cytokines with inhibitory effects on T cell function could be relevant to the phenomenon described. Of particular interest in HIV-1 infection is IL-10, which is elevated in HIV-1-infected individuals and is involved in regulating immunity to HIV-1 [31,32]. Indeed, we have shown that prolonged activation of T cells in the presence of a discrete amount of IL-10 induces the expression of CD94 in T cells. It has also been described that IL-10 interferes with HIV-1 replication [33]. Whether or not these two facts are related, requires further investigation.
Taken together, these results suggest that the enhanced expression of CD94 on HIV-1-infected individuals could be involved in the dysfunction of T and NK cytotoxicity demonstrated in HIV-1-infected patients. These results also suggest that IL-10 could have the capacity to induce the expression of CD94 on CD8+ and CD56+ cells. Whether these changes in the Tc and NK compartment may influence the outcome of individuals with HIV-1 infection is an important issue to a better understanding of the precise role of cytotoxic cells in HIV-1-infected individuals.
Acknowledgments
We are very grateful to Dr M. Lopez-Botet (Madrid) and Professor A. Moretta (Genoa) for kindly providing MoAb HPF1 and MoAb Z199. This work was supported by grants FIS95/1169 (J.P.), SAF 96-0259 (J.P.), FIS98/1051 (R.S.), FIS95/1170 (M.S.) and CajaSur Foundation (M.D.G.). We are also grateful to Dr J. M. Kindelan from the Infectious Diseases Unit at Reina Sofía Hospital for the support provided to this work.
References
- 1.Lewis DE, Tang DS, Adu-Oppong A, Schober W, Rodgers JR. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–20. [PubMed] [Google Scholar]
- 2.Blackbourn DJ, Mackewicz C, Barker E, Levy JA. Human CD8+ cell non-cytolytic anti-HIV activity mediated by a novel cytokine. Res Immunol. 1994;145:653–8. doi: 10.1016/s0923-2494(05)80049-5. [DOI] [PubMed] [Google Scholar]
- 3.Mackewicz CE, Ortega HW, Levy JA. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87:1462–6. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirianni MC, Mezzaroma I, Aiuti F, Moretta A. Analysis of the cytolytic activity mediated by natural killer cells from acquired immunodeficiency syndrome patients in response to phytohemagglutinin or anti-CD16 monoclonal antibody. Eur J Immunol. 1994;24:1874–8. doi: 10.1002/eji.1830240824. [DOI] [PubMed] [Google Scholar]
- 5.Sirianni MC, Tagliaferri F, Aiuti F. Pathogenesis of the natural killer cell deficiency in AIDS. Immunol Today. 1990;11:81–82. doi: 10.1016/0167-5699(90)90032-5. [DOI] [PubMed] [Google Scholar]
- 6.Scott-Algara D, Vuillier F, Cayota A, Dighiero G. Natural killer (NK) cell activity during HIV infection: a decrease in NK activity is observed at the clonal level and is not restored after in vitro long-term culture of NK cells. Clin Exp Immunol. 1992;90:181–7. doi: 10.1111/j.1365-2249.1992.tb07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratcliffe LT, Lukey PT, Mackenzie CR, Ress SR. Reduced NK activity correlates with active disease in HIV- patients with multidrug-resistant pulmonary tuberculosis. Clin Exp Immunol. 1994;97:373–9. doi: 10.1111/j.1365-2249.1994.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantaleo G, Soudeyns H, Demarest JF, et al. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–53. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jewett A, Cavalcanti M, Giorgi J, Bonavida B. Concomitant killing in vitro of both gp120-coated CD4+ peripheral T lymphocytes and natural killer cells in the antibody-dependent cellular cytotoxicity (ADCC) system. J Immunol. 1997;158:5492–500. [PubMed] [Google Scholar]
- 10.Cauda R, Goletti D, Lucia MB, et al. Analysis of natural killer (NK) cell subsets defined by the expression of two novel surface antigens (EB6 and GL183) in AIDS and AIDS-related conditions. Clin Immunol Immunopathol. 1994;70:198–205. doi: 10.1006/clin.1994.1029. [DOI] [PubMed] [Google Scholar]
- 11.De Maria A, Ferraris A, Guastella M, et al. Expression of HLA class I-specific inhibitory natural killer cell receptors in HIV-specific cytolytic T lymphocytes: impairment of specific cytolytic functions. Proc Natl Acad Sci USA. 1997;94:10285–8. doi: 10.1073/pnas.94.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biassoni R, Falco M, Cambiaggi A, et al. Amino acid substitutions can influence the natural killer (NK)-mediated recognition of HLA-C molecules. Role of serine-77 and lysine-80 in the target cell protection from lysis mediated by “group 2” or “group 1” NK clones. J Exp Med. 1995;182:605–9. doi: 10.1084/jem.182.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181:1133–44. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luque I, Solana R, Galiani MD, et al. Threonine 80 on HLA-B27 confers protection against lysis by a group of natural killer clones. Eur J Immunol. 1996;26:1974–7. doi: 10.1002/eji.1830260845. [DOI] [PubMed] [Google Scholar]
- 15.Mandelboim O, Reyburn HT, Sheu EG, et al. The binding site of NK receptors on HLA-C molecules. Immunity. 1997;6:341–50. doi: 10.1016/s1074-7613(00)80336-2. [DOI] [PubMed] [Google Scholar]
- 16.Moretta L, Ciccone E, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: origin, clonality, specificity, and receptors. Adv Immunol. 1994;55:341–80. doi: 10.1016/s0065-2776(08)60513-1. [DOI] [PubMed] [Google Scholar]
- 17.Colonna M, Navarro F, Bellon T, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–18. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braud VM, Allan DS, O'Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 19.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813–8. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N, Llano M, Carretero M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199–204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol. 1997;158:3603–9. [PubMed] [Google Scholar]
- 22.Carretero M, Cantoni C, Bellon T, et al. The CD94 and NKG2-A C-type lectins covalently assemble to form a natural killer cell inhibitory receptor for HLA class I molecules. Eur J Immunol. 1997;27:563–7. doi: 10.1002/eji.1830270230. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Villar JJ, Carretero M, Navarro F, et al. Biochemical and serologic evidence for the existence of functionally distinct forms of the CD94 NK cell receptor. J Immunol. 1996;157:5367–74. [PubMed] [Google Scholar]
- 24.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 25.Melder RJ, Balachandran R, Rinaldo CR, Gupta P, Whiteside TL, Herberman RB. Cytotoxic activity against HIV-infected monocytes by recombinant interleukin 2-activated natural killer cells. AIDS Res Hum Retrovir. 1990;6:1011–5. doi: 10.1089/aid.1990.6.1011. [DOI] [PubMed] [Google Scholar]
- 26.Borrego F, Pena J, Solana R. Regulation of CD69 expression on human natural killer cells: differential involvement of protein kinase C and protein tyrosine kinases. Eur J Immunol. 1993;23:1039–43. doi: 10.1002/eji.1830230509. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Cozar FJ, Molina IJ, Cuadrado MJ, Marubayashi M, Pena J, Santamaria M. Defective B7 expression on antigen-presenting cells underlying T cell activation abnormalities in systemic lupus erythematosus (SLE) patients. Clin Exp Immunol. 1996;104:72–79. doi: 10.1046/j.1365-2249.1996.d01-648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Botet M, Carretero M, Perez-Villar J, Bellon T, Llano M, Navarro F. The CD94/NKG2 C-type lectin receptor complex: involvement in NK cell-mediated recognition of HLA class I molecules. Immunol Res. 1997;16:175–85. doi: 10.1007/BF02786361. [DOI] [PubMed] [Google Scholar]
- 29.Mingari MC, Vitale C, Cantoni C, et al. Interleukin-15-induced maturation of human natural killer cells from early thymic precursors: selective expression of CD94/NKG2-A as the only HLA class I-specific inhibitory receptor. Eur J Immunol. 1997;27:1374–80. doi: 10.1002/eji.1830270612. [DOI] [PubMed] [Google Scholar]
- 30.Mingari MC, Ponte M, Bertone S, et al. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci USA. 1998;95:1172–7. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finnegan A, Roebuck KA, Nakai BE, et al. IL-10 cooperates with TNF-alpha to activate HIV-1 from latently and acutely infected cells of monocyte/macrophage lineage. J Immunol. 1996;156:841–51. [PubMed] [Google Scholar]
- 32.Bergamini A, Bolacchi F, Faggioli E, et al. HIV-1 does not alter in vitro and in vivo IL-10 production by human monocytes and macrophages. Clin Exp Immunol. 1998;112:105–11. doi: 10.1046/j.1365-2249.1998.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masood R, Lunardi-Iskandar Y, Moudgil T, et al. IL-10 inhibits HIV-1 replication and is induced by tat. Biochem Biophys Res Commun. 1994;202:374–83. doi: 10.1006/bbrc.1994.1938. [DOI] [PubMed] [Google Scholar]