Abstract
This cross-sectional study evaluates the correlation between anti-phospholipid antibodies and CD5+ B cells in 110 patients infected with HIV-1. There were 89.1% of the patients who had IgG antibodies against cardiolipin and phosphatidylserine. The prevalence of IgM and IgA antibodies was < 22%. AIDS was associated with lower frequencies of IgM antibodies against cardiolipin (P = 0.05) and IgG-antibodies against cardiolipin and phosphatidylserine (P = 0.011). Drug users had higher IgM antibodies against phospholipids than patients from other risk groups (P = 0.02). A history of thromboembolic events was not accompanied by higher levels of anti-phospholipid antibodies (P > 0.2). No correlation between anti-phospholipid antibodies and CD5+ B cells was detected. Percentage part of CD5+ B lymphocytes was elevated in all patients and absolute CD4+ T lymphocyte counts and HIV p24 antigen were inversely correlated. In advanced disease a significant reduction of anti-phospholipid antibodies was contrasted with persistent elevation of CD5+ B lymphocytes. These observations may reflect immunological dysfunction involving apoptosis and endothelial damage rather than polyclonal B cell hyperstimulation. A possible explanation would be that in HIV infection an increased rate of spontaneous apoptosis in peripheral blood lymphocytes is accompanied by functional and structural changes of mitochondria. Therefore, structurally altered mitochondrial phospholipids could serve as antigen to induce specific humoral immune responses.
Keywords: anti-phospholipid antibodies, CD5+ B cells, cross-sectional study, HIV infection
INTRODUCTION
The most prominent pathogenic feature of infection with HIV is the loss of CD4+ T lymphocytes and the emerging susceptibility to opportunistic infections or tumours. Recent research indicates that immune activation as well as autoimmunity may contribute to clinical features of HIV-associated disease [1]. This is particularly apparent in those HIV-infected individuals who have clinical evidence of autoimmune diseases like immune thrombocytopenia, inflammatory neuropathy and vasculitis. Polyclonal B cell activation was noted early during the AIDS epidemic [2] and was attributed, at least in part, to HIV or its peptides [3–5]. Also, spontaneous immunoglobulin synthesis in vitro [6] and serum autoantibodies [7,8] have frequently been detected. Antibodies against peripheral blood cells and MHC II have been frequently demonstrated, whereas antinuclear antibodies have been rarely observed [9]. This is contrasted by the observation of a disturbed generation of specific antibodies in HIV-infected individuals [10].
Antibodies against phospholipids, particularly anti-cardiolipin antibodies (ACLA) have recently been the subject of special interest, first, because they were associated with distinct clinical symptoms, thromboembolic disease, recurrent fetal loss or thrombocytopenia [11–14], which were also frequently seen in HIV-associated disease, and second, because of their appearance during infectious disease states distinct from an association with syphilis, lyme borreliosis, helminthoses or Mycoplasma infections [15–17]. They were also highly prevalent in HIV infection [18].
B cells bearing the CD5 antigen constitute a subset which is thought to be part of the natural immune system and their role in generating natural occurring autoantibodies is still under discussion [19]. CD5+ B cells are the predominant cell type found in chronic B cell lymphocytic leukaemia [20]. High numbers in the peripheral blood were also reported in rheumatoid arthritis [21], Sjögren's syndrome [22], and recent-onset insulin-dependent diabetes mellitus [23]. In the setting of HIV infection there are few reports concerning the number and the role of CD5+ B lymphocytes in relation to the development of autoimmune phenomenon, lymphoid neoplasia or disease progression [24,25].
At this time, there is no study that focuses on the relationship of CD5+ B lymphocytes and ACLA with each other as well as with clinical disease state, immunological progression, surrogate marker and outcome in HIV-infected individuals. The objective of this study therefore was to investigate the clinical, immunological and laboratory features associated with these laboratory autoimmune phenomena in HIV infection.
SUBJECTS AND METHODS
Subjects
One hundred and ten individuals with confirmed HIV infection receiving continuous medical treatment were consecutively recruited from the out-patient clinic for infectious diseases. After complete physical examination, blood was withdrawn for routine and research laboratory analysis after informed consent. Patients were seen monthly for follow-up visits. Any clinical disease during the course, including HIV-related opportunistic events, was noted. In addition, arctuarial survival was calculated from start of the study to death or last patient contact.
Laboratory measurements
Detection of autoantibodies
The serum material obtained was immediately aliquoted and frozen at −70°C until analysis. Measurements of anti-cardiolipin and anti-phosphatidylserine antibodies (ACLA and APSA, respectively) were performed for all samples with the same test kits with identical lot numbers to minimize methodological errors. Antinuclear antibody (ANA) titres were determined by standard immunofluorescence assays using Hep2 cells as targets in dual logarithmic dilutions of patient's serum. ACLA and APSA were measured by ELISA. Briefly, standard samples, diluted serum samples, positive and negative control sera (each 100 μl/well) were incubated using microtitre plates precoated with cardiolipin or phosphatidylserine (IMTEC Immundiagnostika GmbH, Zepernick, Germany) for 2 h at room temperature. Plates were then washed three times with PBS. Peroxidase-conjugated rabbit antibody to human IgG, IgM, and IgA, respectively, was added in a dilution of 1:1000. After an incubation period of 1 h at room temperature and washing, 200 μl o-phenylenediamine was added as substrate. The reaction was stopped after 10 min by the addition of 100 μl 3 n H2SO4. Optical density (OD) at 492 nm was read and a standard curve constructed for each plate. For this method the lower detection limit was 10 U/ml, the interassay coefficient of variation was < 10% (data not shown). All samples were analysed in duplicate. Given concentrations represent the mean of the two readings. Values > 48 U/ml and > 44 U/ml for IgG ACLA and IgM ACLA, respectively, were considered to be positive. Normal values for IgA ACLA were < 45 U/ml. Positive levels of IgG APSA and IgM APSA comprised values > 20 U/ml and > 39 U/ml, respectively.
Flow cytometry
All measurements were performed immediately after blood was withdrawn. Complete blood counts and cell differentials were performed with an automated cell counter. Flow cytometric determination of lymphocyte surface antigens was performed by direct three-colour immunofluorescence using the FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany) equipped with standard software (FACScan Research, Becton Dickinson) and commercially available MoAbs. Isotype-matched non-specific antibodies were used as negative controls. Lymphocyte phenotyping included the quantification of CD3+ (pan T cell), CD4+ (helper cells), CD8+ (suppressor cells), CD19+ (pan B cells), CD5+ (T and B cell subset), κ and λ light chains on at least 5000 cells. Lymphoid cell population was detected by characteristic forward and 90° scatter light properties. Quality of the gate was determined by immunofluorescence with anti-CD45 (pan leucocyte), anti-CD14 (monocyte), and anti-CD64 (monocytes, macrophages) antibodies. Material was used for further analysis only if the number of CD45high/CD14−/CD64−cells was > 95% in the lymphocyte gate.
Instruments settings were calibrated daily with an automated software using prepared beads (Calibrite and Autocomp Software; Becton Dickinson). Coefficient of variation (CV) for the MoAbs used in the study was < 10% (for anti-κ and anti-λ < 15%). Enumeration of absolute CD4+ T lymphocyte counts was performed according the recommendations of the Centers for Diseases Control and Prevention (CDC) [26].
Statistical analysis
The SPSS-for-Windows Version 6.12 software package was used for data analysis. Descriptive analysis included mean, median, range and s.d. of each variable. The normal distribution of single variables was tested by Kolgomorow–Smirnov analysis. Correlations of clinical and laboratory parameters with results of the flow cytometric and autoantibody determinations were estimated by bivariate linear correlation analysis (two-sided Spearman's rank sum correlation coefficient). Differences between patient groups were compared using the Mann–Whitney U-test and the Kruskal–Wallis one-way anova for independent samples. Relative risk estimations were performed for the association of clinical diseases with laboratory variables at defined cut-off points. Survival was estimated using the method described by Kaplan & Meier [27]. P < 0.05 was assumed to be of significance in all tests used.
RESULTS
General findings
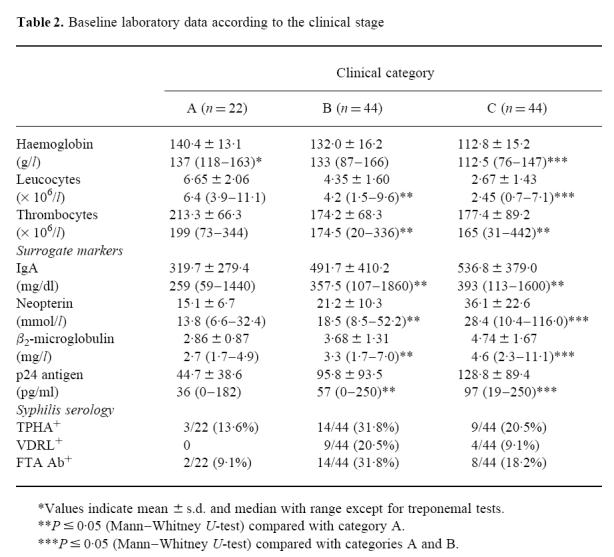
For evaluation, the 110 patients recruited were classified into clinical categories of the disease according to the 1993 classification system of the CDC [28]. Most of the patients were in an advanced stage of HIV infection. Only 14.5% of the patients had a past history of clinically apparent syphilis and 7.3% had thrombosis or thromboembolic diseases (Table 1). Malignant lymphoma was seen in only two subjects, whereas Kaposi's sarcoma was found in four. Almost all symptomatic patients received anti-retroviral treatment (only two out of 22 category A individuals in contrast to 84 out of 88 in categories B and C) either with zidovudine (ZDV), didanosine (DDI), zalcitabine (DDC) or stavudine (D4T) alone or a combination therapy with either ZDV/DDC or ZDV/DDI. Regarding the baseline laboratory parameters (Table 2), severe anaemia, leukopenia or hypoproteinaemia occurred in none of the group A patients. Of the symptomatic individuals 16 (18.2%) had relevant anaemia or leukopenia, whereas none of the patients were found to have hypoproteinaemia. Thrombopenia (thrombocytes < 130/nl) was evident in one (4.5%) category A subject and in six patients each in categories B and C. Serological evidence of Treponema pallidum infection was present in 26 (23.6%) patients. HIV p24 antigen was detectable in all category C as well as in 90.5% and 88.6% of category A and B patients, respectively.
Table 1.
Clinical characteristics of the patients studied
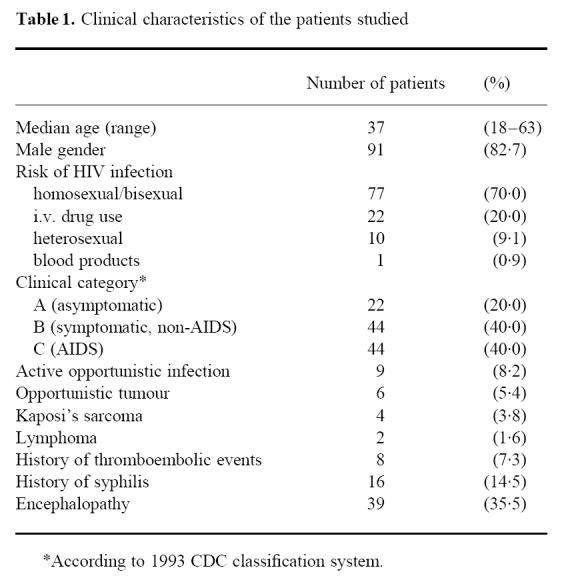
Table 2.
Baseline laboratory data according to the clinical stage
Autoantibodies
Of the individuals examined, 89.1% (21.8%) were found to have IgG ACLA (IgM ACLA), and 89.1% (8.2%) had positive IgG APSA (IgM APSA). Results are summarized in Table 3. Elevated IgA ACLA concentrations were found in 39.1% (41/110 patients). ACLA and APSA positivity was not associated with any of the treponemal serological tests (all P values > 0.2, Fisher's two-tailed exact test). In contrast to this, autoantibody screening revealed only four (3.6%) positive test results for ANA. Anti-mitochondrial and anti-smooth muscle antibodies were found in four patients (3.6%) and in one patient (0.9%), respectively. Anti-Sm antibodies were found in 6.9% of the patients. This is in agreement with our previous results that showed a prevalence of non-organ-specific autoantibodies (ANA, anti-mitochondrial and anti-smooth muscle antibodies) in HIV-infected individuals to be always < 5% (unpublished data).
Table 3.
Positivity for anti-cardiolipin antibodies (ACLA) and anti-phosphatidylserine antibodies (APSA) and clinical stage
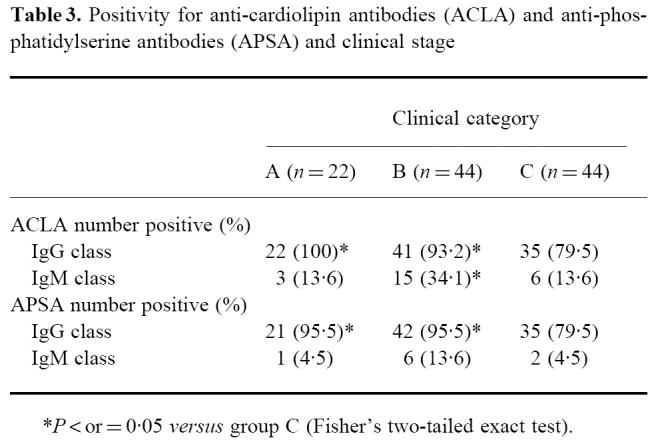
A significantly lower number of patients with clinical AIDS had positive IgM antibodies against cardiolipin compared with those individuals with symptomatic HIV infection (Table 3). Furthermore, the frequency of IgG ACLA and IgG APSA was significantly lower in patients with full-blown AIDS than in those of category A or B (both P = 0.011, Fisher's two-tailed exact test). Similar results were obtained not only for the frequency but also for the absolute concentrations of IgG antibodies if compared according to the clinical (Fig. 1a) or immunological stage (Fig. 1b).
Fig. 1.
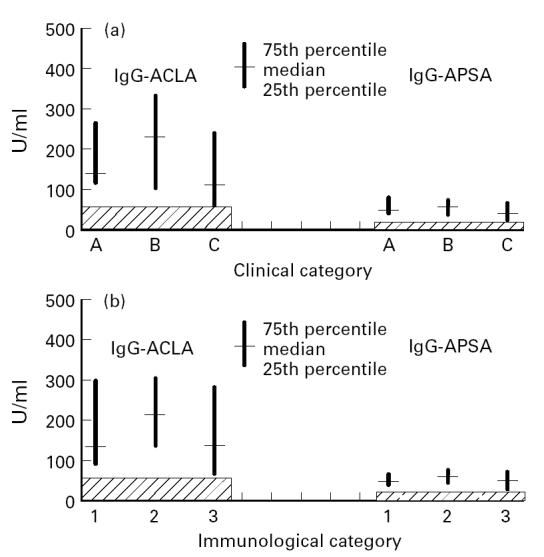
IgG anti-cardiolipin antibodies (ACLA) and IgG anti-phosphatidylserine antibodies (APSA) levels according to the clinical (a) and immunological (b) stage. Measurements of ACLA and APSA were performed as described in Subjects and Methods. The frequency of IgG ACLA and IgG APSA was significantly lower in patients with full-blown AIDS than in those of category A or B (both P = 0.011, Fisher's two-tailed exact test). Similar results were obtained comparing the different immunological groups. The hatched area indicates the negative range.
With regard to the risk factors for the acquisition of HIV infection, i.v. drug users had significantly higher concentrations of IgM antibodies against cardiolipin and phosphatidylserine than individuals from other risk groups (each P < 0.002, Kruskal–Wallis one-way anova). Also, drug abuse was associated with higher concentrations of IgG APSA compared with homo- or bisexual patients (P < 0.05, Mann–Whitney U-test).
Differences regarding the levels or the positivity of autoantibodies were not found in relation to: gender or age, acute or active opportunistic infections, and clinical signs of HIV-related encephalopathy or radiological features suggesting cerebral vasculopathy. Patients with a history of thrombotic or thromboembolic events or thrombopenia had no higher levels of serum autoantibodies (all P > 0.2, Mann–Whitney U-test) except for IgM APSA, where a trend towards higher concentrations was found in thrombopenic subjects (P = 0.06).
Considering the association of survival in individuals with severe immunodeficiency (CD4+ T lymphocytes < 0.2/nl), those with positive IgG APSA tended to have a longer survival compared with IgG APSA− subjects. The difference between these groups, however, was found to be not statistically significant (P = 0.106, log-rank test; Fig. 2). Survival time was not affected by the presence of any other autoantibody determined in this study (all P > 0.20, log-rank test).
Fig. 2.
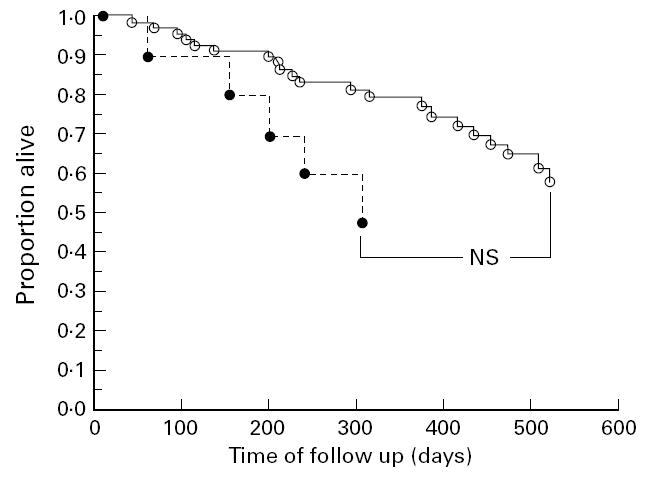
Survival estimations comparing IgG anti-phosphatidylserine antibody (APSA)+ (○) and IgG APSA− (•) individuals. Individuals with severe immunodeficiency (CD4+ T lymphocytes < 0.2/nl) and positive APSA IgG tended to have a longer survival compared with subjects with severe immunodeficiency, but negative APSA IgG (P = 0.106, log-rank test).
Cellular immunology
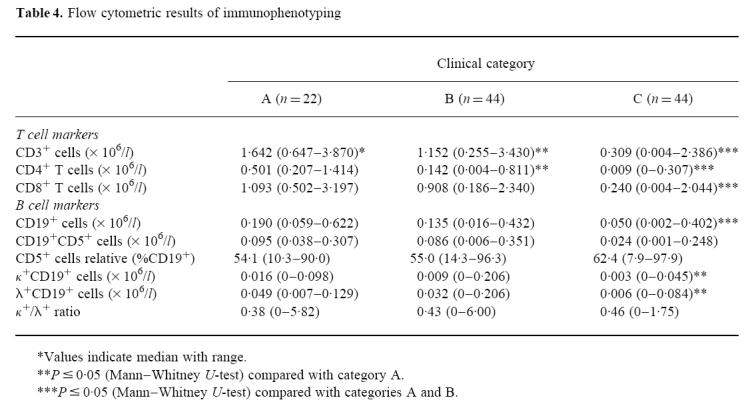
As expected, progression in clinical symptoms was accompanied by a decrease in lymphocyte and T lymphocyte numbers (Table 4). Also, B lymphocyte counts were reduced in advanced HIV infection. CD5 antigen-positive B lymphocytes showed a proportional increase relative to all B lymphocytes in all three patient groups compared with levels from healthy subjects. A decrease in absolute numbers similar to the total B lymphocyte population with advanced clinical stage of HIV infection was also detected. No significant difference was seen regarding gender, age, risk for the acquisition of HIV infection or full-blown AIDS. Immunoglobulin light chain expression on B cells showed no differences between either group, male or female gender, different risk groups and clinical AIDS versus non-AIDS.
Table 4.
Flow cytometric results of immunophenotyping
CD5+ B lymphocytes were inversely correlated with absolute CD4+ T lymphocyte counts (r2 = − 0.18, P = 0.037; Fig. 3) and the immune complex-dissociated HIV p24 antigen in serum (r2 =− 0.19, P = 0.029), whereas no correlation was found with other surrogate markers like serum neopterin, β2-microglobulin (P > 0.2), and IgA (all P values > 0.2).
Fig. 3.
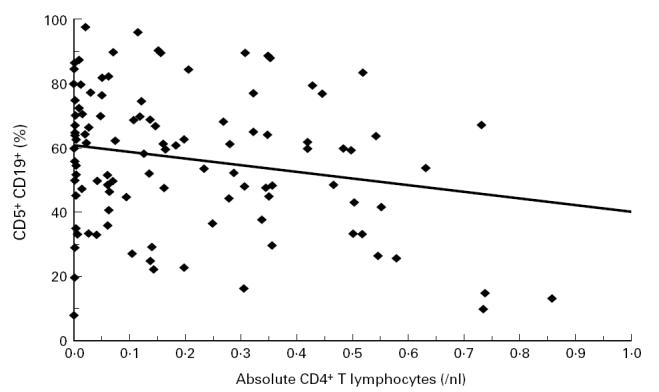
Correlation of CD4+ T lymphocytes with the proportion of B1 lymphocytes in the peripheral blood. CD5+ B lymphocytes were inversely correlated with absolute CD4+ T lymphocyte counts (r2 = − 0.18, P = 0.037) and immune-complex-dissociated HIV p24 antigen in serum (r2 = − 0.19, P = 0.029).
Individuals with a high proportion of CD5+ B lymphocytes and only moderate CD4+ T lymphocyte depletion tended to have a longer survival than those with CD5+ B lymphocytes < 50% of all B lymphocytes (Fig. 4; log-rank test; the difference was not statistically significant: P = 0.087).
Fig. 4.
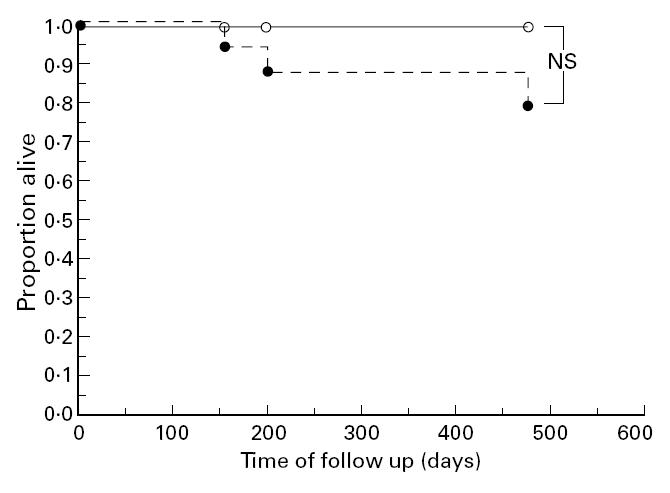
Survival in individuals with immunological stages 2 and 3 compared according to the proportions of CD5+ B lymphocytes. Individuals with a high proportion of CD5+ B lymphocytes and only moderate CD4+ T lymphocyte depletion (○) tended to have a longer survival than those with CD5+ B lymphocytes < 50% of all B lymphocytes (•) (P = 0.087, log-rank test).
Comparisons of cellular and humoral markers of autoimmunity
No correlation could be detected between the absolute or relative number of B lymphocytes or CD19+CD5+ lymphocytes (B1 cells) and ACLA and APSA concentrations. Symptomatic individuals (clinical categories B and C) with positive results for ACLA and APSA IgG testing had lower levels of CD5+ B lymphocytes (P = 0.028, Mann–Whitney U-test). Similar data were obtained in individuals with severe immunodeficiency (CD4+ T lymphocytes < 0.2/nl).
DISCUSSION
Antibodies against cardiolipin and phosphatidylserine are known to be associated with certain clinical conditions including autoimmune diseases like systemic lupus erythematosus [29], thromboembolic events [30], recurrent fetal loss [13], and infectious disease states. Their occurrence in the setting of HIV infection was noted early in the course of the epidemic [18,31,32]. In our work we have detected in almost all HIV-infected individuals at least one variety of anti-phospholipid antibody. The presence of these autoantibodies was not accompanied by evidence of polyclonal B cell stimulation or production of non-specific antibodies such as antinuclear antibodies. Therefore, it appears that anti-phospholipid antibodies are of significance in HIV infection. Anti-phospholipid antibodies may well play a role in HIV-related immunopathogenesis: in the setting of HIV infection an increased rate of spontaneous apoptosis in peripheral blood lymphocytes is reported [33]. Initial events that characterize lymphocyte apoptosis are alterations of the mitochondrial function such as changes of mitochondrial transmembrane potential and increased mitochondrial generation of superoxide anion [34]. This is found in a significant proportion of peripheral lymphocytes in HIV-infected subjects. Furthermore, the same group reported a structural defect of the cardiolipin-containing inner mitochondrial membrane in lymphocytes of HIV-infected individuals. Structurally altered mitochondrial phospholipids are transported by a phospholipid flippase/translocase mechanism [35,36] to the cell surface and could then act as new antigens which induce humoral immune response in the HIV-infected host who already presents with polyclonal B cell activation. This is interesting because the transporter protein belongs to the mdr gene products which encode P-glycoproteins. These proteins are up-regulated in cells which are infected with HIV-1 in vitro [37] and in the presence of opportunistic pathogens in chronically infected cell lines [38]. This hypothesis is strongly supported by results recently published by Silvestris et al. [39], who demonstrated a clear association between the occurrence of APSA and apoptosis in HIV-infected individuals. Additionally, this group also reported an augmented antibody-dependent cell-mediated cytotoxicity (ADCC) of autologous macrophages using IgG APSA compared with non specific IgG, which could increase the clearance of apoptotic T cells.
With regard to the prognostic significance of our results, we found in contrast to Coll Daroca et al. [25] significantly less positive ACLA and APSA in patients with full-blown AIDS. This is also accompanied by a statistical trend for better survival comparing IgG APSA+ with IgG APSA− individuals. Therefore, we hypothesize that the disappearance of ACLA and APSA may reflect the increasing immunodeficiency with loss of T helper cell type 2 functions resulting in a progressive B cell immunodeficiency in the late course of HIV disease. Furthermore, the presence of anti-phospholipid autoantibodies was not associated with thromboembolic diseases or thrombocytopenia, nor was there an increased incidence of other non-specific autoantibodies. In our study, patients with positive ACLA or APSA did not have a higher risk for the development of thromboembolic events or thrombocytopenia. This finding is in accordance with the data published by Weiss et al. [32]. A previously published paper suggests an association between cerebrovascular perfusion deficits as detected by 99mTc-HMPAO SPECT and anti-phospholipid antibodies [40]. In our study 99 patients had cerebral imaging studies (computed tomographic or magnetic resonance imaging scans) within 3 months from the determination of ACLA and APSA. However, these imaging techniques did not reveal an association of microangiopathy with antibodies. Neither was there a correlation to the presence of clinical symptoms of encephalopathy and the occurrence of autoantibodies.
In this study we have found significantly higher antibody levels in i.v. drug users compared with homosexual patients. This could be due to a stronger activation of endothelial tissues by drug injection, the high incidence of bloodstream infections as well as the higher amount of antigen presented to peripheral blood cells in this risk group. All factors may lead to B cell activation with augmented production of ACLA and APSA. Biological false-positive syphilis serology was only rarely seen in our population and reached an incidence of 0.9%. Similar data were recently published in a large retrospective analysis which showed no association with ACLA [41].
The pathogenicity of ACLA and APSA is probably mediated by the interaction of the antibodies with membrane phospholipids or phospholipid-bound cofactors (e.g. apolipoprotein H) from endothelial cells or platelets resulting in increased release of platelet-activating factor, interference with phospholipid-dependent anticoagulant factors (protein S, protein C) and imbalance in the amount of arachidonic acid metabolites. Thus, cofactor binding may be the crucial point in the pathogenesis of the anti-phospholipid antibody syndrome-related clinical manifestations [42,43]. In infectious diseases, including HIV infection, detectable anti-phospholipid antibodies have been shown to be apolipoprotein H-independent [44–46]. This fact may explain the lack of association of clinical symptoms with the detection of ACLA and APSA in HIV-infected individuals. On the other hand, the determination of both subtypes (ACLA and APSA) enabled us to show positivity for both cofactor-dependent or -enhanced (ACLA) and cofactor-independent (APSA) antibodies.
Elevated proportions of B1 lymphocytes expressing the CD5 antigen have been documented in the peripheral blood of HIV-infected individuals [47,48]. These cells are thought to be involved in autoantibody production [49,50] and may represent a part of the natural immune system [51]. However, the prognostic or clinical significance in the setting of HIV infection has not been evaluated so far. In this study, no statistically significant correlation or association of CD5+ B lymphocytes with ACLA and APSA, clinical disease or progression of HIV infection was detected. The observed (i) weak inverse correlation to the levels of CD4+ T lymphocytes and the HIV core antigen serum, and (ii) tendency of a better survival of patients with mild to moderate immunosuppression if CD5+ B lymphocytes comprise the lesser part of the B cell are both of limited value in the clinical assessment of HIV infection.
We conclude that anti-phospholipid antibodies are frequently observed in HIV-infected individuals. These antibodies may be involved in immunopathogenic events during HIV-induced lymphocyte apoptosis and may reflect loss of B cell functionality in the course of the disease. There is no correlation between CD5+ cells and ACLA/APSA or clinical disease.
Acknowledgments
The authors wish to thank Mrs B. Dominik, Mrs U. Gläser and Mrs M. Götze for their expert technical assistance. We thank Alan G. McBride (Cambridge, UK) for critical reading of the manuscript.
References
- 1.Pantaleo G, Fauci AC. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;15:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 2.Lane CH, Masur H, Edgar LC, Wahlen G, Rook G, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 3.Pahwa S, Pahwa R, Saxinger C, Gallo RC, Good RA. Influences of human T lymphototropic virus/lymphdenopathy associated virus on human lymphocytes: evidence of polyclonal B-cell activation by banded viral preparations. Proc Natl Acad Sci USA. 1985;82:8198–202. doi: 10.1073/pnas.82.23.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnittman SM, Lane CH, Higgins S, Folks T, Fauci AS. Direct polyclonal activation of human B lymphocytes by acquired immunodeficiency syndrome virus. Science. 1986;233:1084–6. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- 5.Chirmule N, Oyaizu N, Saxinger C, Pahwa S. Nef protein of HIV-1 has B-cell stimulatory activity. AIDS. 1994;8:733–9. doi: 10.1097/00002030-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Yarchoan R, Redfield RR, Broder S. Mechanisms of B cell activation in patients with acquired immunodeficiency syndrome and related disorders. Contribution of antibody-producing B cells, of Epstein-Barr virus-infected B cells, and of immunoglobulin production induced by human T cell lymphotropic virus type III/lymphadenopathy associated virus. J Clin Invest. 1986;78:439–47. doi: 10.1172/JCI112595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnabend J, Witkin SS, Purtillo DT. Acquired immunodefiency syndrome, opportunistic infections, and malignancies in male homosexuals, A hypothesis of etiologic factors in pathogenesis. JAMA. 1983;249:2370–4. [PubMed] [Google Scholar]
- 8.Muller S, Richalet P, Laurent-Crawford A, et al. Autoantibodies typical of non-organ-specific autoimmune diseases in HIV-seropositive patients. AIDS. 1992;6:933–42. doi: 10.1097/00002030-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Viard JP, Chabre H, Bach JF. Autoantibodies to nucleosomes in HIV-1-infected patients. J AIDS. 1994;7:1286–7. doi: 10.1097/00126334-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Pinching AJ. Antibody responses in HIV infection. Clin Exp Immunol. 1991;84:181–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Asherson RA, Harris EN. Anticardiolipin antibodies-clinical associations. Postgrad Med J. 1986;62:1081–7. doi: 10.1136/pgmj.62.734.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes GRV, Asherson RA, Khamashta MA. Antiphospholipid syndrome-linking many specialities. Ann Rheum Dis. 1989;48:355–6. doi: 10.1136/ard.48.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematodes (SLE) and in non-SLE disorders. Ann Intern Med. 1990;112:692–8. doi: 10.7326/0003-4819-112-9-682. [DOI] [PubMed] [Google Scholar]
- 14.Sammaritano LR, Gharavi AE, Lockshin MD. Antiphospholipid antibody syndrome: immunologic and clinical aspects. Sem Arthritis Rheum. 1990;20:81–96. doi: 10.1016/0049-0172(90)90021-7. [DOI] [PubMed] [Google Scholar]
- 15.Mackworth-Young CG, Loizou S, Walport MJ. Antiphospholipid antibodies and disease. Quart J Med. 1989;269:767–77. [PubMed] [Google Scholar]
- 16.Thomas MA, Frampton G, Isenberg DA, Shoenfeld Y, Akinsola A, Ramzy M, Lilleywhite J, Williams DG. A common anti-DNA antibody isotype and antiphospholipid antibodies in sera from patients with schistosomiasis and filariasis with and without nephritis. J Autoimmun. 1989;2:803–11. doi: 10.1016/0896-8411(89)90006-1. [DOI] [PubMed] [Google Scholar]
- 17.Snowden M, Wilson PB, Longson M, Pumphrey RSH. Antiphospholipid antibodies and Mycoplasma pneumoniae infection. Postgrad Med J. 1990;66:356–62. doi: 10.1136/pgmj.66.775.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stimmler MM, Quismorio FP, McGehee WH, Boylen T, Sharma OP. Anticardiolipin antibodies in acquired immunodeficiency syndrome. Arch Int Med. 1989;149:1833–5. [PubMed] [Google Scholar]
- 19.Becker H, Weber C, Storch S, Federlin K. Relationship between CD5+ B lymphocytes and the activity of systemic autoimmunity. Clin Immunol Immunopathol. 1990;56:219–25. doi: 10.1016/0090-1229(90)90143-e. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder HW, Dighiero W. The pathogenesis of chronic lymphocytic leukemia: analysis of the antibody repertoire. Immunol Today. 1994;15:288–94. doi: 10.1016/0167-5699(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 21.Maini RN, Plater-Zyberk C, Andrew E. Autoimmunity in rheumatoid arthritis. An approach via a study of B lymphocytes. Rheum Dis Clin North Am. 1987;13:319–38. [PubMed] [Google Scholar]
- 22.Dauphinee M, Tovar Z, Talal N. B cells expressing CD5 are increased in Sjögrens's syndrome. Arthritis Rheum. 1988;31:642–7. doi: 10.1002/art.1780310509. [DOI] [PubMed] [Google Scholar]
- 23.Smerdon RA, Peakman M, Hussain MF, Wong FS, Watkins PJ, Leslie RD, Vergani D. CD5+ B-cells at the onset of type 1 diabetes and in the prediabetic period. Diabetes Care. 1994;17:657–64. doi: 10.2337/diacare.17.7.657. [DOI] [PubMed] [Google Scholar]
- 24.Capel P, Janssens A, Clumeck N, Gerard M, Feremans W, Vandevelde D, Fondu P. Anticardiolipin antibodies (ACA) are most often not associated with lupus-like anticoagulant (LLAC) in human immunodeficiency virus (HIV) infection. Am J Hematol. 1991;37:234–8. doi: 10.1002/ajh.2830370404. [DOI] [PubMed] [Google Scholar]
- 25.Coll Daroca J, Gutiérrez-Cebollada J, Yazbeck H, Bergés A, Rubié-Prat J. Anticardiolipin antibodies and acquired immunodeficiency syndrome: prognostic marker or association with HIV infection? Infection. 1992;20:140–2. doi: 10.1007/BF01704601. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 1994 revised guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus (HIV) infection. MMWR Morb Mortal Wkly Rep. 1994;43(RR-3):1–21. [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–65. [Google Scholar]
- 28.Centers for Disease Control and Prevention. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 29.Buttgereit F, Grünewald T, Schüler-Maué W, Burmester GR, Hiepe F. Value of anticardiolipin antibodies for monitoring disease activity in systemic lupus erythematosus and other rheumatic diseases. Clin Rheumatol. 1997;16:562–9. doi: 10.1007/BF02247796. [DOI] [PubMed] [Google Scholar]
- 30.Alarcon-Segovia D. Clinical manifestations of the antiphospholipid syndrome. J Rheumatol. 1992;19:1778–81. [PubMed] [Google Scholar]
- 31.Canoso RT, Zon LI, Groopman JE. Anticardiolipin antibodies associated with HTLV-III infection. Br J Jaematol. 1987;65:495–8. doi: 10.1111/j.1365-2141.1987.tb04157.x. [DOI] [PubMed] [Google Scholar]
- 32.Weiss L, You JF, Giral P, Alhenc-Gelas M, Senger D, Kazatchkine MD. Anti-cardiolipin antibodies are associated with anti-endothelial cell antibodies but not with anti-beta 2 glycoprotein I antibodies in HIV infection. Clin Immunol Immunopathol. 1995;77:69–74. doi: 10.1016/0090-1229(95)90138-8. [DOI] [PubMed] [Google Scholar]
- 33.Oyaizu N, McCloskey TW, Than S, Hu R, Pahwa S. Mechanisms of apoptosis in peripheral blood mononuclear cells of HIV-infected individuals. Adv Exp Biol Med. 1995;374:101–14. doi: 10.1007/978-1-4615-1995-9_9. [DOI] [PubMed] [Google Scholar]
- 34.Macho A, Castedo M, Marchetti P, et al. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus-1 carriers. Blood. 1995;86:2481–7. [PubMed] [Google Scholar]
- 35.Devaux PF. Protein involvement in transmembrane lipid asymmetry. Ann Rev Biophys Biomol Struct. 1992;21:417–39. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- 36.Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994;77:1071–81. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 37.Gollapudi S, Gupta S. Human immunodeficiency virus 1-induced expression of P-glycoprotein. Biochem Biophys Res Commun. 1990;171:1002–7. doi: 10.1016/0006-291x(90)90783-j. [DOI] [PubMed] [Google Scholar]
- 38.Gollapudi S, Gupta S. Mycobacterium tuberculosis induces expression of P-glycoprotein in promonocytic U1 cells chronically infected with HIV-1. Biochem Biophys Res Commun. 1994;199:1181–7. doi: 10.1006/bbrc.1994.1355. [DOI] [PubMed] [Google Scholar]
- 39.Silvestris F, Frassanito MA, Cafforio P, et al. Antiphosphatidylserine antibodies in human immunodeficiency virus-1 patients with evidence of T-cell apoptosis and mediate antibody-dependent cellular cytotoxity. Blood. 1996;87:5185–95. [PubMed] [Google Scholar]
- 40.Rubbert A, Bock D, Schwab J, Marienhagen J, Nuesslein H, Wolf F, Kalden JR. Anticardiolipin antibodies in HIV infection: association with cerebral perfusion defects as detected by 99mRc-HMPAO SPECT. Clin Exp Immunol. 1994;98:361–8. doi: 10.1111/j.1365-2249.1994.tb05498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rusnak JM, Butzin C, McGlasson D, Blatt SP. False-positive rapid plasma reagin tests in human immunodeficiency virus infection and relationship to anticardiolipin antibody and serum immunoglobulin levels. J Infect Dis. 1994;169:1356–9. doi: 10.1093/infdis/169.6.1356. [DOI] [PubMed] [Google Scholar]
- 42.Chamley LW, McKay EJ, Patterson NS. Cofactor dependent and cofactor independent anticardiolipin antibodies. Thromb Res. 1991;61:291–9. doi: 10.1016/0049-3848(91)90106-7. [DOI] [PubMed] [Google Scholar]
- 43.Bevers EM, Galli M. Cofactors involved in the antiphospholipid syndrome. Lupus. 1992;1:51–53. doi: 10.1177/096120339200100201. [DOI] [PubMed] [Google Scholar]
- 44.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Suzuki T, Sumida T, Yasuda T, Koike T. Heterogeneity of anticardiolipin antibodies defined by the anticardiolipin cofactor. J Immunol. 1992;148:3885–91. [PubMed] [Google Scholar]
- 45.Hunt JE, NcNeil HP, Morgan GJ, Crameri RM, Krilis SA. A phospholipid-beta-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not in infection. Lupus. 1992;1:75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 46.Saitoh N, Tsukamoto M, Gohchi K, Asami K, Hashimoto M. Measurement of β2-glycoprotein I (apolipoprotein H)-independent anticardiolipin antibodies in HIV 1-positive and virus 1-negative hemophiliacs. Am J Hematol. 1993;43:146–8. doi: 10.1002/ajh.2830430215. [DOI] [PubMed] [Google Scholar]
- 47.Sampolo A, López-Gómez M, Jiménez-Alonso J, Ortiz F, Samaniego F, Garrido F. CD5+ B lymphocytes in HIV infection: relationship to immunological progression of disease. Clin Immunol Immunopathol. 1993;66:260–8. doi: 10.1006/clin.1993.1034. [DOI] [PubMed] [Google Scholar]
- 48.Vuillier F, Scott-Algarra D, Tortevoye P, Pialoux G, Dighiero G. Serial study of CD5+ and CD5- B cell subpopulations in 335 HIV seropositive patients. Clin Exp Immunol. 1991;85:476–80. doi: 10.1111/j.1365-2249.1991.tb05752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B cell repertoire. Immunol Today. 1989;10:364–8. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 50.Casali P, Notkins AL. Probing the human B cell repertoire with EBV-polyreactive antibodies and CD5+ B lymphocytes. Ann Rev Immunol. 1989;7:513–35. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 51.Hayakawa K, Hardy R. Normal, autoimmune and malignant CD5+ B cells–the Ly-1+ B lineage? Ann Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]


