Abstract
The aim of this work was to study the expression of β1- and β2-integrins, CR1, CD44 and Fcγ receptors on peripheral blood monocytes in RA. The expression of these receptors was measured by flow cytometry, before and after treatment with low-dose prednisolone. Expression of the same receptors was also measured before and after treatment with metyrapone, a substance that inhibits the synthesis of cortisol in the adrenals. The expression of the β2-integrins CD11a, CD11b and CD18, of CD35 (CR1), and of FcγRII and FcγRI (CD32 and CD64) on monocytes was elevated in the RA patients compared with healthy controls, while the expression of the β1-integrins (CD29, CD49d, CD49f) was unaffected. A significant correlation between monocyte expression of CD64 and C-reactive protein (CRP), and blood platelet count, respectively, was found in the group of patients with RA. After 4–6 weeks of treatment with low-dose prednisolone, the expression on the monocytes of CD11a, CD11b, CD18, CD35, CD32 and CD64 was normalized. A significant correlation (r = 0.64, P = 0.02) was found between the decrease in expression of CD11b and clinical improvement after prednisolone treatment. Two days of metyrapone treatment, which significantly lowered the serum cortisol levels, elevated the expression of CD35 and CD49f. Priming of peripheral monocytes seems to be one of the mechanisms behind the recruitment of monocytes to the rheumatoid synovium. One reason for the good clinical effects of prednisolone in RA could be a down-regulation of adhesion and phagocytosis receptors on monocytes.
Keywords: rheumatoid arthritis, monocytes, integrins, prednisolone, metyrapone
INTRODUCTION
RA is a chronic polyarthritic inflammatory disease of unknown aetiology. Macrophages, plasma cells and lymphocytes are the most prominent cells in the rheumatoid synovium. The macrophages are thought to play a central role in the destruction of cartilage and bone in the rheumatoid joints [1,2]. The macrophages are the most important source of cytokines in the rheumatoid joint [3]. The cytokines found in the joints are mostly of the proinflammatory type and derived from macrophages and fibroblasts, i.e. IL-1, IL-6, tumour necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β) [3]. Cytokines from T lymphocytes are either detected at low levels or not at all. Blood monocytes and their adhesion to endothelial cells are important for the recruitment of macrophages to the synovial membrane [1].
The adhesion of monocytes is considered to be mediated by three types of adhesion molecules: the selectins, the integrins and the immunoglobulin superfamily [4–6]. The first step of adhesion, called rolling, is a reversible binding of the leucocytes to the endothelium and is mediated by the selectins, l-selectin on the leucocytes and E- and P-selectin on the endothelial cells. During the rolling the leucocytes become activated and the expression of the integrins is up-regulated. The integrins are glycoproteins consisting of non-covalently associated chains, an α- and a β-chain. The integrins are divided into different groups dependent on different β-chains. The ligands for the integrins may be cell receptors or extracellular matrix proteins. Individual integrins can bind to more than one ligand [4]. Adhesion molecules of the immunoglobulin superfamily are cellular ligands to the integrins.
Blood monocytes express two types of Fcγ receptors, FcγRI (CD64), the high-affinity receptor that binds monomeric IgG, and FcγRII (CD32) the low-affinity receptor that binds IgG complexes. These receptors are important for the phagocytosis, the respiratory burst and secretion [7]. Two CR, CR1 (CD35) and CR3 (CD11b/CD18), are expressed on monocytes. The CR and the Fcγ receptors co-operate in the facilitation of phagocytosis. The CR mediate the binding and the Fcγ receptors the internalization [7].
Glucocorticoids have been used in the treatment of RA for five decades [8] as effective anti-inflammatory agents with possible disease-modifying activity [9]. Glucocorticoids exert at least part of their action at the molecular level by binding to specific receptors in the cytoplasm and then migrating to the nucleus where they bind to selective regulatory sites on DNA. This can result in increased or decreased expression of genes important for the inflammatory process [10]. The glucocorticoid–receptor complex can also interact with AP-1 transcriptional factor. This binding abolishes AP-1 transcriptional activity, probably by preventing it from entering the nucleus [11]. By these mechanisms the glucocorticoids inhibit the transcription of many cytokines, i.e. IL-1, TNF-α and IL-6. The influence of corticosteroids on the monocyte expression of adhesion molecules and phagocytosis receptors in RA has not been studied previously.
The aim of the present study was to investigate the integrin and phagocytosis receptor expression on peripheral blood monocytes in patients with RA and to study the effects of endogenous and exogenous corticosteroids on the expression of these receptors.
SUBJECT AND METHODS
Patients
Twenty-two patients with definite RA (17 women, five men, mean age 61 years, range 29–86 years) according to the ARA criteria [12] were included in the study. The mean duration of disease was 63 months (range 1 month to 43 years). Ten of these patients with definite RA and another three patients with seronegative polyarthritis not fulfilling the criteria for definite RA (three men, mean age 65 years and a mean disease duration of 10 years) were treated with prednisolone; the initial dose of 5–15 mg/day was decreased to 5–7.5 mg/day during the first 2 weeks of treatment. These patients were also treated with second line drugs: chloroquine 250 mg × 1 (n = 6), sulphasalazine 1 g × 2 (n = 2), methotrexate 7.5 mg/week (n = 1) and oral gold 3 mg × 2 (n = 1). Blood samples were collected before the start of the treatment and after 4–6 weeks of treatment. All blood samples were drawn before 10.00 a.m. None of the patients had received glucocorticoid treatment for the past 3 months prior to inclusion in the study and none of the patients had been treated with second line drugs. Most of the patients were treated with non-steroidal anti-inflammatory drugs (NSAIDs).
Eight of the patients with definite RA and another three patients with other inflammatory arthropathies than RA were treated as in-patients with metyrapone for 2 days. Metyrapone inhibits the activity of the enzyme 11β-hydroxylase in the adrenal cortex and thus decreases the synthesis of cortisol. On day 1, oral doses of metyrapone of 750 mg were given at 6.00 a.m., 12.00 a.m., 6.00 p.m. and 12.00 p.m. The first dose of 750 mg on day 2 was given at 6.00 a.m. Blood samples were collected the day before the start of metyrapone treatment and on the second day of metyrapone treatment. All blood samples were drawn at 7.30 a.m. All patients were on NSAIDs, but none had received glucocorticoid treatment for the past 3 months before inclusion in the study. One of the patients was on treatment with sulphasalazine 500 mg × 1.
The patients were assessed according to the Thompson Index [13] of joint inflammation and to the duration of early morning stiffness. The blood samples were analysed for the following: haemoglobin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), leucocyte count, monocyte count and platelet count. The patients were informed according to the Declaration of Helsinki and the study was accepted by the local ethics committee.
Controls
Fifteen healthy female and six healthy male individuals served as controls. Their mean age was 48 years (range 26–65 years). None of the controls had any symptoms of infection or any rheumatic disease.
Preparation of leucocytes
Leucocytes were labelled with MoAbs to cell surface receptors using a method described earlier [14]. Venous blood (2 ml) treated with heparin and processed within 2 h was diluted with 10 ml PBS–citrate buffer with 2% new born calf serum (NBS) and centrifuged for 5 min at 160 g. The supernatant was removed and the remaining 2 ml were incubated for 4 min at 37°C with an equal volume of 0.4% paraformaldehyde to fixate the blood cells. The blood sample was then incubated with 0.83% NH4Cl in 0.01 m Tris–HCl buffer pH 7.4 for 15 min at 37°C to haemolyse the erythrocytes. The cells were then centrifuged for 5 min at 160 g and the supernatant and the erythrocytes were removed, and the remaining leucocytes were then washed twice with PBS–citrate buffer. The cells were diluted with 0.5 ml PBS–citrate–0.2% NBS and counted. The concentration of the granulocytes was adjusted to 1.7–2.5 × 106/ml. The cells were incubated on ice with the following FITC-conjugated antibodies for 30 min: control antibodies for IgG1, IgG2a and IgG2b (Dako, Glostrup, Denmark) and CD11a, CD14, CD16, CD18, CD29 (Dako), CD11b, CD35, CD44, CD49d, CD64 (Immunotech, Marseille, France), CD32 (Medarex, Annandale, NY) and CD49f (Serotec, Raleigh, NC). Then the cells were washed twice with PBS and the samples were analysed by a Coulter Flow Cytometer (Coulter Co. Inc., Hialeah, FL).
Flow cytometric analysis
Monocytes were gated on the basis of their forward scatter and side scatter pattern and checked by staining with anti-CD14. The control antibodies were used to set the background levels. The relative number of positive monocytes and the mean fluorescence intensity (MFI) for each antibody were measured.
Statistical analysis
Values are given as medians and interquartile ranges. Non-parametric tests, Mann–Whitney U-test, Wilcoxon matched pair test and the Spearman rank correlation coefficient were used to analyse the data. P < 0.05 was considered significant. For statistical calculation the software Statistica (Stat. Soft. Inc., Tulsa, OK) was used.
RESULTS
Monocyte cell surface expression
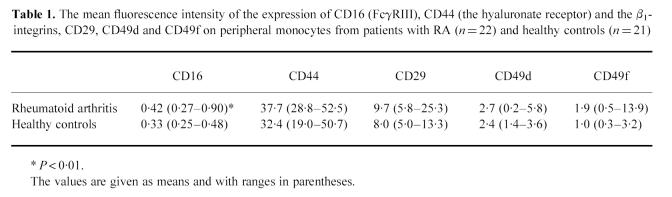
Figure 1a shows the expression of the β2-integrins on monocytes from the RA patients compared with the healthy controls. The expression of CD11a (LFA-1), CD11b (Mac-1, CR-3) and CD18 (the common β2-chain) were all up-regulated in the RA group (P < 0.0001, P < 0.0001, P < 0.001, respectively). The expression of CD35 (CR1) on monocytes was increased (P < 0.0001) in the patient group (Fig. 1b). The expression of the two phagocytosis receptors expressed on the monocytes, CD32 (FcγRII) and CD64 (FcγRI), was also elevated (P = 0.001, P < 0.05, respectively) on monocytes from the patients (Fig. 1b). The relative number (%) of CD16 (FcγRIII)-positive monocytes was the same (mean 11.3%) in both groups. The expression of CD16 on monocytes from the patients, measured as MFI, was, however, higher than that of control monocytes, although at a very low level (Table 1, P < 0.01). Monocyte expression of the β1-integrins and CD44 (hyaluronan receptor) was not significantly different between patients and controls (Table 1).
Fig. 1.
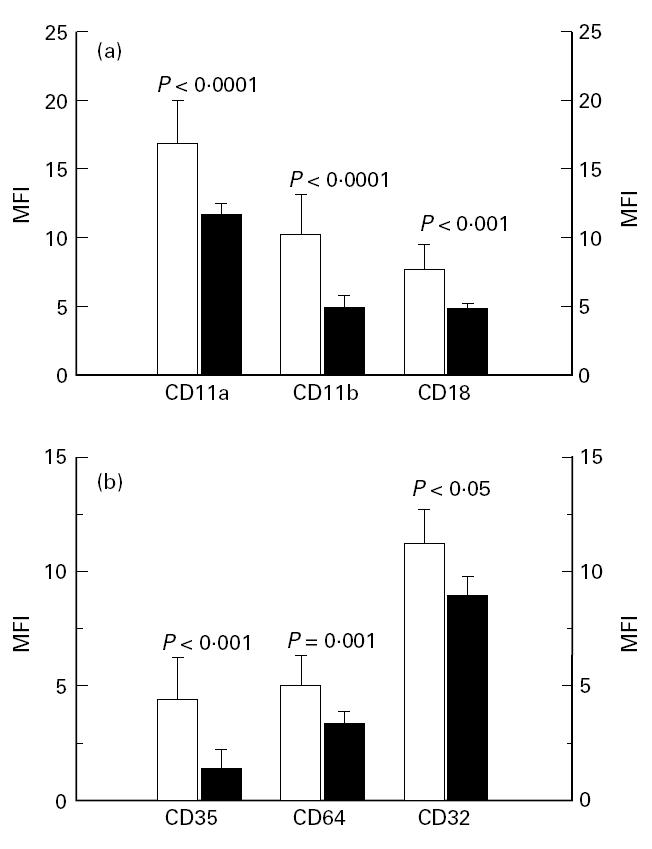
(a) Expression of the β2-integrins CD11a, CD11b and CD18 on monocytes from peripheral blood of 22 patients with definite RA (□) in comparison with 21 healthy controls (▪). The analyses were done by flow cytometry using specific FITC-conjugated MoAbs. The medians and upper quartiles are shown. (b) Expression of complement receptor 1 (CR1, CD35) and FcγRI and FcγRII (CD64, CD32, respectively) on monocytes from peripheral blood of 22 patients with RA (□) in comparison with 21 healthy controls (▪). The analyses were done by flow cytometry using specific FITC-conjugated MoAbs. The medians and upper quartiles are shown. MFI, Mean fluorescence intensity.
Table 1.
The mean fluorescence intensity of the expression of CD16 (FcγRIII), CD44 (the hyaluronate receptor) and the β1-integrins, CD29, CD49d and CD49f on peripheral monocytes from patients with RA (n = 22) and healthy controls (n = 21)
The disease activity of the patients was defined by joint index (mean Thompson Index was 88, range 0–225) and various laboratory data; mean ESR was 42 mm/h (range 8–120 mm/h), mean CRP was 40 mg/l (≤ 10–184 mg/l), mean leucocyte count was 8.0 × 109/l (range 4.3 × 109–18.1 × 109/l) and mean platelet count was 366 × 109/l (range 179 × 109–896 × 109/l).
No correlation was found between joint symptoms and monocyte expression of the receptors. A significant correlation was found between the monocyte expression of CD64 and the serum level of CRP (r = 0.45, P = 0.04) and between the monocyte expression of CD64 and the blood platelet count (r = 0.53, P = 0.003). In the RA group a significant correlation was found between monocyte expression of CD11a and CD32 (r = 0.56, P = 0.02) and between CD18 and CD32 (r = 0.69, P = 0.002). These correlations were not seen in the group of healthy controls.
The effect of prednisolone treatment on monocyte cell surface expression
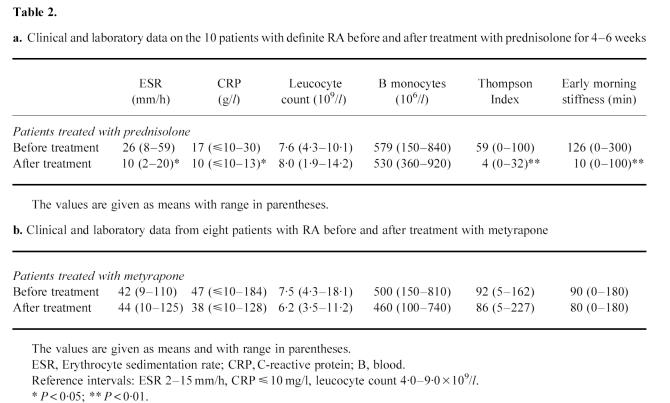
Clinical and laboratory data on 10 patients with definite RA before and after treatment with low-dose prednisolone are shown in Table 2a. The patients improved clinically measured by the Thompson Index and early morning stiffness and laboratory indices of inflammatory activity. After 4–6 weeks of low-dose prednisolone treatment a decreased monocyte expression of the α-chains, CD11a and CD11b, of β2-integrins (P < 0.05, P < 0.01, respectively) was seen (Fig. 2a) as well as a diminished monocyte expression of the common β2-chain (CD18) and of CD35 (P < 0.05, P < 0.001, respectively; Fig. 2b). The expression of α-chains of the β1-integrins CD49d and CD49f also diminished significantly (P < 0.05 and P = 0.05, respectively) after treatment with prednisolone (Fig. 3a). The monocytes also expressed a lower number of Fcγ receptors, CD64 and CD32, after treatment with prednisolone (P < 0.01, P < 0.05, respectively; Fig. 3b). In Figs 2 and 3 the patients with seronegative polyarthritis are also shown. These patients showed the same pattern with lower monocyte expression of measured surface markers after treatment with prednisolone. The monocyte expression of the common β1-chain (CD29) and the hyaluronan receptor (CD44) was not influenced by prednisolone treatment (results not shown).
Table 2.
a. Clinical and laboratory data on the 10 patients with definite RA before and after treatment with prednisolone for 4–6 weeks
b. Clinical and laboratory data from eight patients with RA before and after treatment with metyrapone
Fig. 2.
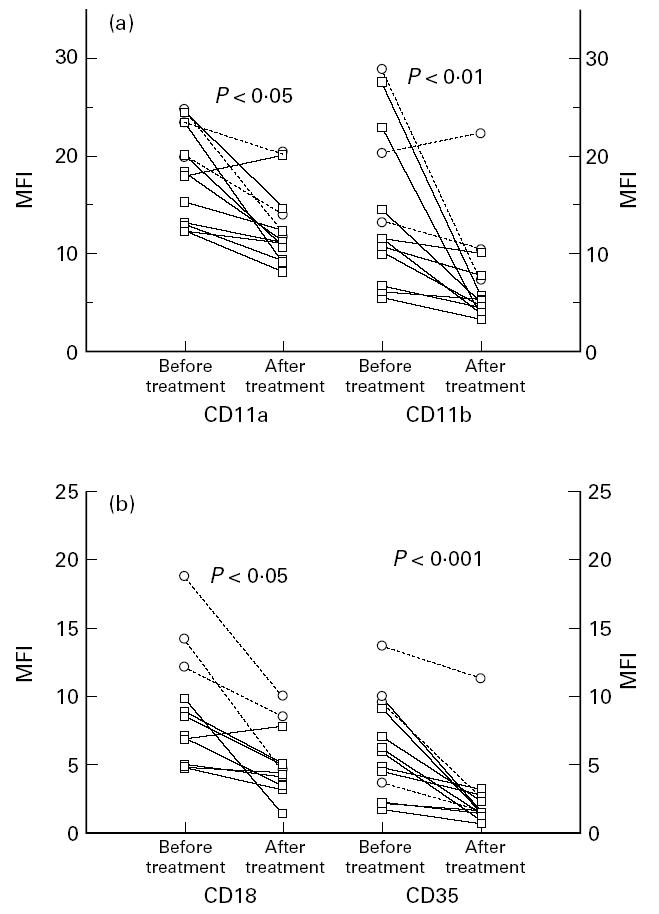
(a) Blood monocyte expression of the α-chains of the β2-integrins CD11a and CD11b in patients with definite RA (□, n = 10) and seronegative polyarthritis (○, n = 3) before and after 4–6 weeks of treatment with low-dose prednisolone. The analyses were done by flow cytometry using specific FITC-conjugated MoAbs. (b) Blood monocyte expression of the common β2-integrin chain (CD18) and the complement receptor 1 (CR1, CD35) in the same patients before and after treatment with low-dose prednisolone. The analyses were done by flow cytometry using specific FITC-conjugated MoAbs. MFI, Mean fluorescence intensity.
Fig. 3.
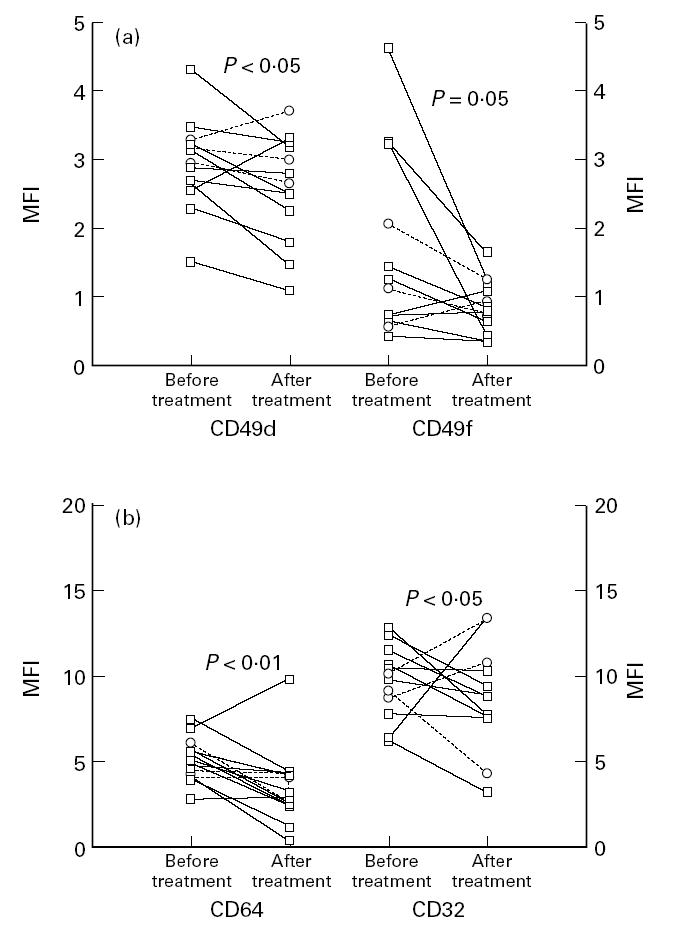
(a) Blood monocyte expression of the α-chains of the β1-integrins, CD49d and CD49f, in patients with definite RA (□, n = 10) and seronegative polyarthritis (○, n = 3) before and after 4–6 weeks on low-dose prednisolone treatment. The analyses were done by flow cytometry using specific FITC-conjugated MoAbs. (b) Blood monocyte expression of the FcγRI and FcγRII (CD64 and CD32) in the same patients before and after prednisolone treatment. The analyses were done by flow cytometry using specific FITC-conjugated MoAbs. MFI, Mean fluorescence intensity.
No difference was seen between the blood monocyte cell surface expression of the receptors measured in the patients with RA after treatment with prednisolone and the control group. A significant correlation was found between prednisolone-induced lowering of monocyte expression of CD11b and clinical improvement defined by the Thompson Index (r = 0.64, P = 0.02).
The effect of metyrapone treatment on monocyte cell surface expression
Eleven patients, eight patients with RA and three patients with polyarthritis, were treated with metyrapone. Clinical and laboratory data on the patients with RA before and after 2 days on metyrapone treatment are shown in Table 2b. The serum cortisol level at 7.30 a.m. on day 2 of metyrapone treatment was significantly lower compared with before the start of treatment (162 nmol/l compared with 438 nmol/l; P < 0.01). When the whole group of the 11 patients was considered, the expression of CD35 was elevated after metyrapone treatment compared with before treatment (P < 0.05). If the polyarthritis patients were eliminated and only the RA patients were evaluated, this change was no longer significant (P = 0.09, Fig. 4). The expression of CD49d was elevated after metyrapone treatment to a significant level in the whole group and also in the group of eight patients with RA (P < 0.05, Fig. 4). The expression of the other receptors (the β2-integrins, Fcγ receptors and the hyaluronan receptor) was not influenced by metyrapone treatment.
Fig. 4.
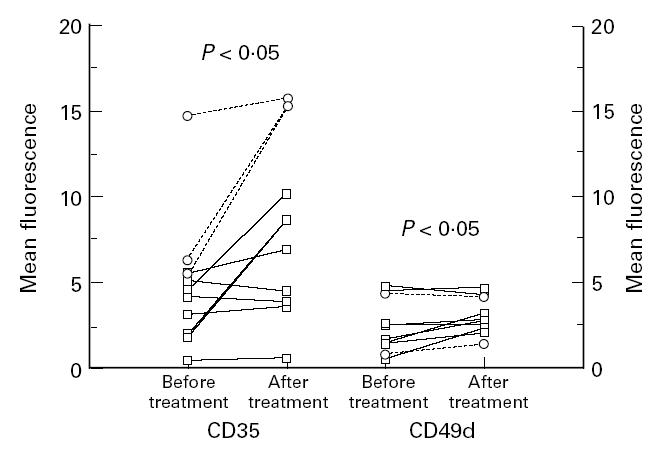
Blood monocyte expression of the complement receptor 1 (CR1, CD35) and CD49d in patients with definite RA (□, n = 8) and seronegative polyarthritis (○, n = 3) before and after 2 days on treatment with metyrapone. The analyses were done by flow cytometry using specific FITC-conjugated MoAbs.
DISCUSSION
The recruitment of monocytes from the blood to tissue macrophages and macrophage-like cell type A and C in the synovial membrane is an important part of the joint destructive process in RA [2]. The adherence of monocytes to endothelium is essential in their development to tissue macrophages [1]. In the present study signs of blood monocyte activation related to adhesion and phagocytosis were observed. Blood monocytes in patients with RA were strongly activated with high expression of the β2-integrins, CR1 and the Fcγ receptors (CD64, CD32 and CD16). Correlation between monocyte expression of CD32 and CD11a and CD18, respectively, was seen in RA. It has been reported that when leucocytes become activated in vitro, these receptors are up-regulated in parallel [15]. When the patients were treated with prednisolone for 4–6 weeks, the expression of the β1- and β2-integrins, CR1 receptor and CD64 and CD32 diminished. The prednisolone treatment normalized the monocyte expression of all the receptors investigated. Lowering of the blood cortisol level with metyrapone treatment for 2 days increased the expression of CD35 and CD49f on the peripheral monocytes.
We report elevated expression of the β2-integrins but not the β1-integrins on peripheral monocytes in RA. This is in concordance with previous results [16–20]. To our knowledge this is the first time that elevated levels of CD11a have been reported on peripheral monocytes in RA. Cytokines are thought to play a central role in the pathogenesis of RA. Their action is complex, with multiple relationships, and it is difficult to know the exact role of each cytokine. The proinflammatory cytokines are the most prominent cytokines in RA, both in the synovial fluid and blood [21]. In vitro the expression of CD11a is stimulated by IL-2, TNF-α and TNF-β, and the expression of CD11b and CD18 is stimulated by IL-2, IL-4, TNF-α and TNF-β [22]. Some of the proinflammatory cytokines, e.g. IL-6, IL-1 and TNF-α, have been measured in high concentration in the blood from patients with RA [23–25] and could in vivo stimulate the expression of the β2-integrins on the monocytes.
Blood monocytes from patients with RA have been shown to be highly adherent to cultured endothelium [20] and to exhibit increased adhesion to plastic, fibronectin and plasma [19]. The β1-integrins are very important for the adhesion of monocytes to extracellular matrix. The fact that the number of β1-integrin receptors on the monocytes from RA patients in the present study was similar to that of monocytes from healthy individuals does not necessarily mean that they are not functionally stimulated. The integrins can change their conformation from an inactive to active state without an increase in the number of receptors [4].
The expression of CD64 and CD32 was elevated and that is also in concordance with previous studies [18,26,27]. Correlation between monocyte expression of CD64 and serum levels of CRP and blood platelet count indicates a relationship between the acute-phase response and up regulation of CD64. Cytokines important for the acute-phase response are IL-6, IL-1 and TNF-α. IL-6 induces thrombocytosis and hepatic production of CRP [28]. IL-1 and TNF-α stimulate the production of CRP [29]. There are, however, no reports on the effect of IL-6, IL-1 and TNF-α on CD64 expression. Interferon-gamma (IFN-γ) and IL-10, on the other hand, induce up regulation of CD64 [30,31], but are not reported to stimulate the production of acute-phase proteins. The mechanism behind the relationship between CD64 expression and acute-phase response can thus not be concluded from present knowledge of the mentioned cytokines and their effect on monocytes. Also in concordance with previous studies was the finding that the peripheral monocytes from RA patients expressed higher levels of CR1 [17,27].
Treatment with low-dose prednisolone significantly lowered the monocyte expression of the β1- and β2-integrins, Fcγ receptors and CR. Glucocorticoids are known to suppress the inflammation process. They inhibit the transcription of many cytokines [32] and also affect the expression of adhesion molecules on different cells. Dexamethasone was shown to inhibit the TNF-α-induced expression of intercellular adhesion molecule-1 (ICAM-1) on synovial fibroblasts [33]. In vitro dexamethasone diminished the TNF-α-induced expression of ICAM-1 and E-selectin and IL-1-induced expression of ICAM-1, vascular cell adhesion molecule-1 (VCAM-1) and E-selectin [34]. In patients with multiple sclerosis treatment with 1 g methylprednisolone lowered the monocyte expression of CD11b [35]. Monocyte HLA-DR expression in multiple sclerosis patients was reduced by 50% following a single dose (500 mg) of intravenous methylprednisolone [36]. Other aspects of monocyte activation can be suppressed in vivo by glucocorticoid treatment. In healthy persons treated with a single dose prednisolone 1.5 mg/kg, the release of TNF-α by monocytes was suppressed [37]. In vitro glucocorticoids suppressed the activation-induced TNF-α release from monocyte-macrophages [38]. Treatment with glucocorticoids also diminished blood levels of IL-6 [39] and IL-8 [40].
The effect of glucocorticoids on monocyte expression of the adhesion molecules seen in the present study could be mediated by the suppressed production of cytokines or a direct effect of the glucocorticoids on the receptor expression on the monocytes. More evidence speaks for the former explanation, i.e. that down-regulation of cytokine production is involved. Another contributing mechanism could be the stimulation of the production of lipocortin-1 mediated by glucocorticoids [41]. Lipocortin-1 inhibits phospholipase A2 and thereby the production of leukotrienes, prostaglandins and platelet-activating factor [32]. The final effect of the glucocorticoid treatment in RA is, however, indicated to be the down-regulation of adhesion and phagocytosis receptors on peripheral monocytes. This can lead to less adhesiveness of the monocytes to the endothelium and consequently a lower number of macrophages in the rheumatoid synovium.
Usually the clinical effect of the most commonly used second line drugs in the treatment of RA is observed after approx. 10–12 weeks. Accordingly we chose to test the effect of prednisolone after 4–6 weeks of treatment. Six of the patients treated with prednisolone also received treatment with chloroquine. Chloroquine inhibits phagocytosis [42] and superoxide production [43] by monocytes. No reports are available on the effect of chloroquine on monocyte adhesion receptors. One patient was treated with methotrexate. Methotrexate reduces the expression of monokines and adhesion molecules in the synovial tissues in RA [44] and inhibits inflammatory cytokine release from blood mononuclear cells [45]. As the exact mechanisms of second line drugs on the rheumatoid inflammatory process are not known, it is impossible to exclude completely the possibility that the second line drugs in some way affect the receptor expression by monocytes in RA. All of the patients that were treated with prednisolone, however, became clinically improved with lower Thompson Index. Furthermore, there was a correlation between the lowering of CD11b expression and the clinical improvement as defined by change of the Thompson Index before and after prednisolone treatment.
The aim of the metyrapone study protocol was to investigate the effect of diminished serum cortisol levels on the monocyte expression of the integrin, complement and phagocytosis receptors. The monocyte expression of CD35 and CD49d was significantly higher after 2 days of metyrapone treatment. In rats that were adrenalectomized or metyrapone-treated, the adhesion and migration were enhanced compared with untreated animals [46]. This suggests that the secreted glucocorticoids can exert a suppressive effect on leucocyte–endothelial interactions. When the patients were treated with prednisolone, more pronounced changes were seen in receptor expression. The time factor could be one explanation, since the prednisolone treatment was ongoing for 4–6 weeks before a new blood sample was taken, while the patients receiving metyrapone were treated for only 2 days.
In the present study the monocytes from patients with RA showed signs of activation in the peripheral circulation concerning adhesion and phagocytosis. The priming of the blood monocytes in RA may be one of the mechanisms behind the recruitment of mononuclear cells to the rheumatoid synovium. Prednisolone seems to exert part of its effect in RA by down-regulation of the adhesion and phagocytosis receptors on monocytes, leading to diminished mononuclear cell infiltration of the synovium. The observed effects of prednisolone on monocyte activity might contribute to the understanding of possible cellular mechanisms behind the disease-modifying role of low-dose corticosteroids recently reported in RA [9].
Acknowledgments
The authors would like to thank Mrs Inger Ohlsson and Mrs Sonja Nordström and the staff of the Department of Rheumatology for the help in collecting the blood samples. This study was supported by the Agnes and Mac Rudbergs foundation, the Swedish Rheumatism Association, and the Medical Faculty of the University of Uppsala.
References
- 1.Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993;11:331–9. [PubMed] [Google Scholar]
- 2.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canete JD, Llena J, Collado A, Sanmarti R, Gaya A, Gratacos J, Blay M, Munoz-Gomez J. Comparative cytokine gene expression in synovial tissue of early rheumatoid arthritis and seronegative spondyloarthropathies. Br J Rheumatol. 1997;36:38–42. doi: 10.1093/rheumatology/36.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Mojcik CF, Shevach EM. Adhesion molecules: a rheumatologic perspective. Arthritis Rheum. 1997;40:991–1004. doi: 10.1002/art.1780400602. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 7.Ranjeny T, Wong R, Lipsky PE. Monocytes and macrophages. In: Harris ED, Kelley WN, Ruddy S, Sledge CB, editors. Textbook of rheumatology. 5. Philadelphia: W.B. Saunders Co.; 1997. pp. 128–45. [Google Scholar]
- 8.Hench P, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex and of pituitary adrenocorticothropic hormone on rheumatoid arthritis. Proc Mayo Clin. 1949;24:181–97. [PubMed] [Google Scholar]
- 9.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 1995;333:142–6. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 10.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198–208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 11.de Waal RMW. The anti-inflammatory activity of glucocorticoids. Molecular Biology Reports. 1994;19:81–88. doi: 10.1007/BF00997151. [DOI] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Thompson PW, Silman AJ, Kirwan JR, Currey HL. Articular indices of joint inflammation in rheumatoid arthritis. Arthritis Rheum. 1987;30:618–23. doi: 10.1002/art.1780300603. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin A, Taylor M, Bernhagen J, Shakoor Z, Mayall S, Noble G, McCarthy D. A method of preparing blood leucocytes for flow cytometry which prevents upregulation of leucocyte integrins. J Immunol Methods. 1992;146:219–28. doi: 10.1016/0022-1759(92)90231-h. [DOI] [PubMed] [Google Scholar]
- 15.Kocher M, Siegel ME, Edberg JC, Kimberly RP. Cross-linking of Fc gamma receptor IIa and Fc gamma receptor IIIb induces different proadhesive phenotypes on human neutrophils. J Immunol. 1997;159:3940–8. [PubMed] [Google Scholar]
- 16.Higaki M, Miyasaka N, Sato K. Increased expression of CD11b (Mo1) on peripheral blood monocytes of patients with rheumatoid arthritis [letter] J Rheumatol. 1992;19:825–6. [PubMed] [Google Scholar]
- 17.McCarthy D, Taylor MJ, Bernhagen J, Perry JD, Hamblin AS. Leucocyte integrin and CR1 expression on peripheral blood leucocytes of patients with rheumatoid arthritis. Ann Rheum Dis. 1992;51:307–12. doi: 10.1136/ard.51.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Highton J, Carlisle B, Palmer DG. Changes in the phenotype of monocytes/macrophages and expression of cytokine mRNA in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1995;102:541–6. doi: 10.1111/j.1365-2249.1995.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liote F, Boval-Boizard B, Weill D, Kuntz D, Wautier JL. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin Exp Immunol. 1996;106:13–19. doi: 10.1046/j.1365-2249.1996.d01-820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazure G, Fernandes T, McCarthy DA, Macey M, Perry JD, Taub NA, Dumonde DC, Brown KA. Blood monocytes in rheumatoid arthritis are highly adherent to cultured endothelium. Int Arch Allergy Immunol. 1995;108:211–23. doi: 10.1159/000237156. [DOI] [PubMed] [Google Scholar]
- 21.Badolato R, Oppenheim JJ. Role of cytokines, acute-phase proteins, and chemokines in the progression of rheumatoid arthritis. Semin Arthritis Rheum. 1996;26:526–38. doi: 10.1016/s0049-0172(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 22.Limb GA, Hamblin AS, Wolstencroft RA, Dumonde DC. Rapid cytokine up-regulation of integrins, complement receptor 1 and HLA-DR on monocytes but not on lymphocytes. Immunology. 1992;77:88–94. [PMC free article] [PubMed] [Google Scholar]
- 23.Arvidson NG, Gudbjörnsson B, Elfman L, Ryden AC, Tötterman TH, Hällgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis. 1994;53:521–4. doi: 10.1136/ard.53.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;2:706–9. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 25.Manicourt DH, Triki R, Fukuda K, Devogelaer JP, Nagant de Deuxchaisnes C, Thonar EJ. Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis Rheum. 1993;36:490–9. doi: 10.1002/art.1780360409. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara S, Hirohata S, Inoue T, Ito K. Phenotypic analysis of peripheral blood monocytes isolated from patients with rheumatoid arthritis. J Rheumatol. 1992;19:211–5. [PubMed] [Google Scholar]
- 27.Gadd SJ, Felzmann T, Majdic O, Maurer D, Petera P, Chen WJ, Smolen J, Knapp W. Phenotypic analysis of functionally associated molecules on peripheral blood and synovial fluid monocytes from arthritis patients. Rheumatol Int. 1992;12:153–7. doi: 10.1007/BF00274935. [DOI] [PubMed] [Google Scholar]
- 28.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–32. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 30.Cassatella MA, Flynn RM, Amezaga MA, Bazzoni F, Vicentini F, Trinchieri G. Interferon gamma induces in human neutrophils and macrophages expression of the mRNA for the high affinity receptor for monomeric IgG (Fc gamma R-I or CD64) Biochem Biophys Res Commun. 1990;170:582–8. doi: 10.1016/0006-291x(90)92131-i. [DOI] [PubMed] [Google Scholar]
- 31.te Velde AA, de Waal Malefijt R, Huijbens RJ, de Vries JE, Figdor CG. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992;149:4048–52. [PubMed] [Google Scholar]
- 32.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–41. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 33.Tessier P, Audette M, Cattaruzzi P, McColl SR. Up-regulation by tumor necrosis factor alpha of intercellular adhesion molecule 1 expression and function in synovial fibroblasts and its inhibition by glucocorticoids. Arthritis Rheum. 1993;36:1528–39. doi: 10.1002/art.1780361107. [DOI] [PubMed] [Google Scholar]
- 34.Aziz KE, Wakefield D. Modulation of endothelial cell expression of ICAM-1, E-selectin, and VCAM-1 by beta-estradiol, progesterone, and dexamethasone. Cell Immunol. 1996;167:79–85. doi: 10.1006/cimm.1996.0010. [DOI] [PubMed] [Google Scholar]
- 35.Gelati M, Corsini E, Dufour A, et al. Reduced adhesion of PBMNCs to endothelium in methylprednisolone-treated MS patients: preliminary results. Acta Neurol Scand. 1997;96:283–92. doi: 10.1111/j.1600-0404.1997.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 36.Crockard AD, Treacy MT, Droogan AG, Hawkins SA. Methylprednisolone attenuates interferon-beta induced expression of HLA-DR on monocytes. J Neuroimmunol. 1996;70:29–35. doi: 10.1016/s0165-5728(96)00100-2. [DOI] [PubMed] [Google Scholar]
- 37.Steer JH, Vuong Q, Joyce DA. Suppression of human monocyte tumour necrosis factor-alpha release by glucocorticoid therapy: relationship to systemic monocytopaenia and cortisol suppression. Br J Clin Pharmacol. 1997;43:383–9. doi: 10.1046/j.1365-2125.1997.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joyce DA, Steer JH, Abraham LJ. Glucocorticoid modulation of human monocyte/macrophage function: control of TNF-alpha secretion. Inflamm Res. 1997;46:447–51. doi: 10.1007/s000110050222. [DOI] [PubMed] [Google Scholar]
- 39.Arvidson NG, Gudbjörnsson B, Larsson A, Hällgren R. The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis. 1997;56:27–31. doi: 10.1136/ard.56.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Brink HR, van Wijk MJ, Geertzen RG, Bijlsma JW. Influence of corticosteroid pulse therapy on the serum levels of soluble interleukin 2 receptor, interleukin 6 and interleukin 8 in patients with rheumatoid arthritis. J Rheumatol. 1994;21:430–4. [PubMed] [Google Scholar]
- 41.Goulding NJ, Godolphin JL, Sharland PR, Peers SH, Sampson M, Maddison PJ, Flower RJ. Anti-inflammatory lipocortin 1 production by peripheral blood leucocytes in response to hydrocortisone. Lancet. 1990;335:1416–8. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- 42.Osorio LM, Fonte L, Finlay CM. Inhibition of human monocyte function by prophylactic doses of chloroquine. Am J Trop Med Hyg. 1992;46:165–8. doi: 10.4269/ajtmh.1992.46.165. [DOI] [PubMed] [Google Scholar]
- 43.Hurst NP, French JK, Bell AL, Nuki G, O'Donnell ML, Betts WH, Cleland LG. Differential effects of mepacrine, chloroquine and hydroxychloroquine on superoxide anion generation, phospholipid methylation and arachidonic acid release by human blood monocytes. Biochem Pharmacol. 1986;35:3083–9. doi: 10.1016/0006-2952(86)90390-4. [DOI] [PubMed] [Google Scholar]
- 44.Dolhain RJEM, Tak PP, Dijkmans BAC, De Kuiper P, Breedveld FC, Miltenburg AMM. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol. 1998;37:502–8. doi: 10.1093/rheumatology/37.5.502. [DOI] [PubMed] [Google Scholar]
- 45.Seitz M, Loetscher P, Dewald B, Towbin H, Rordorf C, Gallati H, Baggioloni M, Gerber NJ. Methotrexate action in rheumatoid arthritis: stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Br J Rheumatol. 1995;34:602–9. doi: 10.1093/rheumatology/34.7.602. [DOI] [PubMed] [Google Scholar]
- 46.Farsky SP, Sannomiya P, Garcia-Leme J. Secreted glucocorticoids regulate leukocyte–endothelial interactions in inflammation. A direct vital microscopic study. J Leukoc Biol. 1995;57:379–86. doi: 10.1002/jlb.57.3.379. [DOI] [PubMed] [Google Scholar]