Abstract
We examined the in vitro effect of Candida albicans on NO production by macrophages. Candida albicans suppressed not only NO production but also expression of inducible NO synthase (iNOS) mRNA by murine IFN-γ and bacterial LPS-stimulated peritoneal macrophages. The suppression was not associated with inhibition but rather stimulation of IL-1β production. This effect was observed when more than 1 × 103/ml of Candida albicans were added to macrophage cultures (1 × 106 cells/ml) and reached a maximal level at 1 × 106/ml. The NO inhibitory effect of Candida albicans was mediated predominantly by as yet unidentified soluble factor(s) and to a lesser extent by direct contact. In addition, heat- or paraformaldehyde-killed Candida albicans did not show this inhibitory activity. Culture supernatant of Candida albicans also inhibited NO production by activated macrophages in a dose-dependent manner, and increased IL-1β production. Finally, the inhibitory effect was not mediated by IL-10 and transforming growth factor-beta (TGF-β), since neutralizing antibodies to these cytokines did not influence Candida albicans-induced reduction in macrophage NO production. Our results suggest that Candida albicans may evade host defence mechanism(s) through a soluble factor-mediated suppression of NO production by stimulated macrophages, and that the effect is independent of production of immunosuppressive cytokines such as IL-10 and TGF-β.
Keywords: Candida albicans, macrophages, nitric oxide, interferon-gamma, suppression
INTRODUCTION
Candida albicans, an ubiquitous fungal microorganism, forms part of the normal microflora in the gastrointestinal tract and vagina even in individuals who do not have an apparent immunological dysfunction [1,2], suggesting the presence of certain mechanisms that evade the host defence system against this pathogen. It has been well documented that the host defence mechanism against mucosal infection with Candida albicans is mediated mainly by cellular immunity [3–6]. In experimental models, protection against candidiasis is closely associated with the synthesis of IL-12 and induction of Th1 cells [7–10]. Neutralization of endogenously synthesized IFN-γ and IL-12 by specific antibodies prevents the development of protective Th1 responses and exacerbates infections with Candida albicans [10,11]. Activated macrophages play a central role in experimentally induced infections in rodents by eliminating microbial pathogens through the generation of NO [12–16]. Recent investigations [17–19] have demonstrated that IFN-γ-activated macrophages required reactive nitrogen intermediates to exhibit effective fungicidal activity against yeast and hyphal forms of Candida albicans.
Microorganisms posses a variety of mechanisms that allow them evade the host defence systems and grow within the area of infection. For example, intracellular parasites survive and multiply within the phagosome and cytoplasm of mononuclear phagocytes [20]. Mycobacterium tuberculosis can survive in phagosomes and resist the microbicidal activity of macrophages by inhibiting phagosome acidification, a process that attenuates the proteolytic activity of acidic proteases, and by preventing phagosome–lysosome fusion through the production of ammonia and cell wall glycolipids [21–23]. On the other hand, Legionella pneumophila and Toxoplasma gondii prevent phagosome–lysosome fusion [22,24], while Listeria monocytogenes and Trypanosoma cruzi escape into the cytoplasm by disrupting phagosomal membrane and survive within the macrophage [25,26]. Recently, we reported that Cryptococcus neoformans, another fungal agent, resists host defence mechanisms by inhibiting NO-mediated fungicidal activity of macrophages [27]. This effect was mostly mediated by a direct contact between the fungus and macrophages and did not involve immunosuppressive cytokines, such as IL-10 and transforming growth factor-beta (TGF-β), which are known to suppress NO production by activated macrophages [28–31].
In the present study, we investigated the ability of Candida albicans to influence the production of NO by macrophages stimulated with IFN-γ and bacterial LPS. Our results show that Candida albicans causes a strong inhibition of NO production by macrophages. Based on our recent results in Cryptococcus neoformans [27], we also investigated the mechanisms causing such inhibition by examining the role of direct contact between the organisms and macrophages and the involvement of soluble factors present in the cultures, e.g. IL-10 and TGF-β, in Candida albicans-mediated inhibition of NO production.
MATERIALS AND METHODS
Animals
Female BALB/c mice were purchased from SLC Japan (Hamamatsu, Japan) and used at the age of 7–12 weeks. All mice were housed in a pathogen-free environment and received sterilized food and water at the Laboratory Animal Centre for Biomedical Science (University of the Ryukyus). The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation at our institution.
Culture medium and reagents
RPMI 1640 medium was obtained from Gibco BRL (Grand Island, NY), fetal calf serum (FCS) from Whittaker (Walkersville, MD). LPS from Escherichia coli serotype O111:B4 was purchased from Sigma Chemical Co. (St Louis, MO). Murine recombinant IFN-γ (specific activity 5 × 106 U/mg) was purchased from Genzyme Diagnostics (Cambridge, MA).
Candida albicans
Candidia albicans, strain RK9232, was isolated from a clinical specimen from a patient with candidial pneumonia at the Ryukyu University Hospital. The isolate was identified as Candida albicans based on common laboratory criteria, including a positive germ tube test. The yeast was grown at 30°C on Sabouraud's dextrose agar plate. After 4 or 5 days of culture, the cells were collected, washed three times in sterile, non-pyrogenic normal saline and counted using a haemocytometer. Killed microorganisms were prepared by autoclaving at 120°C for 1 min or treatment with 1% paraformaldehyde (PFA) for 30 min at room temperature. No live colony was observed on Sabouraud's dextrose agar plate after such treatment.
Preparation of Candida albicans culture supernatant
Candida albicans was cultured at approx. 1 × 106/ml in 200 ml of RPMI 1640 medium supplemented with 10% FCS or FCS-free at 37°C in 5% CO2 until reaching a confluent state, usually for 2 or 3 weeks. The culture supernatant was obtained by centrifugation, followed by filtration through 0.2-μm filters (Sartorius AG, Göttingen, Germany) and kept at −70°C until use.
Preparation of macrophages
Mice were injected intraperitoneally with 1.5 ml of 3% thioglycolate, and peritoneal exudate cells were harvested 4 days later by two cycles of injection of 5 ml of cold RPMI 1640 supplemented with 10 mm HEPES and 10% FCS. The obtained cells were cultured at 1.5–2.0 × 106/ml in FCS-precoated glass dishes for 1 h in 5% CO2 incubator. Adherent cells were collected by dislodging using a rubber policeman after removal of non-adherent cells.
Macrophage cultures
Peritoneal macrophages were cultured at 1 × 106/ml in a 24-well culture plate (Becton Dickinson, Lincoln Park, NJ) for 48 h. In some experiments the culture was set in a 24-well double chamber separated by a 0.45-μm pore membrane (Millicell-HA; Millipore Corp., Bedford, MA).
NO assay
NO is unstable and rapidly converts to nitrite and nitrate. Accordingly, we estimated the level of NO synthesis by macrophages by measuring the amount of nitrite accumulating in the cultures using the method of Stuehr & Nathan [32]. Briefly, 100 μl of supernatant were mixed with the same volume of Griess reagent and absorbance was read at 550 nm using an automated microplate reader. The concentration of nitrite was estimated from a NaNO2 standard curve.
Measurement of IL-1β
The concentration of IL-1β in macrophage culture supernatants was measured by an ELISA kit (purchased from Endogen Inc., Cambridge, MA). The detection limit was 15.6 pg/ml.
Extraction of RNA and reverse transcription-polymerase chain reaction
Total RNA was extracted from the cultured peritoneal macrophages by the acid guanidinium thiocyanate-phenol-chloroform method and subsequently reverse transcription was carried out, as described in our recent study [33]. The obtained cDNA was then amplified in an automatic DNA thermal cycler (Perkin Elmer Cetus, Norwalk, CT) using specific primers: 5′-CATGGCTTGCCCCTGGAAGTTTCTCTTCAAAG-3′ (sense) and 5′-GCAGCATCCCCTCTGATGGTGCCATCG-3′ (antisense) for inducible NO synthase (iNOS), 5′-GTTGGATACAGGCCAAGACTTTGTTG-3′ (sense) and 5′-GATTCAACTTGCGCTCATCTTAGGC-3′ (antisense) for hypoxanthine phosphoribosyl transferase (HPRT). We added 1.0 μl of the sample cDNA solution to 49 μl of the reaction mixture, which contained the following concentrations: 10 mm Tris–HCl pH 8.3, 50 mm KCl, 1.5 mm MgCl2, 10 μg/ml gelatin, dNTP (each at a concentration of 200 μm), 1.0 μm sense and antisense primer, 1.25 U of AmpliTaq DNA polymerase (Perkin Elmer Cetus). The mixture was incubated for 1 min at 94°C, 1 min at 55°C and 1 min 30 s at 72°C. The number of cycles was determined for samples not reaching the amplification plateau (35 cycles for iNOS and 32 cycles for HPRT). The polymerase chain reaction (PCR) products were electrophoresed on 2% agarose gels, stained with 0.5 μg/ml ethidium bromide and observed with a UV transilluminator.
Monoclonal antibodies
A MoAb against murine IL-10 (rat IgG1) [34] was purchased from Genzyme Diagnostics. We confirmed that 10 μg/ml MoAb completely abrogated the biological activity of 1.5 ng/ml murine recombinant IL-10 (Pepro Tech EC Ltd, London, UK) which inhibited macrophage NO production stimulated with 100 U/ml IFN-γ. We also used a MoAb to bovine, murine and human TGF-β1 and -β2 and chicken TGF-β3 (murine IgG1) [35], which was purchased from Genzyme Diagnostics. According to the instructions provided by the manufacturer, 20–30 μg/ml MoAb neutralizes approx. 0.5–1.0 ng/ml of total TGF-β (TGF-β1, -β2 and/or -β3) in an Mv1Lu growth inhibition assay.
Statistical analysis
Data are expressed as mean ± s.d. The unpaired Student's t-test was used to compare differences between groups and P < 0.05 was considered significant.
RESULTS
Candida albicans inhibits production of NO, but not of IL-1β, by macrophages
We initially stimulated murine peritoneal macrophages by IFN-γ (100 U/ml) and LPS (100 ng/ml) for 48 h, then measured nitrite content in the culture supernatants. Stimulation of macrophages resulted in the production of 125.9 ± 4.6 μm nitrite. To examine the effect of Candida albicans on NO production, a variable number of Candida albicans was added to macrophage cultures. As shown in Fig. 1a, Candida albicans showed a dose-dependent inhibition of NO production at 1 × 103–105 cells/ml and caused an almost complete inhibition at 1 × 106 cells/ml.
Fig. 1.
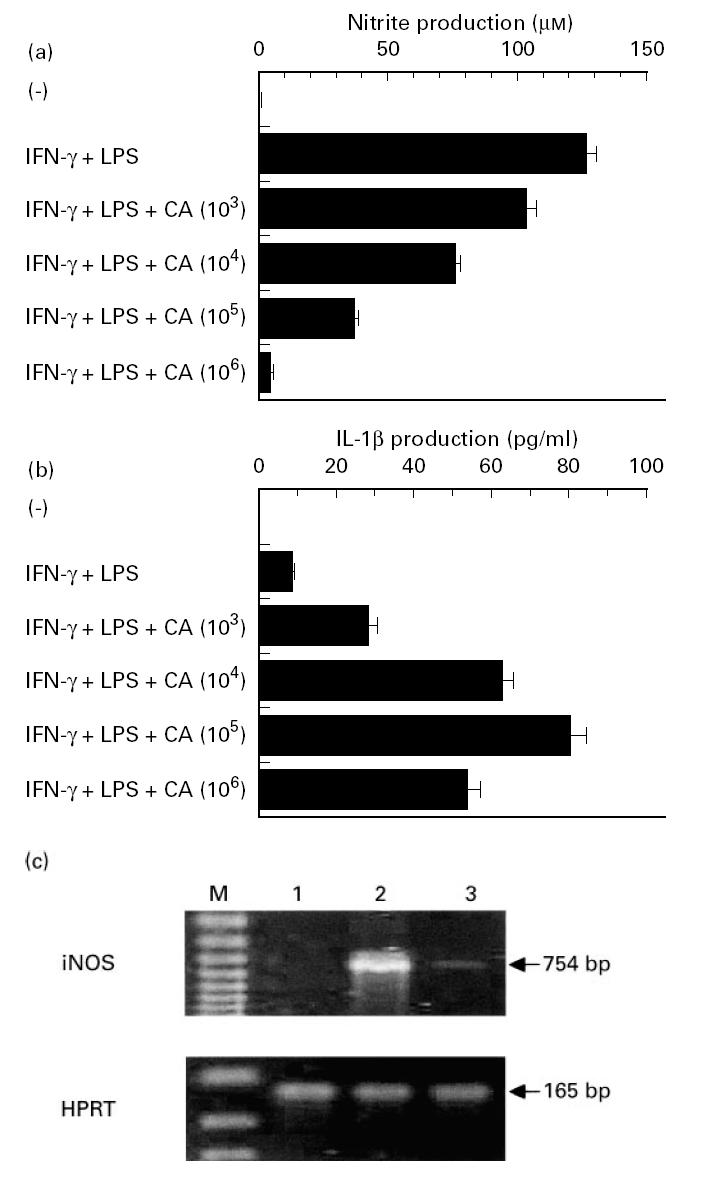
Inhibition of macrophage NO production and iNOS mRNA expression by Candida albicans. (a,b) Peritoneal macrophages were precultured at 1 × 106/ml for 2 h with indicated numbers of Candida albicans. After addition of 100 U/ml IFN-γ and 100 ng/ml LPS, the cultures were maintained for another 48 h, followed by measurement of nitrite and IL-1β content in the culture supernatants. Each bar indicates the mean ± s.d. of triplicate cultures. The experiments were repeated three times with similar results. CA, Candida albicans. (c) In similar experiments, total RNA was extracted from macrophages co-cultured with 1 × 106/ml of Candida albicans, and reverse transcriptase-polymerase chain reaction (RT-PCR) was performed to examine the expression of iNOS and hypoxanthine phosphoribosyl transferase (HPRT) mRNA. The RNA samples were obtained separately from triplicate cultures in each group, and the results are representative of three separate samples. 1, Unstimulated; 2, IFN-γ + LPS; 3, IFN-γ + LPS + Candida albicans; M, DNA size marker.
The reduction of macrophage NO production by Candida albicans did not seem to be due to cytolytic effect on the cells, because the number of viable macrophages, estimated by trypan blue exclusion method, was not significantly different 48 h after initiation of the cultures with and without 1 × 106 cells/ml Candida albicans (percentage viable cells 83.5 ± 4.1% versus 81.1 ± 3.6%). This argument was strengthened by other evidence showing that IL-1β production by activated macrophages was not inhibited by addition of Candida albicans, but rather enhanced in a dose-dependent manner (Fig. 1b).
The inhibitory effect of Candida albicans on macrophage NO production was further confirmed by examining the production of iNOS at an mRNA level using a RT-PCR method. As shown in Fig. 1c, iNOS mRNA was not detected in unstimulated macrophages and treatment with IFN-γ and LPS markedly induced this expression. Compatible with the results in Fig. 1a, Candida albicans clearly suppressed the generation of iNOS mRNA. In the same experiment, HPRT mRNA expression was not influenced by co-culturing with Candida albicans (Fig. 1c).
Necessity of direct contact in Candida albicans-induced suppression of macrophage NO production
To investigate the mechanism of NO inhibition by Candida albicans, macrophage cultures were set in two chambers separated by a membranous septum. The septum acted as a barrier to cell movement between the upper and lower chambers but allowed the diffusion of macromolecules as large as 0.45 μm. As shown in Fig. 2, NO production was strongly inhibited when IFN-γ- and LPS-activated peritoneal macrophages were cultured with Candida albicans in the same chamber. In contrast, when these cells were cultured separately in the lower and upper chambers, only a partial inhibition of the Candida albicans effect was noted and a significant level of inhibition of NO production (approx. 83% of total inhibition) was still present. These results indicate that the NO inhibitory effect of Candida albicans was mediated predominantly by soluble factors and to a lesser extent by a direct contact between the fungus and macrophages.
Fig. 2.
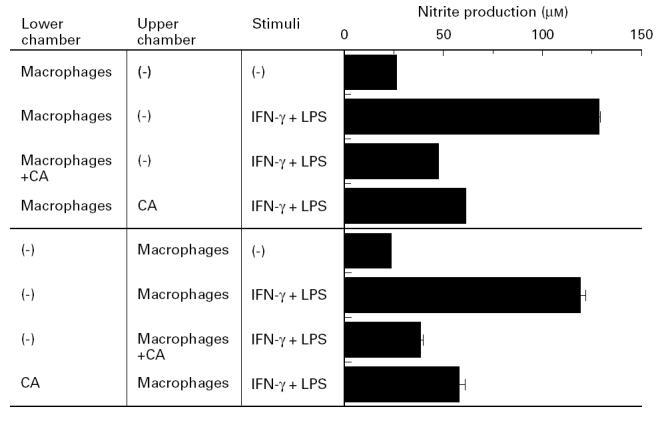
Necessity of direct contact in Candida albicans-induced suppression of macrophage NO production. Each culture was set in a 24-well double chamber separated by a 0.45-μm pores membrane. Peritoneal macrophages (1 × 106) were cultured with 100 U/ml IFN-γ and 100 ng/ml LPS in the lower or upper chamber with or without indicated numbers of Candida albicans in a total volume of 1.0 ml for 48 h, followed by measurement of nitrite content in the culture supernatant. Each bar indicates the mean ± s.d. of triplicate cultures. The experiments were repeated three times with similar results. CA, Candida albicans.
To characterize further Candida albicans-induced suppression, in the next experiment we examined the effect of dead Candida albicans, killed by autoclaving or treatment with 1% PFA, on macrophage NO production. As shown in Fig. 3, live Candida albicans strongly inhibited NO production, while dead Candida albicans did not show such activity. These results confirm the need for live Candida albicans to inhibit NO production.
Fig. 3.
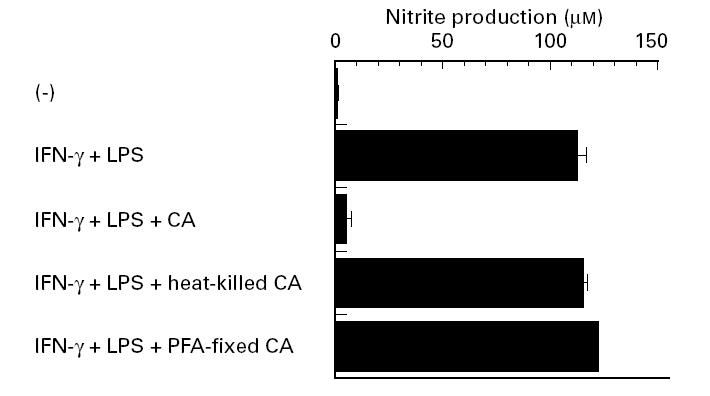
Failure of killed Candida albicans to inhibit NO production. Peritoneal macrophages were precultured at 1 × 106/ml for 2 h with indicated numbers of live Candida albicans or Candida albicans killed by heat or 1% paraformaldehyde (PFA). Following the addition of 100 U/ml IFN-γ and 100 ng/ml LPS, cultures were maintained for another 48 h, and nitrite content in the culture supernatants was measured. Each bar indicates the mean ± s.d. of triplicate cultures. The experiments were repeated three times with similar results. CA, Candida albicans.
Effect of soluble molecules released by Candida albicans on macrophage NO production
To confirm the involvement of soluble molecules secreted by Candida albicans, we examined the effect of culture supernatants of Candida albicans on NO production by IFN-γ- and LPS-stimulated peritoneal macrophages. For this purpose, Candida albicans were cultured to confluence for 2 or 3 weeks in RPMI 1640 supplemented with 10% FCS. As shown in Fig. 4a, culture supernatants of Candida albicans significantly inhibited NO production in a dose-dependent manner, when added at indicated amounts. Diminished production of NO was not due to the cytolytic effect of the culture supernatant on the cells, because the number of viable macrophages, estimated by trypan blue exclusion method, was not significantly different 48 h after initiation of cultures with and without 100% Candida albicans culture supernatant (percentage viable cells 80.2 ± 3.5% versus 78.1 ± 2.3%). This argument was confirmed by another series of experiments which showed failure of the supernatant to inhibit IL-1β production (rather stimulation) by activated macrophages (Fig. 4b).
Fig. 4.
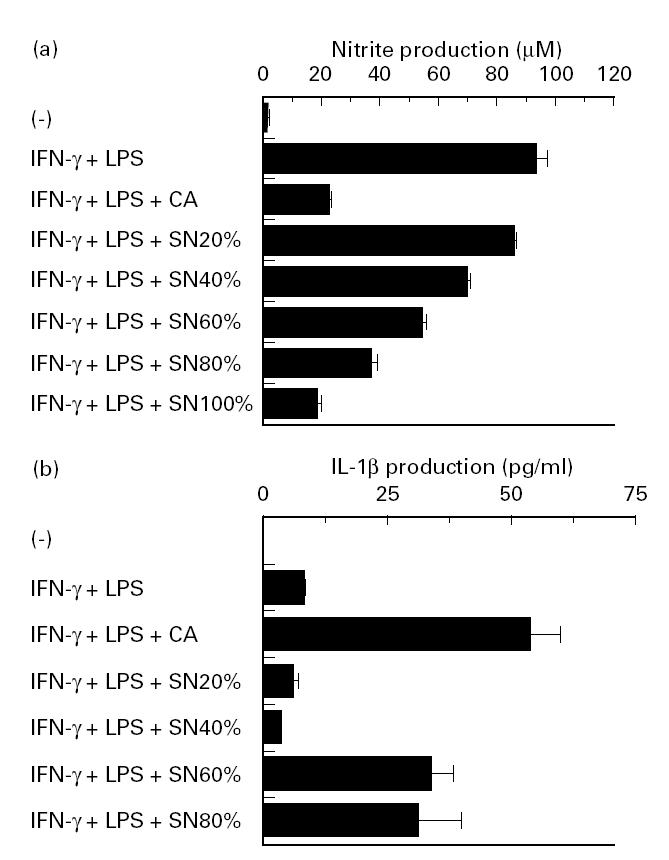
Effect of Candida albicans culture supernatant on NO and IL-1β production. Peritoneal macrophages were precultured at 1 × 106/ml for 2 h with indicated concentrations of Candida albicans culture supernatant prepared in RPMI 1640 with 10% fetal calf serum (FCS). After addition of 100 U/ml IFN-γ and 100 ng/ml LPS, the cultures were maintained for another 48 h, and contents of nitrite (a) and IL-1β (b) in the culture supernatants were measured. Each bar indicates the mean ± s.d. of triplicate cultures. The experiments were repeated three times with similar results. CA, Candida albicans; SN, culture supernatant.
Contribution of IL-10 and TGF-β to Candida albicans-induced suppression of macrophage NO production
IL-10 and TGF-β inhibit NO production by macrophages [28–31]. To examine their role in mediating the inhibitory effect of Candida albicans, we studied the effect of neutralizing antibodies against these cytokines by incubating IFN-γ- and LPS-stimulated macrophages with Candida albicans in the presence of each MoAb or corresponding control IgG. As shown in Fig. 5, the inhibitory effect of Candida albicans on NO production did not change in the presence of either neutralizing anti-IL-10 or anti-TGF-β MoAb or even in the presence of both MoAbs.
Fig. 5.
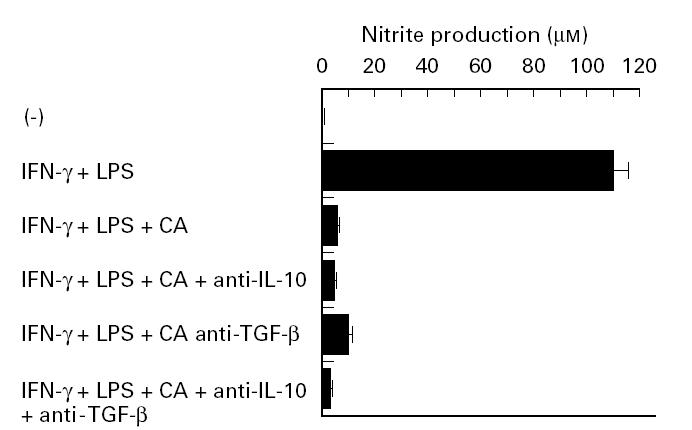
Effect of neutralizing anti-IL-10 and -transforming growth factor-beta (TGF-β) MoAb. Peritoneal macrophages were precultured at 1 × 106/ml for 2 h with the same number of Candida albicans in the presence or absence of neutralizing anti-IL-10 (30 mg/ml) or -TGF-β MoAb (30 mg/ml). As a control of each MoAb, rat or mouse IgG was used at the same doses as above. After addition of 100 U/ml IFN-γ and 100 ng/ml LPS, the cultures were maintained for another 48 h, and nitrite content in the culture supernatant was measured. Each bar indicates the mean ± s.d. of triplicate cultures. The experiments were repeated three times with similar results. CA, Candida albicans.
DISCUSSION
The major findings of the present study were: (i) Candida albicans suppressed the production of NO and iNOS mRNA expression by IFN-γ and LPS-stimulated macrophages; and (ii) such inhibition was mediated mainly by soluble molecules derived from Candida albicans and to a lesser extent by direct contact between the fungus and macrophages; but (iii) not by IL-10 and TGF-β derived from macrophages. Since NO-mediated mechanisms play a central role in macrophage-mediated killing of Candida albicans [17–19], it may be possible that the effects of Candida albicans described in the present study represent putative mechanisms for evasion of the host defence systems and multiplication of the fungus in host tissues.
We have recently demonstrated that Cryptococcus neoformans, another fungal pathogen, also inhibits NO production by macrophages [27]. However, based on the results of double-chamber experiments, Cryptococcus neoformans-induced suppression of NO production was mediated to a large extent by a direct contact of the organism with macrophages, rather than by a soluble factor. In contrast, the present results show that Candida albicans suppressed NO production predominantly by as yet unknown soluble factor and only marginally by direct contact. At present, the precise mechanism underlying the differences in NO inhibition between these two fungi remains to be elucidated. The present study further demonstrates that the presence of live Candida albicans was prerequisite for this inhibition, while dead microorganisms did not show such effect. These results are similar to those of Cryptococcus neoformans [27] and suggest that certain energy-dependent mechanisms may be necessary for induction of the inhibitory activity of Candida albicans.
Because Candida albicans yeast cells germinate and convert to a hyphal form which may then penetrate the cell membrane or become toxic to macrophages [36], it is possible that the inhibition of NO production is due to the cytolytic effect of the organism against macrophages. However, the results of several experiments confirmed that such a mechanism did not play a role in the inhibitory effect of Candida albicans on NO production. First, we showed viability of macrophages 48 h after commencement of culture with Candida albicans or supernatant of the organism, although the growing hyphae prevented us from identifying macrophages and making a correct evaluation of cell viability. Second, expression of HPRT mRNA by macrophages was not influenced by co-culturing with Candida albicans. Third, we showed that co-culture of activated macrophages with the fungus significantly enhanced the production of IL-1β in a dose-dependent manner. Fourth, IL-1β production by activated macrophages significantly increased when these cells were cultured with Candida albicans culture supernatant. Thus, these results confirm that inhibition of NO production was due to a direct action of Candida albicans rather than being a non-specific effect.
In a series of preliminary experiments, we characterized the soluble factors derived from Candida albicans, by separating FCS-free culture supernatant of Candida albicans (which also showed a suppressive effect on macrophage NO production) into two fractions, supernatant and precipitate, following treatment with a saturated concentration of ammonium sulphate. The former fraction was considered to contain non-proteinaceous molecules while the latter was rich in proteins. Our results demonstrate that both fractions exerted an equivalent level of suppression on NO production, suggesting that the responsible molecules may include both proteins and non-proteins (e.g. polysaccharides). Further characterization of the responsible molecules is under study in our laboratory.
A number of macrophage-derived cytokines, including IL-10 and TGF-β, exert anti-inflammatory effects by inhibiting the production of proinflammatory cytokines, such as IL-1, IL-6, and tumour necrosis factor-alpha (TNF-α) [37–40]. In addition, these cytokines also suppress the production of NO by IFN-γ-stimulated macrophages [28–31]. In this regard, Bistoni et al. [41,42] demonstrated that IL-4 and IL-10 inhibited NO-dependent macrophage killing of Candida albicans and that neutralization of IL-10 by specific antibodies up-regulated NO production and protected susceptible mice from infection with Candida albicans. These observations suggest that such anti-inflammatory cytokines, produced by macrophages through stimulation with yeast cells, may be involved in the inhibition of macrophage NO production by Candida albicans. However, our results show that anti-IL-10 and TGF-β MoAbs failed to modify Candida albicans-induced suppression of NO production, indicating that the soluble factor-mediated effects were unrelated to IL-10 and TGF-β, or that the interaction between Candida albicans and macrophages may not cause the release of sufficient amounts of these cytokines to suppress NO production.
Several investigators have demonstrated alterations by Candida albicans of the fungicidal activity of phagocytic cells. For example, Hilger & Danley [41] and Danley et al. [42] indicated that live Candida albicans suppressed the release of H2O2 by neutrophils, while dead organisms did not. On the other hand, Smail et al. [43,44] demonstrated that Candida albicans produced a crude hyphal inhibitory product which inhibited superoxide anion production and release of azurophilic and specific granule components by activated neutrophils. Furthermore, Diamond et al. [45,46] demonstrated that Candida albicans released small peptides which inhibited adhesion of the fungus and neutrophils. Other investigations also described the suppressive effects of Candida albicans on both cellular and humoral immunity [47–52]. Considered together, these observations demonstrate the presence of several mechanisms by which Candida albicans evades the host defence systems and multiplies in host tissue. The present results may describe a new mechanism that allows Candida albicans to resist macrophage fungicidal activity.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Science Research (09670292) from the Ministry of Education, Science, Sport and Culture and by grants from the Ministry of Health and Welfare, Japan. The authors thank Dr F. G. Issa (Department of Medicine, University of Sydney, Sydney, Australia) for editing the manuscript.
References
- 1.Odds FC. 2. London: Baillere-Tindall; 1988. Candida and candidosis. [Google Scholar]
- 2.Edwards JE. Candida species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. New York: Churchill Livingstone Inc; 1995. pp. 2289–306. [Google Scholar]
- 3.Domer JE, Lehrer RI. Introduction to candida: systemic candidiasis. In: Murphy JW, Friedman H, Bendinelli M, editors. Fungal infection and immune response. New York: Plenum Press; 1993. pp. 49–116. [Google Scholar]
- 4.Cantorna M, Balish E. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect Immun. 1991;59:2447–55. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bistoni F, Cenci E, Mencacci A, Schaffolla E, Mpsci P, Puccetti P, Romani L. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J Infect Dis. 1993;168:1449–57. doi: 10.1093/infdis/168.6.1449. [DOI] [PubMed] [Google Scholar]
- 6.Cole GT, Saha K, Seshan KR, Lynn KT, Franco M, Wong PK. Retrovirus-induced immunodeficiency in mice exacerbates gastrointestinal candidiasis. Infect Immun. 1992;60:4168–78. doi: 10.1128/iai.60.10.4168-4178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romani L, Mocci S, Bietta C, Lanfoloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–54. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis: Th responses correlate directly with genetically determined susceptibility or vaccine-resistance. J Immunol. 1993;150:925–31. [PubMed] [Google Scholar]
- 9.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Wolf SF, Puccetti P, Bistoni F. Interleukin-12 but not interferon-γ production correlate with induction of T helper type 1 phenotype in murine candidiasis. Eur J Immunol. 1994;22:909–15. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- 10.Romani L, Mencacci A, Tonnetti R, Spaccapelo R, Cenci E, Puccetti P, Wolf SF, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type-1 differentiation in murine candidiasis. J Immunol. 1994;152:5167–75. [PubMed] [Google Scholar]
- 11.Romani L, Cenci E, Mencacci A, Spaccapelo R, Grohmann U, Puccetti P, Bistoni F. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect Immun. 1992;60:4950–2. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis M. Interferon-gamma-treated macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–7. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 13.Granger DL, Hibbs JB, Jr, Prefect JR, Durack DT. Metabolic fate of l-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990;85:264–7. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–7. [PubMed] [Google Scholar]
- 15.Langermans JAM, van der Hulst MEB, Nibbering PH, Hiemstra PS, Fransen L, van Furth R. IFN-γ-induced l-arginine-dependent toxoplasmatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-α. J Immunol. 1992;148:568–74. [PubMed] [Google Scholar]
- 16.Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992;22:2501–6. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 17.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with C. Albicans. J Immunol. 1994;152:3514–21. [PubMed] [Google Scholar]
- 18.Cenci E, Romani L, Mencacci A, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–8. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez-Torres A, Jones-Carson J, Balish E. Candidacidal activity of macrophages from immunocompetent and congenitally immunodeficient mice. J Infect Dis. 1994;170:180–8. doi: 10.1093/infdis/170.1.180. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz MA. Intracellular parasitism. Curr Opin Immunol. 1988;1:41–46. doi: 10.1016/0952-7915(88)90049-0. [DOI] [PubMed] [Google Scholar]
- 21.Clemens DL. Characterization of the Mycobacterium tuberculosis phagosome. Trends Microbiol. 1996;4:113–8. doi: 10.1016/0966-842X(96)81528-9. [DOI] [PubMed] [Google Scholar]
- 22.Gordon AH, Hart PD, Young MR. Ammonia inhibits phagosome–lysosome fusion in macrophages. Nature. 1980;286:79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- 23.Goren MB, D'Arcy-Hart P, Young MR, Armstrong JA. Prevention of phagosome–lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1976;73:2510–4. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz MA. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome–lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–26. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–53. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley V, Robbins ES, Nussenzweig V, Andrews NW. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990;171:401–13. doi: 10.1084/jem.171.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami K, Zhang T, Qureshi MH, Saito A. Cryptococcus neoformans inhibit nitric oxide production by murine peritoneal macrophages stimulated with interferon-γ and lipopolysaccharide. Cell Immunol. 1997;180:47–54. doi: 10.1006/cimm.1997.1166. [DOI] [PubMed] [Google Scholar]
- 28.Cunha FQ, Moncada S, Liew FY. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-γ in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–9. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 29.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J Immunol. 1992;148:1792–6. [PubMed] [Google Scholar]
- 30.Perrella MA, Yoshizumi M, Fen Z, Tsai JC, Hsieh CM, Kourembanas S, Lee ME. Transforming growth factor-β1, but not dexamethasone, down-regulates nitric oxide synthase mRNA after its induction by interleukin-1β in rat smooth muscle cells. J Biol Chem. 1994;269:14595–600. [PubMed] [Google Scholar]
- 31.Pfeilschifter J, Vosbeck K. Transforming growth factor β2 inhibits interleukin-1β- and tumor necrosis factor α-induction of nitric oxide synthase in rat renal mesangial cells. Biochem Biophys Res Commun. 1991;175:372–9. doi: 10.1016/0006-291x(91)91574-v. [DOI] [PubMed] [Google Scholar]
- 32.Stuehr DJ, Nathan CF. Nitric oxide: a macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–55. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami K, Tohyama M, Xie Q, Saito A. Expression of cytokine and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect Immun. 1997;65:1307–12. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrams JS, Roncarolo MG, Yssel H, Anderson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 35.Ayala A, Meldrum DR, Perrin MM. The release of transforming growth factor-β (TGF-β) following hemorrhage (HEM): its roles as a mediator of host immunosuppression. FASEB J. 1992;6:A1604. [PMC free article] [PubMed] [Google Scholar]
- 36.Louria DB, Brayton RG. Behavior of Candida cells within leukocytes. Proc Soc Exp Biol Med. 1964;115:93–98. doi: 10.3181/00379727-115-28840. [DOI] [PubMed] [Google Scholar]
- 37.Bogdan C, Paik J, Vodovotz Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-β and interleukin-10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]
- 38.Reinhold D, Bank U, Buhling F, Lendeckel U, Ulmer AJ, Flad HD, Ansorge S. Transforming growth factor-beta1 (TGF-β1) inhibits DNA synthesis of PWM-stimulated PBMC via suppression of IL-2 and IL-6 production. Cytokine. 1994;6:382–8. doi: 10.1016/1043-4666(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 39.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Wu P, Siegel MI, Wgan RW, Billah MM. IL-10 inhibits transcription of cytokine gene in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–6. [PubMed] [Google Scholar]
- 41.Hilger AE, Danley DL. Alteration of polymorphonuclear leukocyte activity by viable Candida albicans. Infect Immun. 1980;27:714–20. doi: 10.1128/iai.27.3.714-720.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danley DL, Higler AE, Winkel CA. Generation of hydrogen peroxide by Candida albicans and influence on murine polymorphonuclear leukocyte activity. Infect Immun. 1983;40:97–102. doi: 10.1128/iai.40.1.97-102.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smail EH, Melnick DA, Ruggeri R, Diamond RD. A novel natural inhibitor from Candida albicans hyphae causing dissociation of the neutrophil respiratory burst response to chemotactic peptides from other post-activating events. J Immunol. 1988;140:3893–9. [PubMed] [Google Scholar]
- 44.Smail EH, Kolotila MP, Ruggeri R, Diamond RD. Natural inhibitor from Candida albicans blocks release of azurophil and specific granule contents by chemotactic peptide-stimulated human neutrophils. Infect Immun. 1989;57:689–92. doi: 10.1128/iai.57.3.689-692.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diamond RD, Krzesicki R, Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978;61:349–59. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond RD, Oppenheim F, Nakagawa Y, Krzesicki R, Haudenschild CC. Properties of a product of Candida albicans hyphae and pseudohyphae that inhibits contact between the fungi and human neutrophils in vitro. J Immunol. 1980;125:2797–804. [PubMed] [Google Scholar]
- 47.Piccolella E, Lombardi G, Morelli R. Generation of suppressor cells in the response of human leukocytes to a polysaccharide from Candida albicans. J Immunol. 1981;126:2151–9. [PubMed] [Google Scholar]
- 48.Rivas V, Rogers TJ. Studies on the cellular nature of Candida albicans-induced suppression. J Immunol. 1983;130:376–9. [PubMed] [Google Scholar]
- 49.Cuff CF, Rogers CM, Lamb BJ, Rogers TJ. Induction of suppressor cells in vitro by Candida albicans. Cell Immunol. 1986;100:47–56. doi: 10.1016/0008-8749(86)90005-5. [DOI] [PubMed] [Google Scholar]
- 50.Nelson RD, Herron MJ, McCormack RT. Two mechanisms of inhibition of human lymphocyte proliferation by soluble yeast mannan polysaccharide. Infect Immun. 1984;43:1041–6. doi: 10.1128/iai.43.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Podzorski RP, Gray GR, Nelson RD. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990;144:707–16. [PubMed] [Google Scholar]
- 52.Cuff CF, Packer BJ, Rogers TJ. A further characterization of Candida albicans-induced suppressor B-cell activity. Immunol. 1989;68:80–86. [PMC free article] [PubMed] [Google Scholar]


