Abstract
Lyme disease is a complex disorder that sometimes becomes chronic. There are contradictory reports of experimental Borrelia infections regarding which type of T cell cytokine responses, i.e. Th1 or Th2, are needed to eradicate the Borrelia spirochaetes. In human borreliosis a predominance of Borrelia-specific Th1-like responses has been shown. In this study, spontaneous, as well as Borrelia-specific, secretion of IFN-γ (Th1) and IL-4 (Th2) in Borrelia-seropositive healthy asymptomatic individuals (n = 17) was investigated in peripheral blood by a sensitive ELISPOT assay, and compared with previously reported responses in patients with clinical Borrelia infection (n = 25). The seropositive asymptomatic individuals displayed the same predominance of Borrelia-specific IFN-γ-secreting cells as the patients with clinical Borrelia infection. Interestingly, the proportion of spontaneously IL-4-secreting cells, reflecting the unstimulated in vivo secretion, was lower in the seropositive asymptomatic individuals compared with patients with chronic Borrelia infections (n = 13, P = 0.02), whereas no such difference was found compared with subacute Borrelia infections (n = 12). These findings indicate that IFN-γ secretion alone is not sufficient to eliminate Borrelia spirochaetes in humans, although IFN-γ may still have a beneficial role in borreliosis acting in concert with other mechanisms.
Keywords: Borrelia, T cells, cytokines, IFN-γ, IL-4
INTRODUCTION
Lyme disease is a complex inflammatory disorder, caused by the spirochaete Borrelia burgdorferi, and is transmitted by infected Ixodes ticks. The consequences of infection with B. burgdorferi are diverse [1,2], with a wide range of symptoms from joints, heart and nervous system. The disease in some cases becomes chronic. The pathogenic mechanisms of Lyme borreliosis are not fully known. Since some individuals lack symptoms of Lyme borreliosis, although they are seropositive to Borrelia antigen [3,4], it seems likely that the type of immune reaction that is generated is critical for development of Lyme borreliosis. The chronic forms of Lyme borreliosis are supposed to be a consequence of the host's inability to rid itself of the infecting agent, and may perhaps also be caused by the development of an autoimmune immunological reaction [5]. Furthermore, the neurological manifestations have been proposed to be partly due to cytokine-mediated immunopathological mechanisms [6].
The cytokines secreted by activated T cells have a major influence on the regulation and outcome of immune responses. As a conceptual framework, based on their secreted cytokine profiles and their functional properties, T helper cells (Th) have roughly been divided into two groups. Th1 cells secrete IFN-γ and tumour necrosis factor-beta (TNF-β), promoting a cytotoxic, phagocyte-dependent immune response. Th2 cells secrete IL-4, IL-5 and IL-9, promoting a humoral, phagocyte-independent immune response (for review see [7]). These polarized cytokine patterns were later shown to include also T cytotoxic and γδ T cells.
It has been reported from several experimental studies that susceptibility to Borrelia infection in mice is correlated to the type of T cell cytokine response that is established at the time of infection. Susceptibility to Borrelia infection has been shown to be associated with host secretion of the Th1 cytokine IFN-γ, whereas secretion of the Th2 cytokine IL-4 prevented development of clinical symptoms as well as spirochaete growth [8,9]. Furthermore, early treatment with recombinant IL-4 cured the susceptible animals [10]. In contrast, another group by cytokine treatment showed a significantly decreased infection rate in mice treated with IFN-γ [11]. Th1-like responses in human borreliosis have been reported by us and others [12–15]. The mechanisms influencing the clinical course of the human disease are mainly unknown.
The aim of this study was to investigate the type of cytokine secretion in humans resistant to Borrelia infection and compare it with previously reported responses in patients with clinical Borrelia infection. We assumed healthy individuals seropositive to Borrelia, with no current or previous clinical symptoms of borreliosis, as being resistant. For this purpose, 408 asymptomatic blood donors, without known previous Borrelia infection, were screened for antibodies against B. burgdorferi. Peripheral blood lymphocytes from 17 antibody-positive individuals were analysed regarding spontaneous, as well as Borrelia-specific, secretion of IFN-γ and IL-4, utilizing a previously developed ELISPOT assay [15].
MATERIALS AND METHODS
Screening for Borrelia-seropositive individuals
This study was approved by the local ethical committee of the University Hospital (Linköping, Sweden). After informed consent, blood donors who attended the Department of Transfusion Medicine, University Hospital, were questioned regarding known previous Borrelia infection, tick bites and symptoms of Borrelia infection. Serum samples from individuals who denied knowledge of previous Borrelia infection were screened for IgG antibodies to B. burgdorferi (data on prevalence of antibodies to be published separately). A commercial ELISA kit detecting antibodies to flagella (Lyme borreliosis kit; Dako A/S, Copenhagen, Denmark) was used according to the manufacturer's instructions. A test was considered positive which gave values exceeding the cut-off value given by the manufacturer. Borderline cases, according to the definition by the manufacturer, were not included.
Inhibition of positive ELISA by absorption with flagella antigen
To prove the specificity in the ELISA, all positive samples from the blood donors were absorbed with flagella-enriched fractions, here called FF, of B. garinii strain LAB, prepared as previously described [16,17]. The absorption was done as follows. Sera were diluted 1:50 in sample diluent and FF was added to a final concentration of 100 μg/ml. The samples were then incubated at 4°C overnight, followed by centrifugation at 4000 g for 10 min. The supernatants were further diluted four times and subsequently analysed with the Lyme borreliosis kit. Non-absorbed sera were analysed in parallel. All samples displayed lower absorbance values in flagellin-absorbed compared with non-absorbed sera.
Patients with Borrelia infections and seronegative controls
The results were compared with previously analysed material from patients with Borrelia infection and a control group consisting of 10 healthy individuals who were seronegative regarding IgM and IgG Borrelia antibodies [15]. Among the patients with Borrelia infections were 22 cases with central nervous system (CNS) borreliosis, two with peripheral nervous system (PNS) borreliosis and one with acrodermatitis chronicum atrophicans. Patients who did not fully recover after antibiotic treatment and with a disease course > 3 months were considered as suffering from chronic Borrelia infections (n = 13). The remaining 12 patients, who all fully recovered in response to antibiotic treatment, are referred to as subacute Borrelia infections.
Preparation of cells
Heparinized peripheral blood was separated on Lymphoprep (Nycomed Pharma AS, Oslo, Norway) according to Bøyum [18], followed by washing twice with Hanks' balanced salt solution (HBSS) pH 7.2 (Life Technologies, Paisley, UK). The mononuclear cells were resuspended in tissue culture medium (TCM) consisting of Iscove's modification of Dulbecco's medium (Gibco BRL, Paisley, UK) supplemented with (given as final concentrations in the medium): l-glutamine (Flow Labs, Irvine, UK) 292 mg/l, sodium bicarbonate 3.024 g/l, penicillin 50 IU/ml and streptomycin 50 μg/ml (Flow Labs), 100 × non-essential amino acids 10 ml/l (Flow Labs) and 5% heat-inactivated fetal calf serum (FCS; Flow Labs).
Antigen
An outer surface protein (osp)-enriched fraction consisting of OspA and OspB of B. afzelii strain ACA I, which has been shown to discriminate between patients with Borrelia infection and controls in the IFN-γ ELISPOT assay [19], was used at a previously optimized concentration of 10 μg/ml. Borrelia antigen fractions were prepared as previously described [16,17]. In summary, 1.5 l of B. afzelii strain/ACA I culture (containing 1011 cells in late log phase) was harvested, washed and, after addition of octyl β-d-glucopyranoside (Calbiochem, Novablochem, San Diego, CA) to a final concentration of 2%, incubated at 37°C for 60 min. After centrifugation, the supernatant was incubated at 56°C for 30 min and the resulting flocculent precipitate was separated from the soluble material by a second centrifugation at 48 000 g for 30 min at 37°C. Fraction OF was obtained from the precipitate, which was solubilized with sarkosyl (1% sodium lauryl sarcosinate in TSEA (10 mm Tris pH 7.4, 150 mm NaCl, 10 mm EDTA and 0.05% sodium azide)), after which the resulting solution was centrifuged. The obtained supernatant was dialysed against methanol in glass-distilled water at 20°C and collected. To confirm the presence or absence of the ospA and ospB in the protein fraction, the respective murine MoAbs (H5332 and 84C), obtained from Dr A. G. Barbour and Dr D. D. Thomas (University of Texas Health Science Center, San Antonio, TX), were assayed against whole cell preparations and the fractions by immunoblot analysis [20,21].
Phytohaemagglutinin A (PHA; Boehringer Mannheim, Mannheim, Germany), at a final concentration of 20 μg/ml, was used as a positive control in the stimulation experiments.
Stimulation of lymphocytes and enumeration of IFN-γ- and IL-4-secreting cells
The ELISPOT was performed as described previously [15]. Nitrocellulose-bottomed 96-well microtitre plates (Multiscreen HA; Millipore, Bedford, MA) were coated with 100 μl/well of mouse anti-human IFN-γ 7B61 MoAb or mouse anti-human IL-4 MoAb (both antibodies purchased from Mabtech, Stockholm, Sweden) in sterile PBS pH 7.4 at a concentration of 15 μg/ml overnight at 4°C. The plates were then emptied by suction using a multiscreen vacuum manifold (Millipore) and washed eight times with 100 μl/well of sterile PBS. Unspecific binding sites on the nitrocellulose were blocked by incubation with TCM (containing 5% FCS) for 30 min at 37°C. After emptying, samples of 100 μl cell suspension were applied together with 100 μl of TCM with or without the OF antigen or PHA, respectively. All samples were assayed in triplicates. The cells were then cultured undisturbed at 37°C in a humidified atmosphere with 5% CO2. After 48 h the plates were emptied and washed twice with PBS and twice with PBS containing 0.05% Tween 20 (PBS–T). Thereafter 100 μl of biotinylated anti-human IFN-γ 1-D1K MoAb and biotinylated anti-human IL-4 MoAb (Mabtech), respectively, diluted to 1 μg/ml in PBS–T, were added and incubated for 2 h at room temperature. After four washings with PBS–T, a final incubation was done for 1 h at room temperature with 100 μl/well of streptavidin conjugated with alkaline phosphatase (Mabtech) diluted 1:1000 in PBS–T. Unbound reagent was removed by washing four times with PBS. The spots were developed using an ‘AP conjugate substrate kit’ (BioRad, Hercules, CA). Spots were seen after 5–10 min and the reaction was allowed to proceed for another 5 min before the wells were rinsed with excessive amounts of tap water, emptied and dried overnight at room temperature.
The spots were counted with a dissection microscope. This was done by the same person (C.E.) in all tests performed on blood donors as well as on patients with Borrelia infection. The mean value of the triplicates was calculated, then the value of the non-stimulated cells (the ‘spontaneous spots’) was subtracted from the values of the stimulated cells. An IL-4/IFN-γ ratio was calculated by dividing the number of spontaneously IL-4-secreting cells with the number of IFN-γ-secreting cells. As a control, some wells on each plate were incubated with TCM and antigen, without cells, otherwise treated as the other wells. No spots were found in any of these wells. Stimulation with PHA always elicited strong responses both for IFN-γ and IL-4.
Statistical analysis
The Mann–Whitney U-test was used for comparisons between groups.
RESULTS
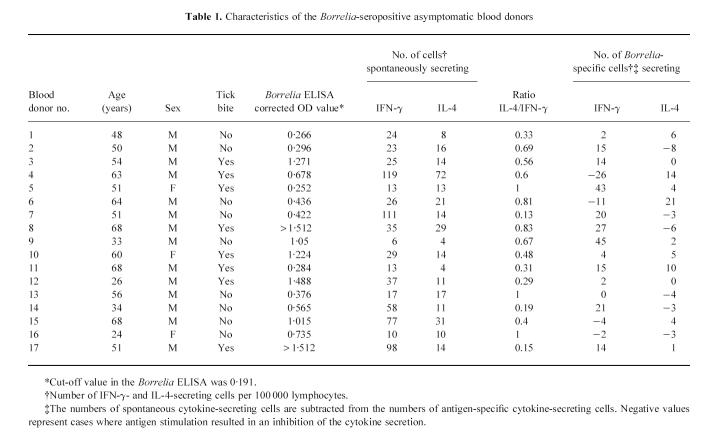
Seventeen (three female, 14 male; age 24–68 years, mean 51.1 years) out of 408 analysed blood donors without known previous Borrelia infection (149 female, 259 male; age 18–74 years, mean 38.8 years) displayed a positive test for IgG antibodies against Borrelia (Table 1). Eight of the seropositive blood donors had experienced one or more tick bites. All seropositive blood donors were clinically examined, and none had clinical signs or symptoms of a current or previous Borrelia infection.
Table 1.
Characteristics of the Borrelia-seropositive asymptomatic blood donors
The number of OF-specific IFN-γ- and IL-4-secreting cells in blood did not differ significantly in the seropositive blood donors compared with patients with Borrelia infection, both groups displaying a predominance of specific IFN-γ-secreting cells (Fig. 1). Seropositive blood donors as well as the previously analysed patients with Borrelia infections [15] displayed larger numbers of OF-specific IFN-γ-secreting cells compared with seronegative controls (P = 0.047 and P = 0.008, respectively). No differences were found in the number of OF-specific IL-4-secreting cells compared with seronegative controls, either in the seropositive blood donors or in the Borrelia infections. Dividing the patients with Borrelia infection into chronic and subacute disease course did not reveal any differences in the number of OF-specific IFN-γ- or IL-4-secreting cells compared with the seropositive blood donors (data not shown).
Fig. 1.
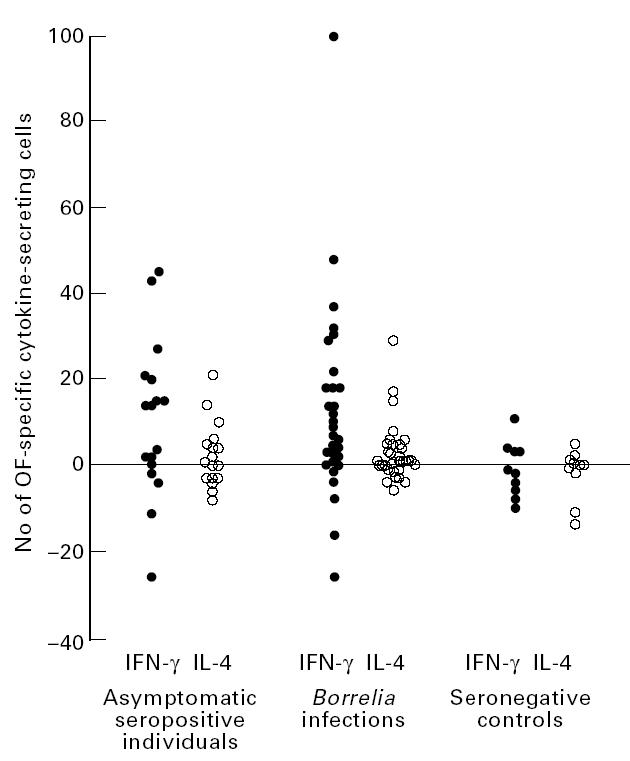
Number of IFN-γ- and IL-4-secreting cells in 100 000 lymphocytes from peripheral blood, after stimulation with outer surface protein (osp) fraction (OF) from Borrelia afzelii in Borrelia-seropositive asymptomatic individuals (n = 17), patients with Borrelia infection (n = 25, some of these analysed at several times during the disease course, data from [15]) and seronegative controls (n = 10, data from [15]). All values are net values where the number of spontaneously IFN-γ- and IL-4-secreting cells, respectively, are subtracted. Negative values represent cases where antigen stimulation resulted in an inhibition of cytokine secretion.
When comparing the spontaneous secretion of IFN-γ and IL-4, the relative proportion of IL-4-secreting cells, displayed as the IL-4/IFN-γ ratio, was found to be significantly lower in the seropositive asymptomatic individuals compared with patients with chronic Borrelia infection (P = 0.017, Fig. 2). This difference was evident early as well as late in the disease course of chronic Borrelia infections (data not shown). No such significant difference was found between the seropositive asymptomatic individuals and patients with subacute Borrelia infection, these groups displaying roughly the same spontaneous cytokine pattern (Fig. 2).
Fig. 2.
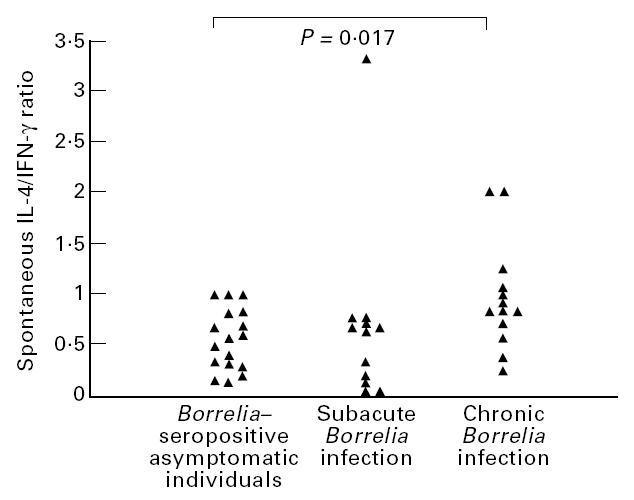
IL-4/IFN-γ ratio of the number of spontaneously IL-4- and IFN-γ-secreting cells in Borrelia-seropositive asymptomatic individuals (n = 17) compared with previous reported results from patients with Borrelia infection with subacute (n = 12) and chronic (n = 13) disease course [15]. The significant P value from comparison by Mann–Whitney U-test is given.
DISCUSSION
The similar patterns of Borrelia-specific cytokine secretion, i.e. a predominance of IFN-γ-secreting cells, found in seropositive asymptomatic individuals and patients with clinical Borrelia infection indicate that Th1-like responses generally occur in Borrelia infections. Since the seropositive asymptomatic individuals, who here are considered as resistant, had similar numbers of Borrelia-specific IFN-γ-producing cells as the patients with chronic Borrelia infection (data not shown), it seems that IFN-γ production alone may not protect from the development of clinical disease. IFN-γ may, however, still have a role in eliminating Borrelia spirochaetes, as cytokines are known to act in complex networks, the net effect depending on other cytokines present.
In this context, the finding of a tendency to lower proportions of spontaneously IL-4-secreting cells in the seropositive asymptomatic individuals compared with patients with chronic Borrelia infections is interesting, since the effects of IL-4 have been reported to dominate over the effects of IFN-γ [22]. In the murine model of Leishmaniasis, where IFN-γ is needed for cure, the mice are not cured if there are elevated levels of IL-4, despite high levels of IFN-γ [23]. Furthermore, in another study of the same model, administration of anti-IL-4 antibodies cured the mice [24]. The spontaneous secretion of cytokines in blood may reflect a background secretion, which is always present, and these patterns may differ between individuals. Based on this assumption, we speculate that individuals with a higher proportion of spontaneously IL-4-secreting cells (i.e. higher spontaneous IL-4/IFN-γ ratio) may not benefit from an increased pathogen-specific secretion of IFN-γ, since the potential eradicating effects of IFN-γ are blocked by the high background level of IL-4.
The data are presented as ‘net values’, where the spontaneous secretion is withdrawn from the stimulated secretion, which sometimes results in negative values. Theoretically, these negative values could be due to an in vitro regulation, since cytokines act in complex networks, and one can down-regulate the secretion of another, resulting in decreased secretion of a certain cytokine. The use of subtracted values provides an opportunity to evaluate antigen-specific secretion, since spontaneous secretion can vary between individuals and also at different times.
The low number of IL-4-secreting cells generally found in this study was not due to technical problems of the IL-4 ELISPOT assay, since we recently showed, using the same assay, increased numbers of antigen-specific IL-4-secreting cells in patients with the Guillain–Barré syndrome during the recovery phase of the disease [25], and we have also found increased numbers of IL-4-secreting cells during normal pregnancy [26]. In addition, stimulation with PHA always elicited large numbers of IL-4-secreting cells.
Different antigen preparations were used for screening of antibodies (flagella antigen) and for stimulation of T cell cytokine responses (outer surface proteins ospA and ospB). This is due to the fact that antibodies and T cells recognize different antigenic epitopes. The ELISA and ELISPOT assays used have been separately developed and optimized for their respective purposes. The Dako flagella ELISA has been reported to have a specificity of 98% [4] and we have previously shown that the OF antigen discriminated best between patients with Borrelia infection and controls in the ELISPOT assay [19].
In conclusion, our findings indicate that Borrelia-specific IFN-γ secretion occurs in asymptomatic as well as clinical Borrelia infections, and that IFN-γ secretion alone is not sufficient to eradicate the Borrelia spirochaetes.
Acknowledgments
We thank Charlotte Masriliez for help with antibody screening of the blood donors. This work was supported by grants from Vårdal stiftelsen, County Council of Östergötland, Tore Nilsons Foundation, The Swedish Society of Medicine and The Medical Research Council (07922).
References
- 1.Steere AC. Medical progress—Lyme disease. N Engl J Med. 1989;321:586–95. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 2.Duffy J. Lyme disease. Ann Allergy. 1990;65:1–13. [PubMed] [Google Scholar]
- 3.Isogai E, Isogai H, Kotake S, et al. Detection of antibodies against Borrelia burgdorferi in patients with uveitis. Am J Ophtalmol. 1991;112:23–30. doi: 10.1016/s0002-9394(14)76207-5. [DOI] [PubMed] [Google Scholar]
- 4.Berglund J, Eitrem R, Ornstein K, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–24. doi: 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Gibofsky A, Patarroyo ME, et al. Chronic Lyme arthritis: clinical and immunogenetic differentiation from rheumatoid arthritis. Ann Intern Med. 1979;93:8–10. doi: 10.7326/0003-4819-90-6-896. [DOI] [PubMed] [Google Scholar]
- 6.Isogai E, Isogai H, Kimura K, et al. Cytokines in the serum and brain in mice infected with distinct species of Lyme disease Borrelia. Microb Pathog. 1996;21:413–9. doi: 10.1006/mpat.1996.0072. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 8.Keane-Myers A, Nickell P. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–8. [PubMed] [Google Scholar]
- 9.Matyniak JE, Reiner SL. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–4. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keane-Myers A, Maliszewski CR, Finkelman FD, et al. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J Immunol. 1996;156:2488–94. [PubMed] [Google Scholar]
- 11.Zeidner N, Dreitz M, Belasco D, et al. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-alpha, interleukin-2, and interferon-gamma. J Infec Dis. 1996;173:187–95. doi: 10.1093/infdis/173.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Yssel H, Shanafelt M-C, Soderberg C, et al. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W-Z, Fredriksson S, Sun J-B, et al. Lyme Neuroborreliosis: evidence for persistent up-regulation of Borrelia burgdorferi-reactive cells secreting interferon-γ. Scand J Immunol. 1995;42:694–700. doi: 10.1111/j.1365-3083.1995.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 14.Oksi J, Savolainen JP, Pènè J, et al. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun. 1996;64:3620–3. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekerfelt C, Ernerudh J, Bunikis J, et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-γ predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–62. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 16.Magnarelli LA, Anderson JF, Barbour AG. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infec Dis. 1989;159:43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Bergström S, Sjöstedt A, Dotevall L, et al. Diagnosis of Lyme borreliosis by an enzyme immunoassay detecting immunoglobulin G reactive to purified Borrelia burgdorferi cell components. Eur J Clin Microbiol Infect Dis. 1991;10:422–7. doi: 10.1007/BF01968022. [DOI] [PubMed] [Google Scholar]
- 18.Bøyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;21(Suppl.):77–79. [PubMed] [Google Scholar]
- 19.Forsberg P, Ernerudh J, Ekerfelt C, et al. The outer surface proteins of Lyme disease Borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–60. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbour AG, Heiland RA, Howe TR. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infec Dis. 1985;152:478–84. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- 21.Shoberg RJ, Jonsson M, Sadziene A, et al. Identification of highly cross-reactive outer surface protein B epitope among diverse geographic isolates of Borrelia spp. causing Lyme disease. J Clin Microbiol. 1994;32:489–500. doi: 10.1128/jcm.32.2.489-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Röcken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–31. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 23.Morris L, Trout AB, McLeod KS, et al. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993;61:3459–65. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadick MD, Heinzel FP, Holaday BJ, et al. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. J Exp Med. 1990;171:115–27. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahle C, Ekerfelt C, Vrethem M, et al. T helper type 2 like responses to P0 and P2 peptides in the recovery phase of the Guillain–Barré syndrome. J Neurol Sci. 1997;153:54–60. doi: 10.1016/s0022-510x(97)00178-0. [DOI] [PubMed] [Google Scholar]
- 26.Ekerfelt C, Matthiesen L, Berg G, et al. Paternal leukocytes selectively increase secretion of IL-4 in peripheral blood during normal pregnancies: demonstrated by a novel one-way MLC measuring cytokine secretion. Am J Reprod Immunol. 1997;38:320–6. doi: 10.1111/j.1600-0897.1997.tb00307.x. [DOI] [PubMed] [Google Scholar]