Abstract
In the field of allergy diagnosis, most in vitro functional tests are focused on basophils. Nevertheless, the very small number of circulating basophils limits these experiments and their clinical benefit remains controversial. As flow cytometry is a valuable tool for identifying cell populations, even at low concentrations, we developed a tricolour flow cytometric method for the study of allergen-induced basophil activation. Identification of cells was based both on CD45 expression and on the presence of IgE on the cell surface, since basophils express high-affinity receptors for IgE (FcεRI). Cell activation upon allergen challenge was assessed by the expression of CD63 antigen on the plasma membrane. Basophil isolation and activation (with the chemotactic peptide formyl-methionyl-leucyl-phenylalanine) were validated in 32 non-allergic patients. In 12 allergic patients, basophil stimulation by a relevant allergen was in most cases positive (10/12). Furthermore a concentration-dependent hook effect was observed. Of the allergic and non-allergic patients, none showed non-specific activation with an irrelevant allergen (specificity 100%). Overall, our preliminary results, even in a small population, suggest that this is a reliable and valuable method for the diagnosis of allergies complementing specific allergen IgE and skin test results. Obviously, additional clinical studies are needed to validate these first results.
Keywords: basophil, CD63, allergy, flow cytometry
INTRODUCTION
A wide variety of allergens are implicated in the induction of atopic diseases. This is the main factor contributing to the difficulty in undertaking clinical and biological investigations in allergic patients. In most cases, a positive skin test and/or positive specific IgE allows identification of the allergen responsible for the hypersensitivity reaction. In the remaining cases, especially for drug allergy, functional in vitro studies are needed such as the lymphocyte transformation test (LTT), histamine release (HR) or enumeration of the percentage of degranulated basophils. Due to their ability to release histamine and other mediators in response to allergen activation, basophils are considered with mast cells to be the key cells in allergic diseases. Most in vitro tests are focused on basophils, as they are circulating cells (in contrast to mast cells). Nevertheless, technical difficulties such as the low number of circulating basophils, the necessity of leucocyte isolation and the difficulty of measuring very low levels of mediators make these methods unreliable and their diagnostic value controversial, as sensitivity and specificity remain to be clearly defined [1–4].
As flow cytometry is a valuable tool for analysing large numbers of cells and for identifying cell populations, even at low concentrations, we developed a tricolour flow cytometric method which could facilitate the study of allergen-induced basophil activation. Identification of cells is based both on CD45 expression, a common leucocyte antigen, and on the presence of IgE on cell surfaces, since basophils express high-affinity receptors for IgE (FcεRI) [1,5,6]. In this selected population cell activation upon allergen challenge is assessed by the expression of CD63 antigen on the plasma membrane [6,7].
SUBJECTS AND METHODS
Subjects
Blood from non-allergic (n = 32) and allergic (n = 12) patients was drawn into vacutainer heparinized tubes. The diagnosis of allergy was based on an evocative clinical history, a positive skin-test (Prick-test; Stallergenes-Pasteur, Fresnes, France) and a positive specific IgE (Cap-System; Pharmacia, Uppsala, Sweden) against the following suspected allergens: latex, d 1 (Dermatophagoides pteronyssinus), cow's milk, honeybee or a positive HR (RIA, Immunotech, Marseille, France) to aspirin for the remaining patient.
Cell preparation
As a first step, experiments were performed on non-allergic subjects in order to validate basophil isolation and activation. For each sample, we prepared a negative (100 μl of whole blood without any drug) and a positive control with 10 μl of chemotactic peptide formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma, St Louis, MO) at 10−5m added to 100 μl of whole blood. Both controls were incubated at 37°C for 30 min.
In a second step, the specificity of the method was assessed among non-allergic patients (n = 10) using different dilutions of allergen preparations designed for Prick tests: d 1 and latex (Stallergenes-Pasteur) at 1:50, 1:100 and 1:500 in Ca/Mg-free PBS (Gibco BRL, Grand Island, NY); 100 μl of whole blood were mixed with 10 μl of each dilution in order to demonstrate any potential non-specific activation.
Lastly, allergic patients (n = 12) were studied. For each sample we prepared a negative control (incubated without any drug or allergen), a positive control with fMLP as previously described, series of tubes with dilutions of the allergen known to induce clinical symptoms (d 1, latex, honeybee, cow's milk or aspirin (Stallergenes-Pasteur) at 1:10, 1:20, 1:50, 1:100, 1:200, 1:500 and 1:1000) and series of tubes with the same dilutions of an irrelevant allergen (known not to be responsible for allergy in this patient), to detect an eventual non-specific activation.
Antibodies
The following MoAbs were purchased: PE-cyanin 5 (PC5)-conjugated anti-human CD45 and PE-conjugated anti-human CD63 with their respective isotype controls and used according to the manufacturer's recommendations: 10 μl/100 μl whole blood (Immunotech). As low concentrations of anti-IgE do not induce detectable basophil activation [1], surface-bound IgE was detected using diluted polyclonal FITC-conjugated anti-human IgE (dilution 1:8 of a 0.5 mg/l solution: Caltag Labs, San Francisco, CA). After allergen stimulation, a 15-min staining was performed at room temperature in a dark chamber. Samples were then lysed using the Q-PREP system (Coulter, Hialeah, FL).
Flow cytometry
Samples were analysed on a Coulter EPICS XL flow cytometer (System II software; Coulter) which was calibrated daily with Immuno-Check fluorospheres (Coulter). Previous experiments have documented a non-specific binding with polyclonal anti-IgE which disturbed basophil isolation using side scatter and surface-bound IgE characteristics [1,8]. Thus, to be sure to exclude cell debris and other IgE-reactive leucocytes, we also defined in a first step a region with side scatter characteristics and CD45+ expression which comprised basophils and in part lymphocytes (Fig. 1). Then, among these cells, we isolated basophils on a biparametric histogram with forward scatter and surface-bound IgE (Fig. 1). Basophils clearly constituted a small distinct population that stained with anti-IgE. After that, we focused only on this population and observed CD63 expression before and after stimulation (Fig. 2). Thus, all results are expressed as a percentage of CD63+ basophils in the total basophil population.
Fig. 1.
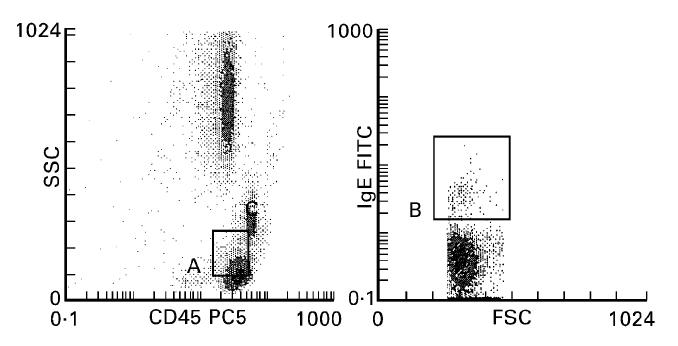
Left: Gating region (A) defined on the basis of side scatter characteristics (ordinate) and CD45 expression (abscissa). Usual basophil localization lies between monocytes and lymphocytes. Right: Gated cells from A expressed on the basis of forward scatter characteristics (abscissa) and IgE expression (ordinate); basophils constituted (region B) a small population above lymphocytes.
Fig. 2.
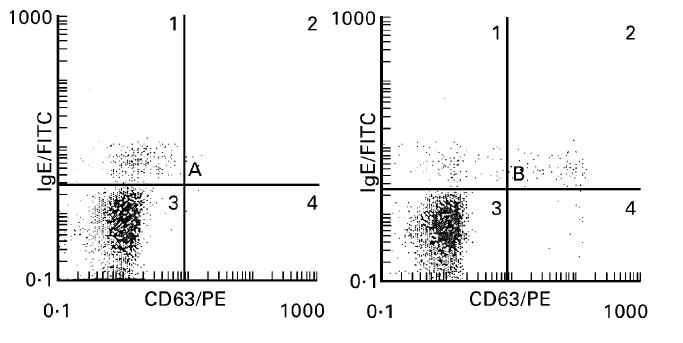
Representative increased expression of CD63 on the basophil surface before (left histogram, A1 8.6%, A2 0.2%, A3 91.2%, A4 0%) and after (right histogram, B1 4.8%, B2 3.3%, B3 90.8%, B4 1.1%) exposure to allergen (latex, 1:100). Gated cells from region B (Fig. 1) are presented on the basis of CD63 (abscissa) and IgE (ordinate) expression. A basophil shift to the right is clearly observed after allergen challenge.
RESULTS
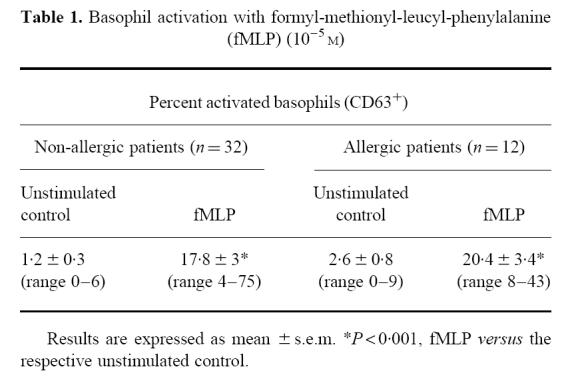
Results of the first experiments are shown in Table 1. In 32 non-allergic patients, a wide range of response to fMLP was observed, since a few patients showed very weak activation (< 10%) in comparison with the others (about 70%). Nevertheless, fMLP clearly activated basophils: 17.8% versus 1.2% for negative controls (P < 0.001, Mann–Whitney test). Such significant results obtained in a cell population defined by several characteristics (forward and side scatter, CD45+, IgE+) which expressed CD63 in response to fMLP as described by Knol et al. [7] allowed us to consider these cells as basophils.
Table 1.
Basophil activation with formyl-methionyl-leucyl-phenylalanine (fMLP) (10−5 m)
Among these non-allergic patients, 10 samples were incubated with three dilutions (1:50, 1:100, 1:500) of latex and d 1. No activation was shown in any of them and all results remained below 3%, reflecting the absence of a non-specific degranulation.
Allergic patients demonstrated very similar values (negative controls or with fMLP) in comparison with those of non-allergic patients (Table 1). Nevertheless, in response to the known responsible allergen, basophil activation was in most cases positive and higher than that observed with fMLP. Individual data are presented in Table 2. Within-run reproducibility (n = 10) with fMLP or specific allergen made on three allergic patients was found to be < 12% for each patient. Of the allergic patients, none showed non-specific degranulation with an allergen known not to be responsible for disease (Table 2). In the allergic group, the most interesting results are shown in Fig. 3, which illustrates data from the allergen dilution series in patients who had a positive response to the appropriate allergen (10 of 12). It was noteworthy that there was a significant ‘hook effect’ depending on the allergen concentration (Fig. 3) which was very indicative of a specific biological effect.
Table 2.
Individual results of allergen-induced basophil activation: expression of CD63 in documented allergic patients (n = 12) with positive skin-test and positive specific IgE (n = 11) or positive histamine release (n = 1, patient 9). For allergen results, the data shown are the highest values obtained from each allergen dilution series
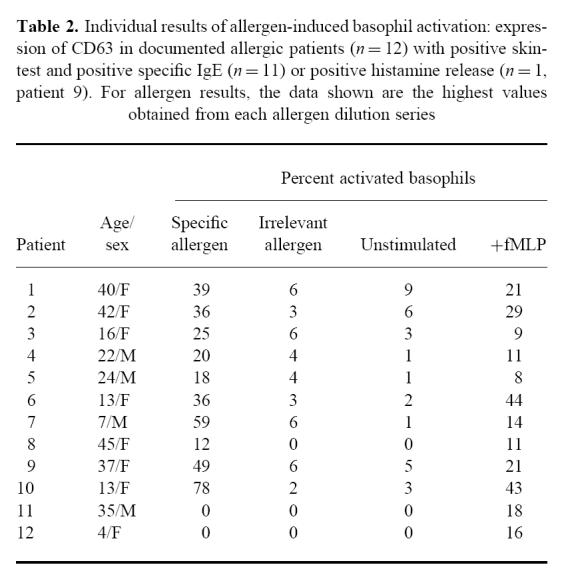
Fig. 3.
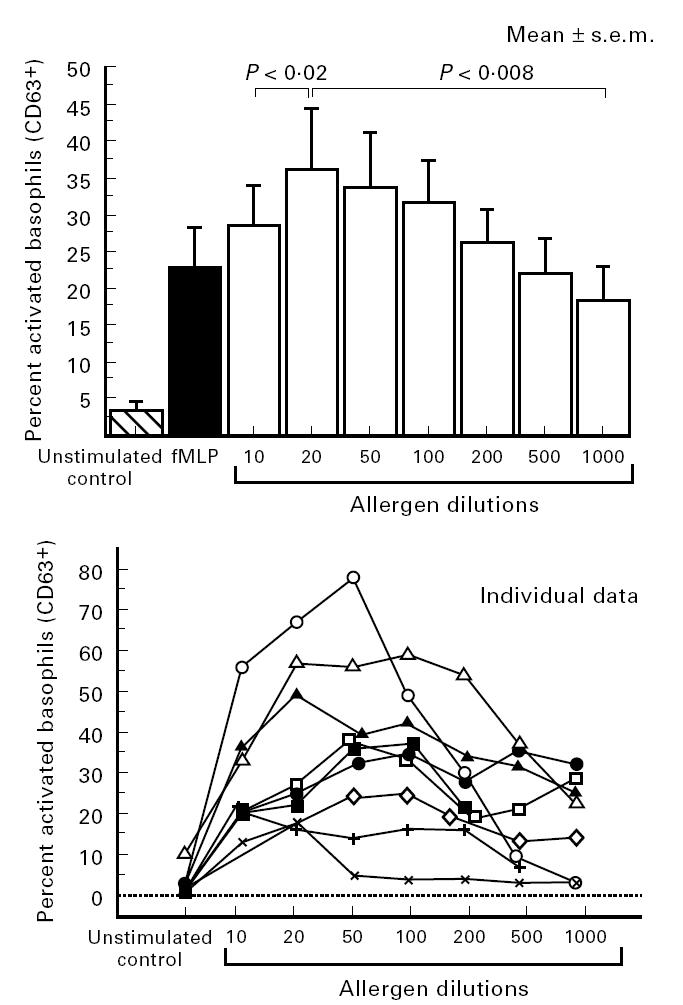
Results of basophil activation depending on allergen concentration in patients (n = 10) who presented a positive response to an appropriate allergen: mean values and individual data. (P values according to the Wilcoxon paired test; one individual curve is missing because results of only three dilutions were available for this patient.)
DISCUSSION
In diagnosis of atopic allergy, skin testing and quantification of serum levels of allergen-specific IgE are considered as the gold standards even if variability in results or discrepancies between interpretation and clinical assessment have been clearly demonstrated. It illustrates the difficulty in defining a consensus in allergy investigation which is largely explained by the multiple factors which have to be taken into account, e.g. a wide variety of responsible allergens, mediation by either IgE or T cells, and variations in disease severity; thus, there is still a need for reliable in vitro methods to complement the usual investigations when they show discrepancies or are not feasible. Basophils and mast cells are key cells in atopic diseases. Consequently, functional in vitro tests are focused on basophils since, in contrast to mast cells, they are circulating cells. Nevertheless, the very low number of circulating basophils constitutes a limitation to these experiments. Concentration steps are often necessary (Ficoll gradient, elutriation) and this directly increases the variability of the results. Another approach is to measure mediators entrapped in basophil secretion granules (such as histamine) after allergen-induced activation. But HR is costly in terms of both materials (radioisotopes) and laboratory technician time. To date, the real benefit brought by HR remains controversial [3]. Thus, the aim of our study was to perform an easy flow cytometry test for activated basophil detection, without any previous cell separation steps, using a model consisting of whole blood without pretreatment (free of any additional mediators such as IL-3), with a short incubation time in order to provide a method with a reasonable degree of standardization which could be used in different laboratories.
Our work was largely based on two previous very interesting studies. Knol et al. [7,9] detailed expression of CD63 on the human basophil surface after anti-IgE or fMLP challenge. They demonstrated that CD63 is anchored in the basophilic granule membrane (which contains histamine) and its exposure to the outside of the cells reflects cell degranulation because of fusion between the granule and plasma membrane. Furthermore, they demonstrated a strong correlation between CD63 expression and HR. The authors considered the study of CD63 as a reliable way to monitor basophil activation because of an all or nothing response. In addition, Gane and co-workers [1,8] proposed a method to isolate basophils by flow cytometry using IgE detection on cell surfaces (due to IgE binding to FcεRI) coupled to CD45 expression to exclude non-specific binding of polyclonal antibodies against IgE. Dendritic cells have similar side scatter to basophils and also express FcεRI. Nevertheless, there is a very small number of circulating dendritic cells, and furthermore, Gane et al. found a purity of > 80% when sorting basophils using these characteristics [1].
To our knowledge, we report here the first description of basophil activation detection by a tricolour flow cytometric method using CD45, CD63 and IgE+ expression. After first gating on CD45 and side scatter characteristics, basophils defined a small IgE+ population (Fig. 1) in which it was easy to assess increased CD63 expression as a marker of activation (Fig. 2). Our results with fMLP clearly demonstrated this. In the 22 patients (allergic and others), non-specific activation with irrelevant allergens was not detected, thus providing an interesting specificity of 100%. Ten out of 12 allergic patients, in response to appropriate allergen, showed activation of basophils (up to 78%). The two remaining patients gave false-negative results. In cases of positive tests in allergic patients, the most remarkable result is the description of a hook effect, depending on allergen concentration, which was very indicative of a specific biological effect.
Overall, our results are very consistent with the description of a reliable and easy method to detect basophil activation in response to various allergens (d 1, latex, cow's milk, aspirin and honeybee). The specificity and sensitivity, obtained even in a small population, allowed us to conclude that this test could be a valuable and economical tool (up to four-fold less expensive than HR in our laboratory) for the difficult diagnosis of allergies, complementing specific allergen IgE and skin test results. Obviously, additional clinical studies are needed to validate these first results.
In the future, the method could be improved by detecting antigen on basophil surface using, if available, MoAb against FcεRI [10,11] or against eotaxin receptor [12]. As data support participation of T lymphocytes in cases of drug allergy [13], it is equally possible to imagine complementary and more reliable flow cytometric methods [14] in place of the lymphocyte transformation test. Taken together, these data indicate promising developments in flow cytometry in the diagnosis of allergies in the clinical immunology laboratory.
Acknowledgments
The authors thank Drs A. Noiret and G. Guerrier for their collaboration, Professor J. Whicher for reading this manuscript and Dr J. P. Poizat for scientific discussion on flow cytometry.
References
- 1.Gane P, Pecquet C, Lambin P, Abuaf N, Leynadier F, Rouger P. Flow cytometric evaluation of human basophils. Cytometry. 1993;14:344–8. doi: 10.1002/cyto.990140316. [DOI] [PubMed] [Google Scholar]
- 2.Salkie ML. Role of clinical laboratory in allergy testing. Clin Biochem. 1994;27:343–55. doi: 10.1016/0009-9120(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 3.Bernady A, Taytard A, De Tunon Lara JM. IgE specifiques et histamino-liberation. Rev Fr Allergol. 1996;36:884–8. [Google Scholar]
- 4.Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. 1997;27:175–81. [PubMed] [Google Scholar]
- 5.Bochner BS, Sterbinsky SA. Altered surface expression of CD11 and Leu 8 during human basophil degranulation. J Immunol. 1991;7:2367–73. [PubMed] [Google Scholar]
- 6.Fureder W, Agis H, Sperr WR, Lechner K, Valent P. The surface membrane antigen phenotype of human blood basophils. Allergy. 1994;49:861–5. doi: 10.1111/j.1398-9995.1994.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 7.Knol EF, Mul FPJ, Jansen H, Calafat J, Roos DR. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 8.Gane P, Pecquet C, Crespeau H, Lambin P, Leynadier F, Rouger P. Flow cytometric monitoring of allergen induced basophil activation. Cytometry. 1995;19:361–5. doi: 10.1002/cyto.990190411. [DOI] [PubMed] [Google Scholar]
- 9.Knol EF, Mul FPJ, Kuijpers TW, Verhoeven AJ, Roos DR. Intracellular events in anti-IgE nonreleasing human basophils. J Allergy Clin Immunol. 1992;90:92–103. doi: 10.1016/s0091-6749(06)80015-1. [DOI] [PubMed] [Google Scholar]
- 10.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity receptors (FcεRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 11.Grattan CEH, Walpole D, Francis DM, Niimi DM, Dooston G, Edler S, Corbett MF, Barr RM. Flow cytometric analysis of basophil numbers in chronic urticaria: basopenia is related to serum histamine releasing activity. Clin Exp Allergy. 1997;27:1417–24. doi: 10.1046/j.1365-2222.1997.1630972.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhaugherti BL, Siciliano SJ, Demartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterisation of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–53. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauri Hellweg D, Bettens F, Mauri D, et al. In vitro and in vivo T-cell response to drugs in drug allergic individuals. J Immunol. 1995;155:462–72. [PubMed] [Google Scholar]
- 14.Yamamura Y, Rodriguez N, Schwartz A, Eylar E, Bagwell B, Yano N. A new flow cytometric method for quantitative assessment of lymphocyte mitogenic potentials. Cell Mol Biol. 1995;41:121–32. [PubMed] [Google Scholar]


