Abstract
Intraperitoneal injection of pristane induces a lupus-like disease in BALB/c and other non-autoimmune mice characterized by autoantibody production and the development of immune complex disease closely resembling lupus nephritis. Two subsets of autoantibodies are induced by pristane: IgG anti-DNA and -chromatin autoantibodies are strongly IL-6-dependent, whereas IgG anti-nRNP/Sm and -Su antibodies are not. The present studies were carried out to examine the role of T cells in establishing this dichotomy between the production of anti-nRNP/Sm/Su versus anti-DNA/chromatin autoantibodies. Autoantibody production and renal disease were evaluated in athymic (nude) mice treated with pristane. BALB/c nu/nu mice spontaneously developed IgM and IgG anti-single-stranded (ss)DNA and -chromatin, but not anti-nRNP/Sm or -Su, autoantibodies. Pristane treatment increased the levels of IgG anti-chromatin antibodies in nu/nu mice, but did not induce production of anti-nRNP/Sm or -Su antibodies. In contrast, BALB/c nu/+ and +/+ control mice did not spontaneously produce autoantibodies, whereas anti-nRNP/sm and -Su autoantibodies were induced by pristane in approx. 50% of nu/+ and +/+ mice and anti-DNA/chromatin antibodies at lower frequencies. Nude mice spontaneously developed mild renal lesions that were marginally affected by pristane, but were generally milder than the lesions developing in pristane-treated nu/+ and +/+ mice. The data provide further evidence that two distinct pathways with different cytokine and T cell requirements are involved in autoantibody formation in pristane-induced lupus. This dichotomy may be relevant to understanding differences in the regulation of anti-DNA versus anti-nRNP/Sm autoantibodies in systemic lupus erythematosus, as well as the association of anti-DNA, but not anti-nRNP/Sm, with lupus nephritis.
Keywords: pristane, systemic lupus erythematosus, autoantibodies, autoimmunity, mouse models
INTRODUCTION
Intraperitoneal injection of pristane induces a lupus-like disease in BALB/c and other non-autoimmune mice characterized by autoantibody production and the development of immune complex-mediated glomerulonephritis closely resembling lupus nephritis [1,2]. Analysis of the autoantibodies induced by pristane in IL-6-deficient versus intact mice suggests that the cytokine requirements of different subsets of the lupus-associated autoantibodies differ. Pristane induces IgG autoantibodies against chromatin, single-stranded (ss)DNA, and double-stranded (ds)DNA in wild-type, but not IL-6-deficient, BALB/c mice, suggesting that humoral immunity to chromatin is strongly IL-6-dependent. In contrast, the frequency of anti-nRNP/Sm and -Su autoantibodies is similar in IL-6-deficient versus intact animals [3].
The present study was carried out to examine further the dichotomy between IL-6-dependent and independent autoantibody responses. IL-6 transgenic mice develop hypergammaglobulinaemia, which is at least partly due to a T cell-independent activation of IgG and IgA production [4,5]. It was of interest therefore to see if IL-6-dependent autoantibodies (anti-chromatin or DNA) can be induced in T cell-deficient mice by pristane treatment.
MATERIALS AND METHODS
Mice
Female BALB/c ByJ nu/nu mice and age/sex-matched controls (+/+ and nu/+) (Jackson Laboratory, Bar Harbor, ME), aged 10–12 weeks, were injected once intraperitoneally with 0.5 ml of pristane (2,6,10,14-tetramethylpentadecane; Sigma Chemical Co., St Louis, MO) or with an equal volume of PBS [1]. All mice were housed under specific pathogen-free (SPF) conditions in barrier cages with autoclaved bedding and water and γ-irradiated food. Serum samples were collected from the tail vein before treatment, 2 weeks later, and then at 4-week intervals. At 7 months, kidneys were processed for light, electron and immunofluorescence microscopy. Proteinuria was measured with Albustix (Miles Labs, Elkhart, IN). Values ≥ 3+ (300 mg/dl) were graded as significantly elevated.
Immunoprecipitation
Immunoprecipitation of 35S-methionine-labelled cell extract from K562 (human erythroleukaemia) cells was performed as described using human prototype sera specific for Su or nRNP/sm as standards [1,2].
ELISAs
Sera were tested for various autoantibodies as described [2,3]. Affinity-purified intact U small nuclear (sn)RNPs served as antigen in the anti-nRNP/Sm ELISA. Y2 (IgG2a anti-Sm-B + D MoAb) culture supernatant (gift of Dr Joan Steitz, Yale University, New Haven, CT; 1:10–1:50 000 dilutions) served as a standard. Alkaline phosphatase-conjugated goat anti-mouse IgG (γ-chain-specific, 1:1000 dilution; Southern Biotechnology, Birmingham, AL) was the second antibody. The reaction was developed with p-nitrophenyl phosphate substrate, and absorbance at 405 nm was determined.
For the anti-ssDNA ELISA, microtitre plates were coated with 3 μg/ml heat-denatured calf thymus DNA, mouse sera were diluted 1:500, and binding was detected with alkaline phosphatase-conjugated goat anti-mouse IgG or IgM antibodies [3]. For the anti-chromatin ELISA, polyvinyl chloride microtitre plates were coated with 1.0 μg/ml chicken erythrocyte chromatin in Tris–HCl pH 8.0, sera were diluted 1:500, and binding was detected using alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology) [3]. The A405 nm of a 1:1000 dilution of a high-titre MRL/lpr reference serum was assigned a value of 2048 units, and a 211-fold dilution a value of 2 units. Data were analysed by the Mann–Whitney test.
Renal pathology
Renal tissue was evaluated by a renal pathologist (J.C.J.) in a blinded manner. For light microscopy, tissue was fixed in 4% paraformaldehyde and 4-μm paraffin sections were stained with haematoxylin and eosin. Sections were graded as follows: 1+, mild focal mesangial hypercellularity alone; 2+, moderate mesangial hypercellularity; 3+, complex endocapillary hypercellularity sometimes with mild sclerosis or necrosis; 4+, severe endocapillary proliferative glomerulonephritis with necrosis or crescent formation. A score ≥ 1+ was positive.
For immunofluorescence, tissue was embedded in OCT Compound (Miles) and 4-μm unfixed frozen sections were stained with 1:20 goat anti-mouse IgG1, 2a, 2b, or 3, or IgM (Southern Biotechnology), or with rabbit anti-mouse C3 (Cappel, Durham, NC). Glomerular staining was graded according to intensity on a 0–4+ scale (0 = no staining, 4+ = maximum intensity staining). Background was defined as the strongest level of staining observed in PBS-treated control mice (nu/+, +/+). The distribution of staining in mesangial regions and capillary walls was recorded. Scores ≥ 1+ were positive for all isotypes except IgM (positive ≥ 3+), which was present at up to 2+ background levels in control mice. The Fisher exact test with the Bonferroni correction for multiple comparisons was used for statistical analysis.
Immune complex deposits were evaluated by electron microscopy using renal tissue fixed in 2.5% glutaraldehyde/cacodylate buffer and embedded in Poly/Bed 812 resin (Polysciences, Warrington, PA). Thin sections were viewed with a Jeol Gem-100F electron microscope.
RESULTS
The production of autoantibodies against chromatin and DNA in pristane-induced lupus is strongly IL-6-dependent, whereas production of anti-nRNP/Sm autoantibodies is not [3]. Since IL-6 may stimulate IgG and IgA production in the absence of T cells, the thymus-dependence of anti-DNA/chromatin versus anti-nRNP/Sm and -Su autoantibody production in pristane-induced lupus was investigated in nude mice.
Anti-ssDNA and chromatin antibodies
Two weeks after treating BALB/c nu/nu and control nu/+ and +/+ mice with pristane or PBS, IgM anti-ssDNA antibody levels were measured by ELISA (Fig. 1, top). PBS-treated BALB/c mice (both nu/nu and controls) spontaneously produced low levels of IgM anti-ssDNA antibodies (Fig. 1, top). The level of these antibodies increased 2 weeks after pristane treatment in the nude mice (Fig. 1 top, P < 0.05 by Mann–Whitney test for pristane- versus PBS-treated group). There was no significant difference between the mean level of IgM anti-ssDNA antibodies between the pristane-treated nu/nu mice and controls (nu/+, +/+).
Fig. 1.
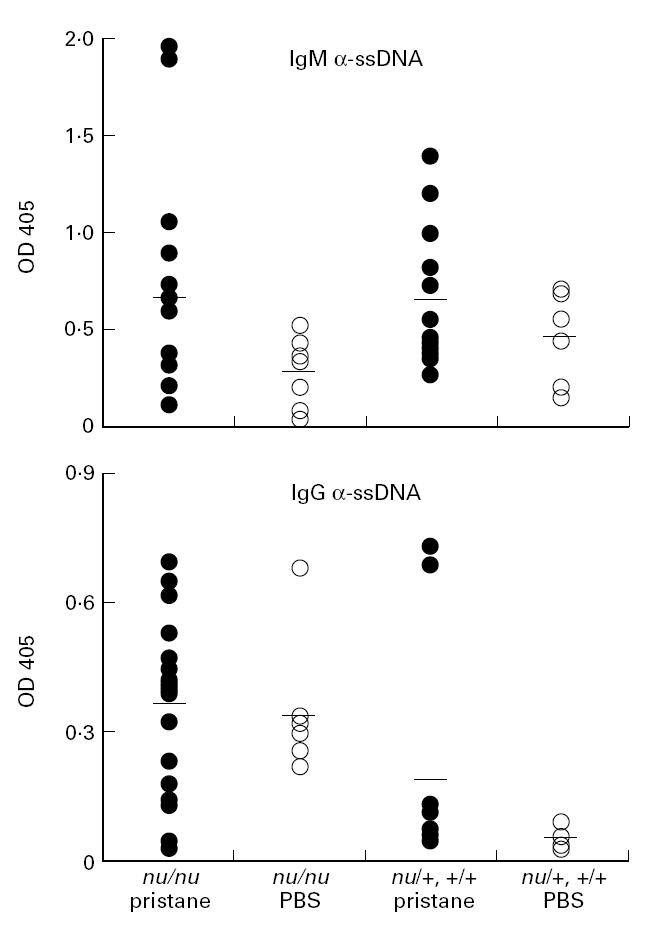
Anti-ssDNA antibodies in pristane-treated mice. Top, levels of IgM anti-ssDNA antibodies by ELISA in sera obtained 2 weeks after pristane or PBS treatment. Bottom, levels of IgG anti-ssDNA antibodies by ELISA in sera obtained 5 months after pristane or PBS treatment. Groups of mice are indicated below. All sera were tested at a 1:500 dilution.
Five months after treatment, PBS-treated nu/nu, but not PBS-treated nu/+ or +/+, mice, produced IgG anti-ssDNA antibodies spontaneously (Fig. 1, bottom). Pristane induced the production of high levels of IgG anti-ssDNA antibodies in two of 12 nu/+ or +/+ mice and lower levels in several more mice, but these antibodies were found at much lower levels in PBS-treated controls. The levels of IgG anti-ssDNA antibodies were significantly higher in pristane-treated nu/+ or +/+ mice than in the PBS-treated group (P < 0.001 by Mann–Whitney test). There was no clear effect of pristane treatment on the levels of IgG anti-ssDNA antibodies in sera from nu/nu mice, since levels were elevated in both pristane- and PBS-treated mice.
Low levels of IgG anti-chromatin autoantibodies also were produced spontaneously in an age-dependent fashion by PBS-treated nu/nu mice (Fig. 2, top). There was little or no spontaneous production of IgG anti-chromatin antibodies by nu/+ and +/+ mice (Fig. 2, bottom). Pristane treatment induced high levels of IgG anti-chromatin autoantibodies in nu/+ and +/+ mice. A low level of IgG anti-chromatin antibodies was present in three of 12 nu/+ and +/+ mice initially, but was absent at 6 months in the same mice. Furthermore, pristane greatly enhanced the production of anti-chromatin autoantibodies in nu/nu mice.
Fig. 2.

Anti-chromatin antibodies in pristane-treated mice. Top, levels of IgG anti-chromatin in sera of nu/nu mice were determined by ELISA at 0 and 6 months after treatment with either pristane (•) or PBS (○). Bottom, levels of IgG anti-chromatin in nu/+and +/+ controls. Sera were tested at a 1:500 dilution. Data are expressed as units of activity in undiluted serum.
Taken together, these data indicate that IgG as well as IgM autoantibodies to ssDNA and chromatin are produced spontaneously in nude mice, consistent with previous reports that nude mice produce IgG and IgM antinuclear antibodies [6]. In addition, pristane treatment enhanced the production of IgM anti-ssDNA and IgG anti-chromatin autoantibodies in nu/nu mice, suggesting that a component of the pristane-induced autoantibody response against chromatin/DNA is T cell-independent.
Anti-nRNP/Sm and -Su autoantibodies
In contrast to anti-DNA/chromatin autoantibodies, the frequencies of IgG anti-nRNP/Sm and -Su autoantibodies are similar in IL-6-deficient and intact mice [3], suggesting that they may be produced by different mechanisms. To explore that possibility further, the frequencies of anti-nRNP/Sm and -Su autoantibodies were compared in pristane-treated nu/nu mice versus nu/+ and +/+ controls.
Consistent with previous data [1,2], sera from six of 12 nu/+ and +/+ mice immunoprecipitated the U1-A, B′/B, U1-C, D, E/F, and G proteins, indicative of anti-nRNP/Sm activity at 6 months after pristane treatment; six of 12 also immunoprecipitated the 100/102-kD Su proteins (Table 1). In contrast, neither anti-nRNP/Sm nor anti-Su antibodies were detected in pristane-treated nu/nu mice. As indicated in Table 1, sera from three of 17 pristane-treated nu/nu and one of 12 nu/+, +/+, as well as one of seven PBS-treated nu/nu mice, immunoprecipitated core histones.
Table 1.
Frequency of autoantibodies by immunoprecipitation†

In agreement with the immunoprecipitation studies (Table 1), 6/12 nu/+ and +/+ mice were anti-nRNP/Sm-positive by ELISA 6 months after pristane treatment versus 0/17 nu/nu mice (P = 0.0019 by Fisher exact test, Fig. 3). None of the PBS-treated mice developed anti-nRNP/Sm antibodies. Thus, pristane did not induce the production of IgG anti-nRNP/Sm or -Su autoantibodies in nude mice, nor did nude mice produce them spontaneously.
Fig. 3.
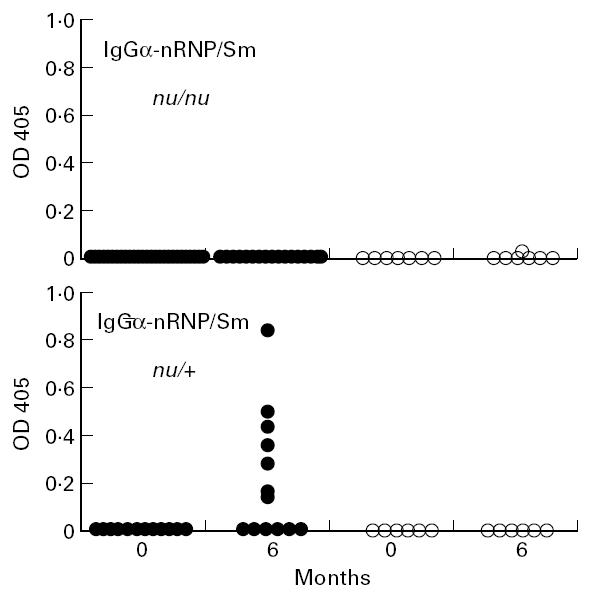
Anti-nRNP/sm autoantibodies in pristane-treated mice. Sera obtained from the indicated groups of mice at 0 and 6 months after pristane (•) or PBS treatment (○) were tested at a 1:500 dilution for IgG anti-nRNP/sm activity by ELISA.
Glomerulonephritis
Autoantibodies to DNA and chromatin are associated with nephritis in murine as well as human lupus [7]. In contrast, anti-nRNP/Sm autoantibodies correlate with a diminished risk of renal involvement [8]. In view of the spontaneous and pristane-enhanced production of anti-DNA/chromatin autoantibodies in pristane-treated nude mice, we examined the development of renal lesions in these mice.
Kidneys from 12 pristane- and seven PBS-treated nu/nu and 11 pristane- and six PBS-treated nu/+ or +/+ mice were examined 7 months after treatment (Table 2). Glomerular disease, as assessed by light microscopy, ranged from no abnormalities to marked glomerular hypercellularity with focal necrosis in pristane-treated nu/nu animals. None of the PBS-treated nu/+ mice had glomerular hypercellularity, and only one of seven PBS-treated nu/nu mice had mild hypercellularity; proteinuria was absent (Table 2). Two of 12 pristane-treated nu/nu mice had more advanced glomerular pathology and marked proteinuria. However, the frequency of light microscopic changes was not statistically different in pristane- versus PBS-treated nude mice, which developed mild renal lesions spontaneously.
Table 2.
Renal lesions in pristane-treated nude mice
Likewise, by indirect immunofluorescence, there was little difference in renal immunoglobulin deposition between pristane- and PBS-treated nude mice (Table 2). Four of seven PBS-treated nu/nu mice spontaneously developed glomerular IgG deposits, three in a mesangial only (Fig. 4A, top) and one with a capillary distribution (Fig. 4B, top). In contrast, a capillary ± mesangial staining pattern for IgG was seen in four of 12 pristane-treated nu/nu mice, and two additional mice exhibited mesangial only staining. A high intensity granular IgG and C3 capillary staining was seen in the two pristane-treated nu/nu animals with advanced glomerular pathology and proteinuria, suggesting a correlation between capillary wall immune complex localization and advanced renal disease, as in human lupus nephritis [9]. In many nu/nu kidneys (pristane- or PBS-treated) linear IgG staining without associated complement staining was seen. Pristane-treated nu/+ mice had a higher frequency of IgG2a, IgG2b and C3 deposits than similarly treated nu/nu mice (P < 0.05 by Fisher exact test) (Table 2).
Fig. 4.
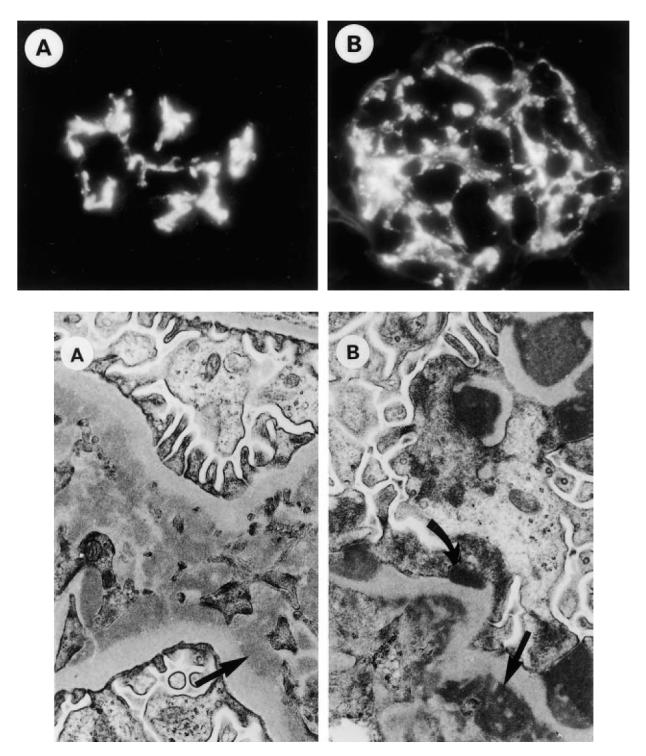
Renal pathology. Top, frozen sections of renal tissue from PBS-treated BALB/c nu/nu mice were stained with FITC-conjugated goat anti-mouse IgG1. (A) Diffuse mesangial staining. (B) Mesangio-capillary distribution of IgG1. Note that relatively marked renal immune complex deposition may occur spontaneously in nude mice. Bottom: (A) Tissue from a PBS-treated BALB/c nu/nu mouse exhibiting numerous mesangial electron-dense deposits (arrow). (B) Tissue from a pristane-treated BALB/c nu/nu mouse exhibiting mesangial (straight arrow) and subepithelial (curved arrow) dense deposits.
Immune complex deposits also were apparent by electron microscopy (Fig. 4, bottom). A mesangial immunofluorescence pattern correlated with mesangial electron-dense deposits (Fig. 4A, bottom), whereas capillary granular staining correlated with subepithelial and subendothelial electron-dense deposits (Fig. 4B, bottom). Taken together, the light, fluorescence, and electron microscopic findings indicate that nude mice spontaneously developed a mild immune complex-mediated glomerulonephritis along with IgG and IgM anti-DNA and chromatin autoantibodies. Pristane treatment altered the severity of the spontaneous lesions in nude mice only marginally (Table 2). In contrast, IgG2a and C3 deposition was increased in the glomeruli of pristane-treated nu/+ and +/+ mice compared with PBS-treated controls as well as pristane-treated nu/nu mice.
DISCUSSION
The origin of autoantibodies in pristane-induced lupus is controversial. Their high titres, the predominance of IgG1, 2a, and 2b, and their selectivity for lupus autoantigens are consistent with the possibility that they are T cell-dependent and antigen-driven. However, their induction by a non-specific inflammatory stimulus led to the suggestion that they might be natural autoantibodies [10–12]. We recently reported that the induction of anti-chromatin, -ssDNA, and -dsDNA autoantibodies by pristane is highly IL-6-dependent, whereas the induction of anti-nRNP/Sm and -Su autoantibodies is not [3]. As shown by the present studies, the strong anti-chromatin/DNA responses of nude mice, both spontaneous and pristane-induced, stand in sharp contrast to the complete inability to detect anti-nRNP/Sm and -Su autoantibodies in the sera of either PBS- or pristane-treated nude mice. These data suggest that there is a dichotomy in the T cell requirements for the induction of anti-chromatin/DNA versus anti-nRNP/Sm/Su autoantibodies by pristane.
Spontaneous autoimmunity in athymic mice
Nude mice have greatly reduced numbers of T cells with a limited V-region repertoire [13,14]. With age, they develop a limited population of αβ and γδ T cells extrathymically [15,16]. However, these cells have abnormal phenotypic and functional properties [15]. The thymic rudiment in nude mice is incapable of supporting normal T cell development, resulting in the virtual absence of T cell-dependent immune responses [17] and T cell helper/inducer function [13]. Consequently, nude mice can be used to evaluate thymus dependence in experimental disease models [18].
The autoimmune manifestations induced by pristane in nude mice occur on a background of mild spontaneous autoimmunity, as indicated by the production of IgG anti-ssDNA, -histone, and -chromatin antibodies by PBS-treated nude mice (Figs 1 and 2,Table 1). Spontaneous production of IgG autoantibodies in nude mice, first reported over 20 years ago, may be due to the lack of T cell regulation of anti-self responses [6,19]. In addition to antinuclear antibodies, nude mice develop renal immunoglobulin deposits [6,19] and marked polyclonal B cell activation in response to non-specific stimuli such as peptidoglycan or lipopolysaccharide (LPS), resulting in the production of anti-ssDNA antibodies and rheumatoid factor [20,21]. These may represent low-affinity, polyreactive, ‘natural’ autoantibodies bearing germ-line immunoglobulin variable region sequences that are produced by the B1 cell subset, which is intact in nude mice [22,23].
Autoimmunity to chromatin in nude mice
Superimposed on this background of spontaneous autoimmunity is the enhanced production of IgM anti-ssDNA and IgG anti-chromatin autoantibodies induced by pristane in nude mice (Figs 1 and 2). This may reflect the influence of pristane on residual T cells in athymic mice, cytokine-mediated, but T cell-independent, stimulation of natural autoantibody production [20,24], or both.
The role of T cells in anti-DNA antibody production in lupus is controversial. Anti-DNA antibody production and renal disease are attenuated in NZB/W F1 nude mice [25], chromatin-responsive T cells providing help for anti-DNA antibody production have been reported [26], and anti-CD4 treatment ameliorates anti-DNA antibody production and nephritis [27]. On the other hand, transfer of NZB/W pre-B cells into severe combined immunodeficient (SCID) mice causes hypergammaglobulinaemia as well as IgM and IgG anti-DNA autoantibody production and proteinuria, consistent with a primary B cell defect [28,29]. This may reflect the production of anti-DNA antibodies by T cell-dependent as well as -independent mechanisms [30].
Production of anti-chromatin/DNA autoantibodies in pristane-induced lupus is strictly IL-6-dependent [3], and this cytokine can stimulate T cell-independent immunoglobulin production [5,31]. We speculate that a component of the anti-chromatin/DNA response in pristane-treated nude mice reflects IL-6 stimulation of natural autoantibody production. However, our data do not exclude the possibility that anti-DNA/chromatin autoantibody production is dependent on the small subset of T cells remaining in nude mice, or that both T cell-dependent and -independent mechanisms are involved.
Autoimmunity to nRNP/Sm and Su antigens is deficient in nude mice
Unlike anti-chromatin/DNA, neither IgG nor IgM anti-nRNP/Sm/Su autoantibodies were detected in PBS-treated nude mice, suggesting that regulation of the two subsets differs. This interpretation also is supported by the IL-6 independence of anti-nRNP/Sm, but not anti-DNA/chromatin, autoantibody production [3].
Pristane failed to induce anti-nRNP/Sm or -Su autoantibodies in nude mice, as determined by two highly sensitive assays (Fig. 3,Table 1), indicating that thymic T cell maturation is obligatory for the production of this autoantibody subset. Not only were high-affinity IgG autoantibodies immunoprecipitating the U1 snRNP not detected, low-affinity IgG (Fig. 3) or IgM (not shown) autoantibodies reactive with immobilized antigen on surfaces were undetectable as well. Thus, the residual T cells in nude mice were incapable of supporting the production of anti-nRNP/Sm or -Su autoantibodies. The requirement for a population of thymically derived T cells strongly argues that the production of anti-nRNP/Sm and -Su antibodies, unlike anti-DNA/chromatin activity, is not due to enhanced production of natural autoantibodies following non-specific inflammation.
Renal lesions in nude mice
Nephritis in NZB/W and MRL/lpr mice is T cell-dependent [32,33]. Interestingly, nude mice spontaneously develop mild immune complex-mediated glomerulonephritis [6,19]. The predominance of IgG in the glomerular deposits and the normal or high levels of serum IgG1, IgG2a, and IgG2b in many nude mice (not shown) was somewhat unexpected in view of previous reports that these isotypes are nearly undetectable [34]. However, others have shown that IgG2a, IgG2b, and especially IgG1, levels may be normal or increased in nude mice [35,36]. The explanation for the high levels of ‘T cell-dependent’ isotypes is unclear, but may reflect non-specific microbial stimulation [35].
Spontaneous renal immunoglobulin deposition in nude mice was not influenced greatly by pristane treatment, but was generally milder than that in pristane-treated nu/+ and +/+ mice (Table 2). Although nude mice produce IgG anti-chromatin/DNA antibodies, we have not been able to show a clear relationship between the production of any particular autoantibody and severity of the renal lesions. In view of the IL-6 dependence of anti-DNA/chromatin autoantibody production and the development of mesangial proliferative lesions and glomerular immunoglobulin deposits in IL-6 transgenic mice [4], it is possible that overproduction of IL-6 induced by pristane [37] promotes the development of a subset of autoantibodies as well as renal disease.
In conclusion, studies of pristane-induced lupus in nude mice provide further evidence that there are two distinct pathways of autoantibody production. IgG anti-DNA/chromatin antibodies were produced spontaneously in nude mice at low levels and at much higher levels after pristane treatment. In contrast, IgG anti-nRNP/Sm and -Su autoantibodies required thymic T cell maturation, since in nude mice they were produced neither spontaneously nor after pristane treatment. Similarly, a dichotomy in the induction of anti-DNA/chromatin versus anti-nRNP/Sm autoantibodies by pristane was apparent in IL-6-deficient mice [3]. It will be of interest in the future to define the relationship between these two autoantibody pathways and the development of end organ damage, such as lupus nephritis.
Acknowledgments
We are grateful to Dr Philip Cohen (University of North Carolina, Division of Rheumatology and Immunology) for critically reading the manuscript, Mr Robert Cheek for assistance with the anti-chromatin ELISA, Dr Dwight Bellinger (University of North Carolina, Division of Laboratory Animal Medicine) for assistance with the SPF mouse colony, and Dr Paul Stewart (University of North Carolina, Biostatistics Department) for assistance with statistical analysis. This work was supported by research grants from the Lupus Foundation of America and from the United States Public Health Service (AR44731, AR42573, and DK28492). H.B.R is an Arthritis Foundation Postdoctoral Fellow.
References
- 1.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–6. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh M, Kumar A, Kanwar YS, Reeves WH. Antinuclear antibody production and immune complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci USA. 1995;92:10934–8. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. IL-6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–51. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oka Y, Rolink AG, Suematsu S, Kishimoto T, Melchers F. An interleukin-6 transgene expressed in B lymphocyte lineage cells overcomes the T cell-dependent establishment of normal levels of switched immunoglobulin isotypes. Eur J Immunol. 1995;25:1332–7. doi: 10.1002/eji.1830250530. [DOI] [PubMed] [Google Scholar]
- 6.Monier J, Spetjian M. Spontaneous antinuclear autoimmunisation in swan and nude mice: comparative study. Ann Immunol. 1975;126:63–75. [PubMed] [Google Scholar]
- 7.Lefkowith JB, Gilkeson GS. Nephritogenic autoantibodies in lupus: current concepts and continuing controversies. Arthritis Rheum. 1996;39:894–903. doi: 10.1002/art.1780390605. [DOI] [PubMed] [Google Scholar]
- 8.Winn DM, Wolfe JF, Lindberg DA, Fristoe FH, Kingsland L, Sharp GC. Identification of a clinical subset of systemic lupus erythematosus by antibodies to the Sm antigen. Arthritis Rheum. 1979;22:1334–7. doi: 10.1002/art.1780221203. [DOI] [PubMed] [Google Scholar]
- 9.Appel GB, Cohen DJ, Pirani CL, Meltzer JI, Estes D. Long-term follow-up of patients with lupus nephritis: a study based on the classification of the World Health Organization. Am J Med. 1987;83:877–85. doi: 10.1016/0002-9343(87)90645-0. [DOI] [PubMed] [Google Scholar]
- 10.Alarcon-Segovia D, Cabral AR. Autoantibodies in systemic lupus erythematosus. Curr Opin Rheumatol. 1996;8:403–7. doi: 10.1097/00002281-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hahn BH. Animal models of systemic lupus erythematosus. In: Wallace DJ, Hahn BH, Quismorio FP, Klinenberg JR, editors. Dubois' lupus erythematosus. 5. Baltimore: Williams & Wilkins; 1996. pp. 339–80. [Google Scholar]
- 12.Winska-Wiloch H, Muller S, Katz DR, Wilkinson L, Hutchings PR, Isenberg DA. Immunogenic properties of synthetic fragments of Sm-D protein in normal and lupus mice. Lupus. 1997;6:656–67. doi: 10.1177/096120339700600807. [DOI] [PubMed] [Google Scholar]
- 13.Hunig T. T-cell function and specificity in athymic mice. Immunol Today. 1983;4:84–87. doi: 10.1016/0167-5699(83)90125-1. [DOI] [PubMed] [Google Scholar]
- 14.Abromson-Leeman SR, Jayaraman S, Dorf ME. Characterization of T cell clones from an athymic mouse. J Immunol. 1990;144:2451–8. [PubMed] [Google Scholar]
- 15.Kennedy JD, Pierce CW, Lake JP. Extrathymic T cell maturation. Phenotypic analysis of T cell subsets in nude mice as a function of age. J Immunol. 1988;148:1620–9. [PubMed] [Google Scholar]
- 16.Matsuzaki G, Kenai H, Fujise S, Koyabashi N, Kishihara K, Nomoto K. Extrathymic development of self-reactive gamma/delta T cells in athymic BALB/c nu/nu mice. Cell Immunol. 1996;173:49–54. doi: 10.1006/cimm.1996.0250. [DOI] [PubMed] [Google Scholar]
- 17.Stedra J, Cerny J. Distinct pathways of B cell differentiation. I Residual T cells in athymic mice support the development of splenic germinal centers and B cell memory without an induction of antibody. J Immunol. 1994;152:1718–26. [PubMed] [Google Scholar]
- 18.Gilkeson GS, Pritchard AJ, Pisetsky DS. Cellular requirements for anti-DNA production induced in mice by immunization with bacterial DNA. Eur J Immunol. 1990;20:1789–94. doi: 10.1002/eji.1830200825. [DOI] [PubMed] [Google Scholar]
- 19.Morse HC, III, Steinberg AD, Schur PH, Reed ND. Spontaneous ‘autoimmune disease’ in nude mice. J Immunol. 1974;113:688–97. [PubMed] [Google Scholar]
- 20.Dziarski R. Opposing effects of xid and nu mutations on proliferative and polyclonal antibody and autoantibody responses to peptidoglycan, LPS, protein A and PWM. Immunology. 1984;53:563–74. [PMC free article] [PubMed] [Google Scholar]
- 21.Izui S, Eisenberg RA, Dixon FJ. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981;153:324–38. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364–8. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa K, Hardy RR. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- 24.Lymberi P, Blancher A, Clavas P, Avrameas S. Natural autoantibodies in nude and normal outbred (Swiss) and inbred (BALB/c) mice. J Autoimmun. 1989;2:283–95. doi: 10.1016/0896-8411(89)90270-9. [DOI] [PubMed] [Google Scholar]
- 25.Mihara M, Ohsugi Y, Saito K, et al. Immunologic abnormality in NZB/NZW F1 mice. Thymus-independent occurrence of B cell abnormality and requirement for T cells in the development of autoimmune disease, as evidenced by an analysis of the athymic nude individuals. J Immunol. 1988;141:85–90. [PubMed] [Google Scholar]
- 26.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–81. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wofsy D, Seaman WE. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985;161:378–91. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reininger L, Radaszkiewicz T, Kosco M, Melchers F, Rolink AG. Development of autoimmune disease in SCID mice populated with long-term ‘in vitro’ proliferating (NZB X NZW) F1 pre-B cells. J Exp Med. 1992;176:1343–53. doi: 10.1084/jem.176.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reininger L, Winkler TH, Kalberer CP, Jourdan M, Melchers F, Rolink AG. Intrinsic B cell defects in NZB and NZW mice contribute to systemic lupus erythematosus in (NZB x NZW) F1 mice. J Exp Med. 1996;184:853–61. doi: 10.1084/jem.184.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casali P, Burastero SE, Balow JE, Notkins AL. High-affinity antibodies to ssDNA are produced by CD-5 cells in systemic lupus erythematosus patients. J Immunol. 1989;143:3476–83. [PubMed] [Google Scholar]
- 31.Mihara M, Fukui H, Koishihara Y, Saito M, Ohsugi Y. Immunologic abnormality in NZB/W F1 mice. Thymus-independent expansion of B cells responding to interleukin-6. Clin Exp Immunol. 1990;82:533–7. doi: 10.1111/j.1365-2249.1990.tb05485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh DR, Ho A, Rahemtulla A, Fung-Leung WP, Griesser H, Mak TW. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995;25:2558–62. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- 33.Connolly K, Roubinian JR, Wofsy D. Development of murine lupus in CD4-depleted NZB/NZW mice: sustained inhibition of residual CD4+ T cells is required to suppress autoimmunity. J Immunol. 1992;149:3083–8. [PubMed] [Google Scholar]
- 34.Wortis HH. Immunological studies of nude mice. Contemp Top Immunobiol. 1974;3:243–63. doi: 10.1007/978-1-4684-3045-5_10. [DOI] [PubMed] [Google Scholar]
- 35.Mink JG, Radl J, van den Berg P, Haaijman JJ, van Zwieten MJ, Benner R. Serum immunoglobulins in nude mice and their heterozygous littermates during ageing. Immunology. 1980;40:539–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Klein-Schneegans A-S, Kuntz L, Trembleau S, Fonteneau P, Loor F. Serum concentrations of IgM, IgG1, IgG2b, IgG3 and IgA in C57BL/6 mice and their congenics at the nu (nude) locus. Thymus. 1990;16:45–54. [PubMed] [Google Scholar]
- 37.Shacter E, Arzadon GK, Williams J. Elevation of interleukin-6 in response to a chronic inflammatory stimulus in mice: inhibition by indomethacin. Blood. 1992;80:194–202. [PubMed] [Google Scholar]



