Abstract
Cytotoxic cells possess specialized granules which contain perforin and a group of serine proteinases termed granzymes. Granzyme-positive cells have been identified in synovial fluid and tissue of patients with RA, where they may play an important role as mediators of granule-mediated apoptosis, extracellular proteolysis, and cytokine induction. The aim here was to define further the involvement of cytotoxic cells in RA. Plasma and synovial fluid samples from the knee joint were obtained from 31 RA patients. The disease controls included 20 osteoarthritis (OA) patients and 10 reactive arthritis (ReA) patients. A recently developed capture ELISA was used to detect soluble granzymes A and B in all patients. Compared with OA and ReA disease controls, markedly increased levels of soluble granzymes A and B were detected in both plasma and synovial fluid of RA patients (P < 0.00001). When values for soluble granzymes A and B in plasma and synovial fluid were used simultaneously as independent variables, logistic regression analysis indicated that a diagnosis of RA could be predicted correctly in 84% of the RA patients and a diagnosis of non-RA in 90% of the controls. The markedly elevated levels of soluble granzymes A and B in plasma and synovial fluid of RA patients strongly suggest that cytotoxic cells are active participants in the pathogenesis of RA. Moreover, the results suggest that measurement of granzymes may assist the laboratory evaluation of patients with arthritis. Larger studies in patients with early disease may clarify the role of this test system in differential diagnosis.
Keywords: granzymes, cytotoxic cells, rheumatoid arthritis, osteoarthritis, reactive arthritis
INTRODUCTION
RA is a chronic inflammatory disease that targets synovial tissue and is characterized by hyperplasia of the synovial lining layer and by infiltration with T cells, plasma cells, and macrophages [1]. The number of cytotoxic cells, primarily natural killer (NK) cells, is also increased in rheumatoid synovial tissue [2].
NK cells and cytotoxic T cells possess specialized granules, which contain perforin and a family of homologous serine proteinases termed granzymes [3–5]. Granzyme (Gran) A is a serine proteinase with trypsin-like activity, while GranB expresses the unusual preference for aspartic acid residues. The presence of granzyme-positive cells has been demonstrated previously in synovial tissue from patients with RA, osteoarthritis (OA), and reactive arthritis (ReA) by immunohistologic and molecular biological techniques [2,6–8]. The expression of GranB, especially, was found to be higher in rheumatoid synovium than in synovial tissue from patients with OA or ReA [2,8]. Previous work has also shown that granzyme-positive cells are present in rheumatoid synovial fluid [9,10].
Granzymes may be released extracellularly during degranulation of cytotoxic cells [11,12]. Delivered intracellularly by perforin, the granzymes induce apoptosis in the target cell [4]. GranA may also stimulate the production of tumour necrosis factor-alpha (TNF-α), IL-6 and IL-8 [13,14]. Moreover, GranB can aggravate degradation of extracellular matrix [15–17]. Thus, granzymes may play a crucial role in synovial inflammation and joint destruction, although the significance for the pathogenesis of RA is at present unclear.
We recently developed a capture ELISA that detects soluble GranA and GranB at pg levels in plasma and synovial fluid [12]. Using this technique, the aim here was to define further the involvement of cytotoxic cells in RA. Furthermore, the potential of measurement of soluble granzymes as a diagnostic tool for RA was investigated. Patients with inflammatory OA and ReA served as controls.
PATIENTS, MATERIALS AND METHODS
Patients
Sixty-one patients with active arthritis of at least one knee joint were investigated. Thirty-one patients fulfilled the American College of Rheumatology (ACR) criteria for RA [18]. The disease controls included 20 OA patients, who fulfilled established criteria [19], and 10 patients with ReA induced by Yersinia enterocolitica. The diagnosis of ReA was based on the clinical findings, demonstration of class-specific circulating antibodies to Yersinia outer proteins [20,21], and the exclusion of other rheumatic diseases. All patients were followed for at least 2 years to verify the diagnosis. Clinical assessment included the patient's assessment of pain in the joint that was aspirated (0–10 cm on a visual analogue scale (VAS)), the Ritchie articular index [22], and the swollen joint count. Groups of small joints, e.g. metacarpophalangeal joints, were considered as a single unit. Disease duration in the RA patients was measured from the first clinical signs of arthritis, irrespective of which joint was initially affected. Laboratory assessments included measurement of serum levels of C-reactive protein (CRP) and IgM rheumatoid factor. For diagnostic purposes, x-rays of knees, hands, and feet were obtained at entry and after follow up.
Most patients were treated with non-steroidal anti-inflammatory drugs (NSAIDs) and none received corticosteroids or cytotoxic disease-modifying drugs (DMARDs), such as azathioprine, methotrexate, or cyclophosphamide at inclusion. All patients gave informed consent and the study protocol was approved by the Committee of Medical Ethics of the Leiden University Medical Centre.
ELISA for granzymes
Plasma and synovial fluid samples from the knee joint were obtained from all patients; they were coded and stored at −70°C until analysis. The ELISA to detect sGranA and sGranB has recently been described in detail [12]. Briefly, 100 μl of purified mouse MoAb GA29 (CLB; 2 μg/ml) or MoAb GB11 (CLB; 2 μg/ml), for the detection of sGranA and sGranB, respectively, were coated onto microtitre plates (Nunc Maxisorp, Roskilde, Denmark) for 16 h at 4°C. After washing and blocking steps and pretreatment of the samples and standards (purified GranA and GranB at different concentrations) with 40 μg/ml hyaluronidase (Sigma, St Louis, MO) for 30 min, wells containing 100 μl of diluted test samples were incubated for 1 h. The plates were then incubated with biotinylated GA28 (CLB; 0.5 μg/ml) or GB10 MoAb (CLB; 0.5 μg/ml) for the detection of sGranA and sGranB, respectively, for 1 h. Streptavidin–horseradish peroxidase (HRP) (CLB) was added and the plates were developed using hydrogen peroxide as substrate and tetramethylbenzidine (Merck, Darmstadt, Germany) as dye. The absorbance at 450 nm was determined with a Titertek plate (Labsystems, Helsinki, Finland). The concentrations of soluble granzymes were expressed in pg/ml. Interference by rheumatoid factors was excluded by controls, including replacement of biotinylated GA34 or GB10 MoAb by biotinylated GB10 MoAb or GA34, respectively. Moreover, addition of purified rheumatoid factors to the test samples did not alter the levels of granzymes detected.
Statistical analysis
For statistical analysis and the figure, values below the detection limit were set at 1 pg/ml. The mean and median values and the s.d. were calculated and the following non-parametric tests were used: Wilcoxon signed ranks test for matched pairs, Kruskal–Wallis test for several group means, Mann–Whitney two-sample test, and the Spearman rank correlation. Logistic regression analysis was applied to investigate the possibility of allocating a patient into one of two groups (RA versus non-RA) on the basis of the levels of soluble granzymes. Furthermore, the sensitivity and specificity of the measurements for the diagnosis RA were calculated.
RESULTS
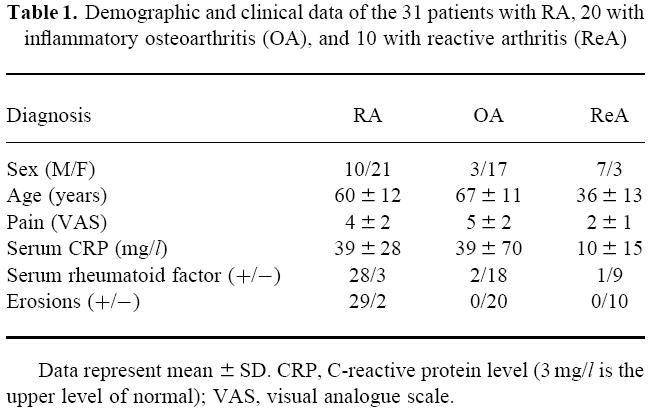
Clinical and demographic data are presented in Table 1. The duration of disease for the RA group was 52 ± 83 months (mean ± s.d.) (range 2–408 months), whereas the median disease duration was 16 months. Thirteen RA patients had a disease duration of ≤ 12 months. The mean duration of disease for the ReA group was 9 ± 11 months (range 1–36 months), whereas the median disease duration was 3 months. Six ReA patients had a disease duration of ≤ 12 months. Data on disease duration are not provided for the OA group, because it is difficult to determine the onset of OA in a reliable way. The mean Ritchie articular index was 8 ± 6 and the mean swollen joint score was 6 ± 3 in the RA patients. The ReA patients had on average lower scores for local pain than the patients with either RA or OA (P < 0.01). There were no differences in local joint swelling between the three groups, since the patients were selected because of this. The sex and age distribution for the three diagnostic groups was as expected. The mean serum levels of CRP were relatively high in the OA group due to high levels (> 40 mg/l) in five OA patients selected on the basis of active inflammation of the knee joint. Rheumatoid factor was detected in 90% of the RA patients and in 10% of the controls. The mean serum levels of IgM rheumatoid factor were as follows: RA, 48 ± 59 U/ml; ReA, 1 ± 1 U/ml; and OA, 2 ± 2 U/ml. Joint erosions were present in 29 of the 31 RA patients and absent in the controls. After 2 years of follow up, all RA patients had erosive disease, whereas erosions were again absent on the radiographs in the ReA and OA patients.
Table 1.
Demographic and clinical data of the 31 patients with RA, 20 with inflammatory osteoarthritis (OA), and 10 with reactive arthritis (ReA)
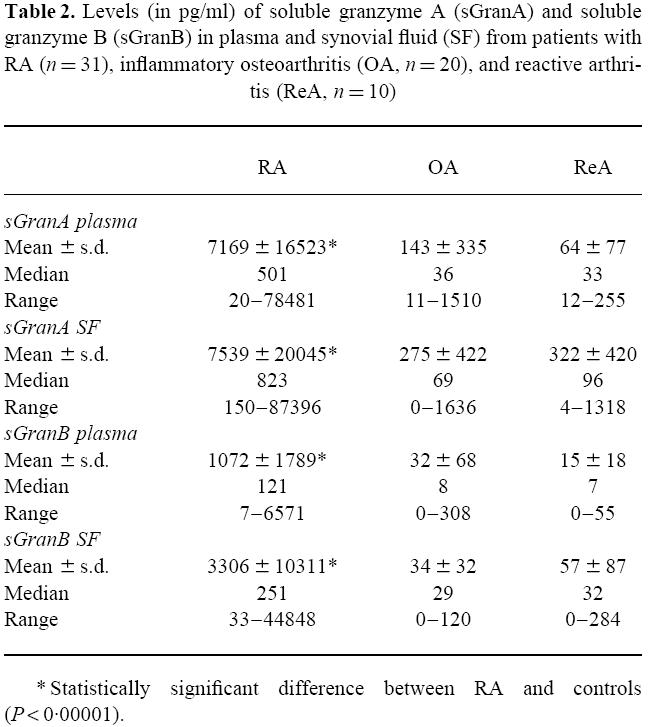
The descriptive statistics of the soluble granzyme measurements are shown in Table 2. The levels of sGranA and sGranB in plasma and synovial fluid of RA patients were markedly elevated compared with the two control groups (P < 0.00001), while levels in OA and ReA patients were similar. Although the sGranA levels tended to be higher in synovial fluid than plasma, the difference was not statistically significant (P = 0.18). The sGranB levels were higher in synovial fluid than plasma (P < 0.002). Positive correlations were noted between sGranA levels in plasma and synovial fluid (ρ = 0.54, P < 0.001); sGranB levels in plasma and synovial fluid (ρ = 0.78, P < 0.001); sGranA and sGranB levels in plasma (ρ = 0.96, P < 0.001); and the sGranA and sGranB levels in synovial fluid (ρ = 0.84, P < 0.001).
Table 2.
Levels (in pg/ml) of soluble granzyme A (sGranA) and soluble granzyme B (sGranB) in plasma and synovial fluid (SF) from patients with RA (n = 31), inflammatory osteoarthritis (OA, n = 20), and reactive arthritis (ReA, n = 10)
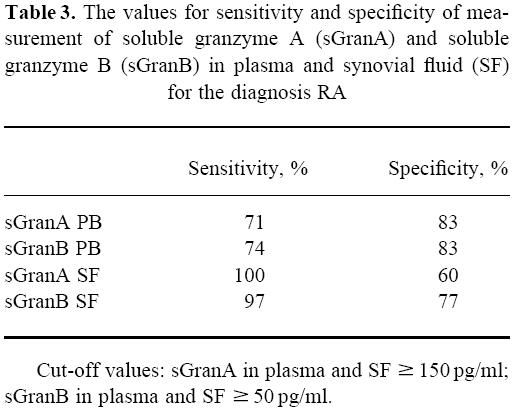
When values for sGranA and sGranB in plasma and synovial fluid were used simultaneously as independent variables, logistic regression analysis indicated that a diagnosis of RA could be predicted correctly in 26 of the 31 patients (84%) and a diagnosis of non-RA in 27 of the 30 patients (90%). In most RA patients, the levels of sGranA and sGranB in plasma and synovial fluid exceeded 150 pg/ml and 50 pg/ml, respectively (Fig. 1). The sensitivities and specificities at these cut-off values are shown in Table 3.
Fig. 1.
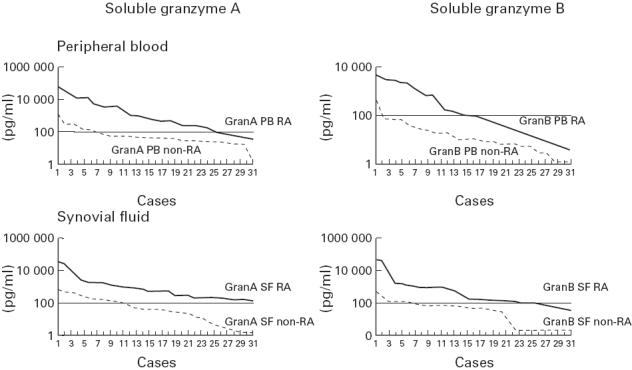
Levels (in pg/ml) of soluble granzymes A and B in plasma and synovial fluid from patients with RA (n = 31) compared with patients with other arthritides (inflammatory osteoarthritis, n = 20; and reactive arthritis, n = 10). The figure shows the individual data in a ranked order from the RA patients versus the non-RA patients.
Table 3.
The values for sensitivity and specificity of measurement of soluble granzyme A (sGranA) and soluble granzyme B (sGranB) in plasma and synovial fluid (SF) for the diagnosis RA
Elevated soluble granzyme levels were found in the RA patients regardless of disease duration. There were highly significant (all P < 0.005) correlations between soluble granzyme levels and serum levels of rheumatoid factor (sGranA plasma: ρ = 0.66; sGranA synovial fluid: ρ = 0.53; sGranB plasma: ρ = 0.66; sGranB synovial fluid: ρ = 0.74). However, increased levels of soluble granzymes were also present in the three rheumatoid factor-negative RA patients. Within the RA group, weakly positive correlations were found between soluble granzyme levels on the one hand and serum CRP levels, Ritchie articular index and the number of swollen joints on the other. These correlations were not statistically significant.
DISCUSSION
The increased number of granzyme-positive cytotoxic cells in synovial tissue distinguishes RA from other forms of arthritis [2,8]. However, the role if any for these cytotoxic cells in the pathogenesis of RA has remained unclear. Using a new ELISA which detects soluble GranA and GranB [12], we tested the hypothesis that RA synovium, secondary to the presence of lytically active cytotoxic cells, produces increased levels of these serine proteinases. Compared with OA and ReA disease controls, significantly higher levels of sGranA and sGranB were indeed detected in both plasma and synovial fluid, suggesting that this effector arm of the immune response is active within the joint. Furthermore, since synovial fluid exceeded plasma levels, the elevated plasma levels probably reflect synthesis and secretion from synovial tissue.
OA is a degenerative disease of articular cartilage where synovial inflammation is considered a consequence of tissue damage [23]. ReA, on the other hand, is usually a non-erosive immune-mediated synovitis. The absence of elevated levels in these disease states might suggest that activation of cytotoxic cells may be relatively specific to RA. The importance of this effector mechanism in the pathogenesis of RA is further underscored by the observation that levels of sGranA/B are considerably higher in RA than in patients with acute viral infections (Epstein–Barr virus (EBV) or HIV) [12], where the role of granule-mediated killing is well established [24,25]. Whether the increased levels of soluble granzymes point to an aetiological role of viruses in the pathogenesis of RA remains to be elucidated.
The selective increase in soluble granzyme levels in RA patients compared with disease controls raises the possibility that measurement of granzymes may assist the laboratory evaluation of patients with arthritis. At present, rheumatoid factor is the most frequently ordered for this purpose but the clinical utility of this test is limited. The sensitivity and specificity of sGranB levels in synovial fluid appears particularly promising. Larger studies in consecutive patients with early disease may clarify the role of this test system in differential diagnosis.
Although our results establish that extracellular granzymes are detectable in RA, the stimuli responsible for the secretory response is conjectural. It is generally accepted that release of the granzymes requires interaction with a target cell that induces granule exocytosis. Consequently, perforin delivers the granzymes intracellularly where the proteinases induce apoptosis by caspase-dependent (GranB) and -independent (GranA) pathways. Potential ‘targets’ that could elicit this response include the fibroblast-like synoviocytes, infiltrating macrophages, or subsets of T cells and B cells. The hyperplastic state of the rheumatoid synovium has been attributed to a dysregulated apoptotic response, perhaps as a consequence of impaired death receptor function (e.g. Fas–FasL) [26,27], and might be perpetuated by non-immunologic factors such as mutations of the p53 suppressor gene [28]. Therefore, activation of granule-mediated apoptosis may be a reactive attempt [29–31] to normalize this state of ineffective apoptosis by deleting fibroblast-like synoviocytes. Alternatively, the cytolytic response may be directed toward macrophages or lymphoid subsets. This possibility is supported by the recent report that mice deficient in perforin and hence in granule-mediated apoptosis have an accelerated development of autoimmune disease when crossed against MRL/lpr mice [32]. Finally, while granzyme release is clearly stimulus-dependent, activated cytotoxic T cells also have been reported in vitro to constitutively secrete these proteinases [33]. To learn whether similar events occur in vivo, experiments should be performed to measure accurately granzyme release from freshly explanted synovium.
In addition to their documented role in granule-mediated apoptosis, other functions have been proposed for the granzymes as mediators of extracellular proteolysis and cytokine induction [10,13–16,34]. Since GranB-positive cells are detectable at the cartilage–pannus junction where synovium invades cartilage and bone [17], secreted granzymes could participate in matrix dissolution [10,15,16,34]. In this regard the only potential non-caspase substrate for GranB is the aggrecan proteoglycan which is cleaved 400-fold more efficiently by the serine proteinase than by human stromelysin-1 [16]. Similarly, GranB has the capacity to degrade proteoglycans in whole articular cartilage explants and in a three-dimensional collagenous network synthesized by chondrocytes [17]. The apparent absence of an extracellular inhibitor would suggest that GranB could interact with articular cartilage to degrade proteoglycans and thereby act in concert with other proteolytic enzymes to mediate tissue damage. In line with this hypothesis, markedly increased sGranB levels were only found in RA and not in non-erosive joint disease.
GranA has been reported to stimulate production of TNF-α, IL-6, and IL-8 by monocytes [13] and IL-6 and IL-8 by fibroblasts [14]. TNF-α, IL-6 and IL-8, abundantly expressed in the synovium, are mainly produced by macrophages and fibroblast-like synoviocytes [1,35–37]. TNF-α is a potent proinflammatory cytokine with stimulating effects on other cytokines [38]. The role of IL-6 in the pathogenesis of synovial inflammation is at present unclear. IL-8 is a chemokine that plays a key role in the migration of neutrophils into the joints [36]. Therefore, the high level of sGranA in the RA joint could promote synovial inflammation due to its effects on cytokine production.
Taken together, the elevated levels of sGranA and sGranB in plasma and synovial fluid of RA patients strongly suggest that cytotoxic cells are active participants in the pathogenesis of RA. With the availability of GranA and GranB knock-out mice [25,39], the influence of these granule-associated serine proteinases on the development of arthritis in various murine models is now amenable to experimental analysis.
Acknowledgments
We are indebted to Dr W. Post (Department of Medical Statistics, Leiden University Medical Centre) for helpful comments. P.P.T. is supported by a NATO-Science Fellowship and the Dutch Arthritis Foundation (Nationaal Reumafonds) and C.J.F. is supported by the Illinois Chapter of the American Arthritis Foundation.
REFERENCES
- 1.Tak PP, Smeets TJM, Daha MR, et al. Analysis of the synovial cellular infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–25. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 2.Tak PP, Kummer JA, Hack CE, et al. Granzyme positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994;37:1735–43. doi: 10.1002/art.1780371205. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths GM, Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J Cell Biol. 1993;120:885–96. doi: 10.1083/jcb.120.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froelich CJ, Dixit VM, Yang XH, Yang X. Lymphocyte granule-mediated apoptosis: matters of viral mimicry and deadly proteases. Immunol Today. 1998;19:30–36. doi: 10.1016/s0167-5699(97)01184-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu CC, Young LHY, Young JDE. Mechanisms of disease: lymphocyte-mediated cytolysis and disease. N Engl J Med. 1996;335:1651–9. doi: 10.1056/NEJM199611283352206. [DOI] [PubMed] [Google Scholar]
- 6.Kummer JA, Tak PP, Brinkman B, et al. Expression of granzymes A and B in synovial tissue from patients with rheumatoid arthritis and osteoarthritis. Clin Immunol Immunopathol. 1994;73:88–95. doi: 10.1006/clin.1994.1173. [DOI] [PubMed] [Google Scholar]
- 7.Muller-Ladner U, Kriegsmann J, Tschopp J, Gay RE, Gay S. Demonstration of granzyme A and perforin messenger RNA in the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:477–84. doi: 10.1002/art.1780380404. [DOI] [PubMed] [Google Scholar]
- 8.Smeets TJM, Dolhain RJEM, Breedveld FC, Tak PP. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998;186:75–81. doi: 10.1002/(SICI)1096-9896(199809)186:1<75::AID-PATH142>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths GM, Alpert S, Lambert E, McGuire J, Weissman IL. Perforin and granzyme A expression identifying cytolytic lymphocytes in rheumatoid arthritis. Proc Natl Acad Sci USA. 1992;89:549–53. doi: 10.1073/pnas.89.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young LH, Joag SV, Lin PY, et al. Expression of cytolytic mediators by synovial fluid lymphocytes in rheumatoid arthritis. Am J Pathol. 1992;140:1261–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Takayama H, Trenn G, Humphrey W, JR, Bluestone JA, Henkart PA, Sitkovsky MV. Antigen receptor-triggered secretion of a trypsin-type esterase from cytotoxic T lymphocytes. J Immunol. 1987;138:566–9. [PubMed] [Google Scholar]
- 12.Spaeny-Dekking EHA, Hanna WL, Wolbink AM, et al. Extracellular granzymes A and B in man: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–6. [PubMed] [Google Scholar]
- 13.Sower LE, Froelich CJ, Allegretto N, Rose PM, Hanna WD, Klimpel GR. Extracellular activities of human granzyme A—monocyte activation by granzyme A versus alpha-thrombin. J Immunol. 1996;156:2585–90. [PubMed] [Google Scholar]
- 14.Sower LE, Klimpel GR, Hanna W, Froelich CJ. Extracellular activities of human granzymes. 1. Granzyme A induces IL-6 and IL-8 production in fibroblast and epithelial cell lines. Cell Immunol. 1996;171:159–63. doi: 10.1006/cimm.1996.0187. [DOI] [PubMed] [Google Scholar]
- 15.Simon MM, Simon HG, Fruth U, Epplen J, Muller-Hermelink HK, Kramer MD. Cloned cytolytic T-effector cells and their malignant variants produce an extracellular matrix degrading trypsin-like proteinase. Immunol. 1987;60:219–30. [PMC free article] [PubMed] [Google Scholar]
- 16.Froelich CJ, Zhang X, Turbov J, Hudig D, Winkler U, Hanna WL. Human granzyme B degrades aggrecan proteoglycan in matrix synthesized by chondrocytes. J Immunol. 1993;151:7161–71. [PubMed] [Google Scholar]
- 17.Ronday HK, Tak PP, Bank R, et al. Granzyme B degrades cartilage matrix in vitro and is present at the pannus–cartilage junction. Arthritis Rheum. 1997;40:S75. [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Altman R, Asch ES, Bloch DA, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 20.Heesemann J, Eggers C, Schroder J. Serological diagnosis of yersiniosis by immunoblot technique using virulence-associated antigen of enteropathogenic Yersiniae. Contrib Microbiol Immunol. 1987;9:285–9. [PubMed] [Google Scholar]
- 21.Tak PP, Visser LG, Hoogkamp Korstanje JA, et al. Unusual manifestations of Yersinia enterocolitica infections diagnosed using novel methods. Clin Infect Dis. 1992;15:645–9. doi: 10.1093/clind/15.4.645. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie DM, Boyle JA, McInnes JM, et al. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968;37:393–406. [PubMed] [Google Scholar]
- 23.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–71. [PubMed] [Google Scholar]
- 24.Mullbacher A, Ebnet K, Blanden RV, et al. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–7. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–87. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 26.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995;96:1631–8. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tak PP, Firestein GS. Apoptosis in rheumatoid arthritis. In: Winkler JD, editor. Apoptosis and inflammation. Basel: Birkhäuser Publishing Ltd; 1999. in press. [Google Scholar]
- 28.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berke G. The Fas-based mechanism of lymphocytotoxicity. Hum Immunol. 1997;54:1–7. doi: 10.1016/s0198-8859(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 30.Talanian RV, Yang XH, Turbov J, et al. Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J Exp Med. 1997;186:1323–31. doi: 10.1084/jem.186.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medema JP, Toes REM, Scaffidi C, et al. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T lymphocyte-induced apoptosis. Eur J Immunol. 1997;27:3492–8. doi: 10.1002/eji.1830271250. [DOI] [PubMed] [Google Scholar]
- 32.Peng SL, Moslehi J, Robert ME, Craft J. Perforin protects against autoimmunity in lupus-prone mice. J Immunol. 1998;160:652–60. [PubMed] [Google Scholar]
- 33.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–9. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 34.Simon MM, Kramer MD, Prester M, Gay S. Mouse T-cell associated serine proteinase 1 degrades collagen type IV: a structural basis for the migration of lymphocytes through vascular basement membranes. Immunol. 1991;73:117–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Chu CQ, Field M, Allard S, Abney E, Feldmann M, Maini RN. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992;31:653–61. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- 36.Rathanaswami P, Hachicha M, Wong WL, Schall TJ, McColl SR. Synergistic effect of interleukin-1 beta and tumor necrosis factor alpha on interleukin-8 gene expression in synovial fibroblasts. Evidence that interleukin-8 is the major neutrophil-activating chemokine released in response to monokine activation. Arthritis Rheum. 1993;36:1295–304. doi: 10.1002/art.1780360914. [DOI] [PubMed] [Google Scholar]
- 37.Koch AE, Kunkel SL, Strieter RM. Cytokines in rheumatoid arthritis. J Invest Med. 1995;43:28–38. [PubMed] [Google Scholar]
- 38.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 39.Ebnet K, Hausmann M, Lehmann-Grube F, et al. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995;14:4230–9. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


