Abstract
Theileria annulata is a tick-borne protozoan parasite which causes the disease bovine tropical theileriosis. In immunized or drug-treated animals, the pathogenic macroschizont stage of the parasite is destroyed by MHC class I-restricted cytotoxic T lymphocytes (CTL). Here we show that although CD8+ T cells increase greatly in number and display activation markers during an acute infection, they exhibit no killing of infected cells. During the ineffectual response, efferent lymph cells' ability to proliferate to IL-2 drops, coinciding with loss of MoAb binding to CD2 by CD8+ cells. When animals were treated with the anti-parasite drug ‘Butalex’, IL-2 responses, anti-CD2 antibody binding by CD8+ cells and strong CTL activity were restored within 24 h. The initial activation of CD4+ T cells by parasite-infected cells altering the IL-2 production in the draining lymph node is the likely cause of the failure of CTL responses.
Keywords: Theileria annulata, IL-2, T cells, cytotoxicity
INTRODUCTION
Theileria annulata is a tick-transmitted protozoan parasite, affecting cattle in Southern Europe, North Africa, India, the Middle East, Southern former USSR and China, and is the causative agent of tropical theileriosis. This disease is of particular importance, as high-yielding ‘exotic’ European cattle imported into endemic areas are extremely susceptible to disease. Animals can develop immunity to the disease through parasite challenge and treatment with the drug ‘Butalex’ (buparvaqoune injection, Mallinckrodt Veterinary, UK; from here on referred to as Butalex) or use of an attenuated live vaccine. However, infection of naive susceptible cattle leads to rapid disease progression, with death commonly within 2–3 weeks of experimental infection depending on the dose of parasite given [1,2].
The principal cells infected and transformed by sporozoites (infective stage) are of macrophage/monocyte lineage [3–5], and infected cells develop in the lymph node draining the site of infection. The majority of disease pathology is associated with the macroschizont stage—full disease symptoms can be produced by this stage [6,7].
Parasite clearance in vaccinated or recovered drug-treated animals is thought to be principally effected by MHC class I-restricted cytotoxic T lymphocytes (CTL) [8,9], which have been shown to lyse macroschizont-infected cells. It has not been possible to isolate such CTL from naive animals undergoing primary infection [9]. Recently, we have shown that infection with T. annulata interferes with T cell development in the local draining lymph node by disrupting normal T cell development pathways—T cells are activated ‘non-specifically’ by parasitized cells, rather than interacting with normal antigen-presenting cells (APC) [10] (Macroschizont-infected cells possess an activity akin to a superantigen, although not yet fully characterized [10,11]). Non-protective T cell responses result in an initial ‘burst’ of CD25+ T cells in the node, but this is not sustained, and the numbers of T cells expressing CD25 drop to background levels within a week of infection [11]. The effects that the failure to develop normal helper T cell responses have on CTL function are unknown.
The aim of this study was to investigate the mechanisms underlying the failure of naive animals to produce functional CTL during T. annulata infection. Using efferent lymphatic cannulation, we have examined in detail the comparative development of CTL in lymph efferent from nodes draining the site of infection of naive and immune cattle. During infection of naive cattle the ineffective T cell responses were examined until the severity of disease symptoms necessitated drug treatment. The animals were subsequently assessed during the recovery period when immune mechanisms were fully functional.
MATERIALS AND METHODS
Animals and cannulations
Three Friesian × Brown Swiss or Charolais calves aged 3–8 months were used in this study. Two were previously unexposed to T. annulata (animals 1 and 2), and one was immune to the parasite, having undergone cell line immunization and sporozoite challenge (animal 3). The efferent lymphatic duct of the animals' prescapular lymph node was cannulated as previously described [12].
Infection with T. annulata and drug treatment
The two naive animals were inoculated with 0.1 tick equivalents (TE) of T. annulata sporozoites (Gharb strain [13]), in the shoulder above the cannulated lymph node 24 h post-cannulation. Both animals developed severe clinical tropical theileriosis, and were treated with Butalex (5 mg/kg) [14] (Malinkrodt Veterinary) as soon as food intake decreased (day 10 post-infection animal 1, day 11 animal 2). Both animals made a full recovery. The immune animal was cannulated on the right shoulder, and baseline measurements taken. Unfortunately this cannula became blocked. The animal was therefore re-challenged with sporozoites on the left shoulder and cannulated 5 days post-infection. Baseline day ‘0’ levels shown for this animal therefore refer to the first cannulation, and the re-challenge values to the second cannulation.
Lymph collection
Lymph was collected into sterile plastic bottles containing 5–10 U/ml heparin (Leo Laboratories, UK) and antibiotics to give a final concentration of penicillin 20 U/ml and streptomycin 20 μg/ml when the bottle was full. The bottle was changed two to four times per day depending on flow rate. Fresh lymph was collected for 1 h each morning for use in functional studies. Remaining lymph was pooled aseptically and re-infused via a jugular cannula to maintain fluid, electrolyte and protein balance, and to avoid any interference with the development of systemic immunity.
Cell preparation
Efferent lymph leucocytes (ELL) were prepared by centrifuging lymph at 800 g for 10 min, re-suspending cells in RPMI 1640 medium, and then separation over Ficoll–Hypaque (‘Lymphoprep’; Nycomed, Oslo, Norway) as previously described (for peripheral blood mononuclear cells (PBMC) [15]). The viability of efferent lymph cells was always ≥ 98% as assessed by trypan blue exclusion.
FACS analysis
Cells were stained for dual-colour FACS analysis as previously described [10]. Analysis concentrated on the expression of activation markers on different T cell subsets. Efferent lymph cells were stained using anti-CD2, CD4, CD8 and WC1 (γδ T cells) (MoAbs IL-A26 [16]; CC8 [17]; SBU-T8 [18]; CC15 [19], respectively). Activation marker expression by T cells was detected using MoAb to CD25 (IL-A111 [20]) and MHC class II (DR) (J11 [21]). Cells were analysed using a Becton Dickinson (Oxford, UK) FACScan and PC Lysys software.
Cytotoxicity assays
Anti-T. annulata cytotoxic activity was assayed using chromium release as previously described [8]. Briefly, ELL were incubated for 4 h with target cells labelled with 0.5 mCi/ml 51Cr (as sodium chromate) (Amersham, Aylesbury, UK). The ratio of effector:targets was varied from 80:1 to 5:1. Targets were previously generated autologous T. annulata macroschizont-infected cell lines or uninfected autologous concanavalin A (Con A) blasts as previously described [8]. Chromium release was assessed by scintillation counting using a Wallac 1450-Microbeta scintillation counter.
Proliferation assays
The ability of ELL to respond to IL-2 and autologous T. annulata macroschizont-infected cells was assessed by thymidine uptake assays essentially as previously described [10]. ELL were grown in 96-well flat-bottomed plates (Nunc, Roskilde, Denmark) and stimulated with medium alone, or 40 U/ml hIL-2 (Boehringer, Mannheim, Germany) for 72 h. ELL also were cultured with autologous irradiated T. annulata macroschizont-infected cells as previously described [6]. Cells were pulsed with 3H-dThd (Amersham) in the last 6 h of culture, and uptake was measured by liquid scintillation counting in a Wallac 1450 Microbeta.
RESULTS
Proliferative capacity of ELL
In the naive animals 1 + 2, ELL proliferated to IL-2, with both showing highest responses at day 8. This declined rapidly by day 10, but was strongly regained following drug treatment (Fig. 1a). In the immune animal, IL-2 responses were maximal at day 5, with a steady decline to baseline levels by day 17 (Fig. 1b)
Fig. 1.
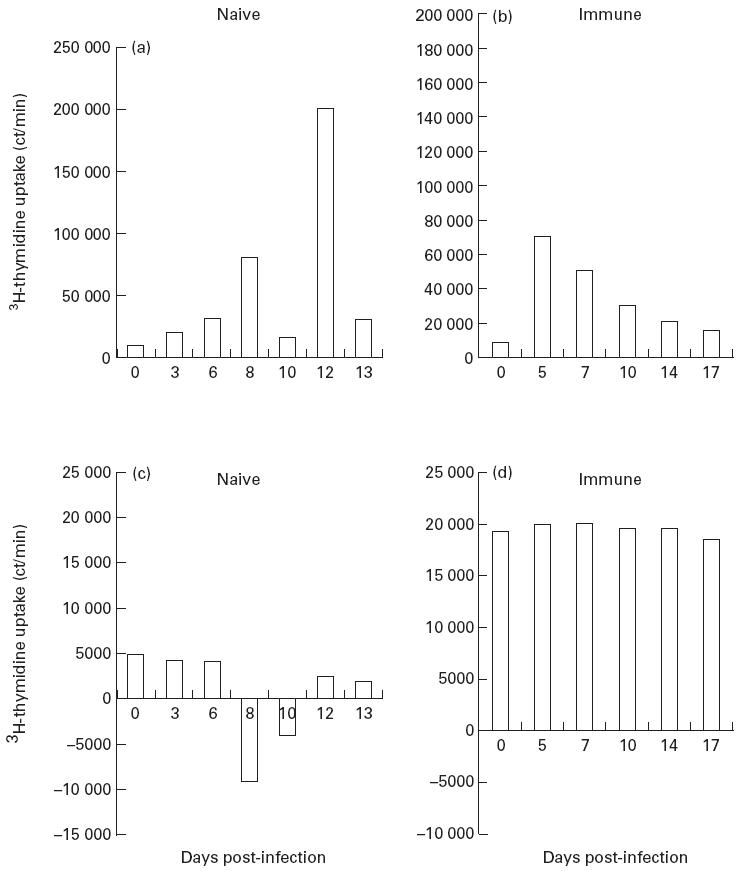
Proliferation of efferent lymph leucocytes (ELL) from naive animal 2 and immune animal 3 to (a,b) IL-2 and (c,d) autologous Theileria annulata-infected cells. Values expressed are net ct/min, i.e. test values minus background ct/min, thus negative values indicate proliferation of ELL lower than background controls.
ELL from naive animals displayed normal ‘non-specific’ proliferative responses of T cells to autologous macroschizont-infected cells [10] at day 0, and in the first few days of infection. As infection progressed, no proliferation was induced in ELL by autologous infected cells until after Butalex treatment, which restored levels of proliferation to approximately those seen before infection (Fig. 1c). The immune animal showed high proliferative responses against autologous infected cells at day 0, which remained essentially constant throughout infection (Fig. 1d).
Kinetics of CD4+ cells
Previous studies of acute tropical theileriosis have indicated a transient appearance of activated T cells in the draining lymph node. Activated CD4+ cells are seen in the lymph node medulla followed by an apparent migration from the node of these cells—CD25+CD4+ cells are not seen in infected nodes by day 8 [10,22]. This pattern was seen in the efferent lymph of naive animals 1 and 2, with a transient increase in the relative percentage of efferent lymph CD4+ cells peaking at days 7 and 8, respectively (Fig. 2a). The peak of CD4+ cells coincided with their highest expression of CD25 and MHC class II (≥ 37% CD4+CD25+; ≥ 40% CD4+ MHC class II+). The peak of CD25+ cells coincided with the day 8 peak of IL-2 responsiveness. The levels of CD4+ cells, and their expression of activation markers (results not shown), rapidly dropped to below baseline levels thereafter (Fig. 2a).
Fig. 2.
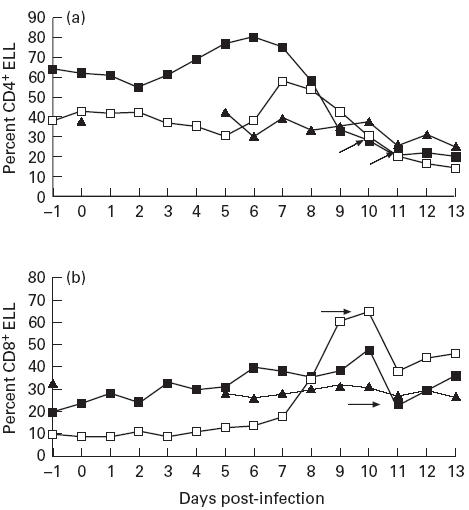
Relative percentages of (a) CD4+ and (b) CD8+ lymphocytes in the efferent lymph of cattle post Theileria annulata sporozoite challenge. Arrows indicate day of Butalex treatment of naive animals. ▪, Naive 1; □, naive 2; ▴, immune 3. ELL, Efferent lymph leucocytes.
In the immune animal number 3, the relative percentage of CD4+ cells fluctuated by ± 20% from baseline post-challenge, with a steady decrease by day 12 (Fig. 2a). This was accompanied by an increase in the expression of MHC class II and CD25 by small numbers (approx. 10%) of these cells (data not shown), dropping to normal again from day 12.
Kinetics of CD8+ cells
The relative percentage of CD8+ cells in ELL of animals 1 and 2 increased steadily post-infection, with a transient drop on day 11, corresponding to severest disease. This drop was partially reversed post-drug treatment (Fig. 2b). CD8+ cells showed a steady increase in expression of the activation marker MHC class II, with > 80% of these cells expressing MHC class II by day 11 of infection in both animals (results not shown).
As for CD4, the immune animal number 3 showed a small stable increase in MHC class II expression by CD8+ cells maintained until day 12, when numbers steadily decreased to baseline levels (data not shown). No significant increase in CD25 expression by CD8+ cells was seen from any of the animals during infection, irrespective of the levels of CTL activity detected in lymph (data not shown).
There was no increase seen in the numbers of, or activation marker expression by, γδ T cells at any point of infection in any of the animals (data not shown).
CTL activity is absent from the blood and lymph of infected naive animals
No cytotoxic activity was observed in the blood or efferent lymph of the naive animals during the course of T. annulata infection in direct cytotoxicity assays against autologous T. annulata-infected cells (Fig. 3). In contrast, anti-parasite cytotoxic responses were detected in the immune animal by 5 days post-infection, reaching a peak in efferent lymph at day 10 (Fig. 3). Peak cytotoxicity levels in blood were found 24–48 h after the peak in lymph (Fig. 3). No cytotoxicity was detected against autologous uninfected Con A blasts at any point from any animal (results not shown).
Fig. 3.
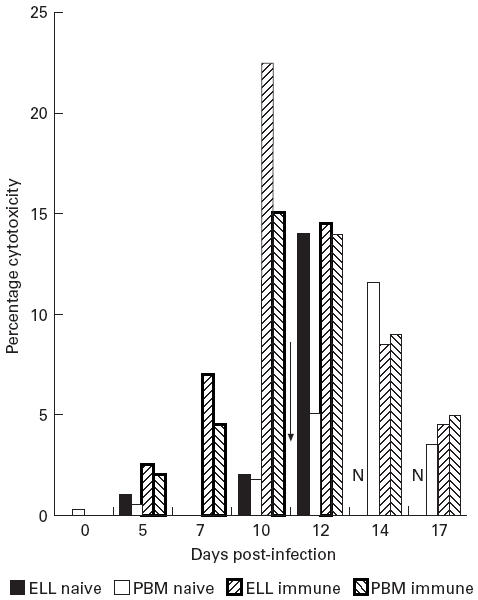
Killing of autologous Theileria annulata-infected cells by efferent lymph leucocytes (ELL) of naive animal 2 and immune animal 3. Effector:target ratio of 40:1. Arrow indicates day of Butalex treatment of naive animal. N, No lymph results post-day 13 in naive animal.
Butalex treatment restores cytotoxic function in acutely infected animals
Treatment of acutely infected animals with Butalex, which rapidly kills the intracellular macroschizont [14], led to a virtually instant appearance of cytotoxic activity in lymph from animals 1 and 2, peaking 48 h post-drug treatment (Fig. 3). Due to the high output rate from the infected lymph nodes (up to 300 ml/h from infected nodes [23]) it was decided on animal welfare grounds to terminate the cannulation of the naive animals by 14 days post-infection. CTL activity was subsequently measured from PBMC (isolated from venous blood using 20% acid citrate dextrose (ACD) as anticoagulant) for the remainder of the experiments. Following drug treatment, CTL activity in PBMC was comparable to ELL with a 24–48 h delay. Cytotoxic activity of PBMC steadily decreased from day 14, but was still detectable 7 days post-drug treatment. (Fig. 3).
Loss and restoration of CTL activity is associated with expression of CD2
In the immune animal number 3, which produced high levels of CTL activity, CD8+ T cells remained > 98% CD2+ throughout the course of parasite challenge (results not shown). In the naive animals, > 96% of CD8+ cells expressed CD2 on day 0. However, there was a marked loss of CD2 surface expression by CD8+ cells following infection, peaking 9–11 days post infection—(60% of all ELL CD8+ cells from both animals did not stain with anti-CD2 on the day of drug treatment (Fig. 4). At the peak of CD2−CD8+ cells in the lymph, no CTL activity was detected.
Fig. 4.
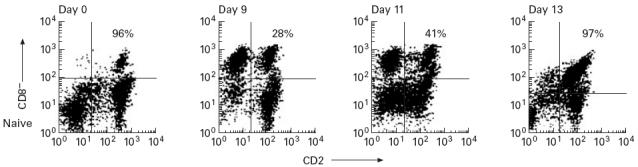
Expression of CD2 by CD8+ cells of naive animal 2 (percentages relate to numbers of CD8+CD2+ cells). Only 28% of CD8+ cells are recognized by anti-CD2 MoAb at day 9, and this lack of MoAb binding is maintained until day 11 (day of Butalex treatment, sample taken pre-Butalex). By day 13, when cytotoxic T lymphocyte (CTL) activity is at its peak, CD2 expression is completely restored.
CD2 expression by CD8+ cells was restored 24–48 h after Butalex treatment (Fig. 4), and coincided with the appearance of CTL activity in blood and lymph.
DISCUSSION
T cell responses are severely disrupted during acute T. annulata infection [10]. ‘Non-specific’ activation, primarily of CD4+ T cells, by parasite-infected APC in the draining lymph node results in the production of interferon-gamma (IFN-γ), but this response does not provide protection [10,11]. Although it was apparent that the alteration of T cell responses may have been responsible for the lack of anti-parasite CTL in naive animals, the mechanisms underlying this failure were not clear. In this study, we show that IL-2 responses are not sustained in the infected lymph nodes, and this is accompanied by a loss of CD2 expression by T cells and the failure to generate CTL responses.
Characteristics of a successful immune response
The successful response to challenge by the immune animal was characterized by both proliferative and cytotoxic responses against macroschizont-infected cells. A small increase in both numbers of, and activation marker expression by, CD4+ and CD8+ T cells corresponded to high IL-2 responsiveness by day 5. Strong CTL responses developed from this point on. These results again emphasize the previously reported importance of T cells in parasite clearance [8,9].
It seems likely that ‘simple’ activation of T cells and the production of IL-2 are the principal requirements for anti-parasite immunity. It has already been demonstrated that IFN-γ (and presumably activation of innate defence mechanisms) does not confer protection in primary acute infections, and indeed may exacerbate infection [11]. Although anti-parasite antibodies are produced following recovery from infection or immunization with recombinant antigen, satisfactory protection cannot be engendered by antibody alone [24]. Skewing of immune responses to a Th1 or Th2 cytokine profile therefore does not appear to be successful in anti-parasite responses. It seems likely that the protective response is similar to the Th0 responses in Fasciola infection of cattle [25].
The immune animal's ELL showed consistently higher proliferative responses to autologous T. annulata-infected cells than the ‘non-specific’ T cell activation induced in naive ELL. The elevated proliferation may signify anti-parasite memory contained in ELL from immune animals, although attempts to isolate these ‘recall’ T cells have proved unsuccessful to date (J.D.M. Campbell and E.J. Glass, unpublished observations).
IL-2 responses are not sustained in the lymph node
Previous studies indicated that although draining lymph node CD4+ cells expressed CD25 soon after infection, these cells were then lost from the node by approx. 8 days post-acute infection [10,22]. The experiments described here show that the activated T cells leave the node via the efferent lymphatic, with the ability of ELL to respond to IL-2 virtually ablated by 10 days of infection. The short window of CD4+ T cell activation and IL-2 responsiveness is due to the non-specific activation of T cells by parasitized APC [10]. Even when IL-2-responsive, ELL showed profoundly suppressed proliferative responses to T. annulata-infected cells, confirming previous observations that the activated T cells seen early in infection are extremely unlikely to mediate anti-parasite immunity [10,11].
Modulation of CTL function
The failure to detect CTL responses from acutely infected animals does not appear to be simply due to this immune mechanism not being stimulated. The numbers of CD8+ T cells in efferent lymph of acutely infected animals increase vastly, and virtually all express the activation marker MHC class II (BoLA DR) by days 9–10 post-infection. This is the phenotype of CTL mediating anti-parasite immunity in animals vaccinated against T. annulata [23]. This response is greatly exaggerated, compared with the low levels of activated CD8+ cells seen in ELL from an immune animal. The differences may be due to the rapid expansion required in the generation of a primary response compared with the recall of memory cells from a precursor pool of greatly increased frequency in the secondary response. There are two linked factors apparent from this study which could account for the failure of this greatly expanded pool of CD8+ cells to mediate CTL activity: the loss of IL-2 responsiveness and the modulation of CD2.
The peak of cytotoxic activity in the immune animal corresponded to the peak of IL-2 responsiveness in the node. While IL-2 responses were initially high in the naive animals, they dropped dramatically with the exit of CD4+CD25+ cells in the efferent lymph. Either these cells were responsible for the IL-2 responses observed (and the CD8 cells were not responding) or the CD4 cells were facilitating proliferation by the CD8 cells. Either way, this response was removed by day 8 of infection, which will have contributed to the CTL inactivity.
There is a clear association between the ability of ELL to mediate CTL activity, and the ability of anti-CD2 MoAb to bind to CD8+ cells. CD2 binding to LFA-3 on target cells is an essential step in the killing of targets by CTL [26]. In the immune animal, with efficient CTL responses, no variation in binding of anti-CD2 MoAb was observed. In the naive animals, despite the extremely large numbers of activated CD8+ cells in ELL, no CTL activity was detected pretreatment, coinciding with the loss of CD2 MoAb binding to CD8+ cells. Following anti-parasite drug treatment, IL-2 responses, CTL activity and CD2 MoAb binding were restored. The ability to detect peak CTL activity directly after drug treatment strongly suggests that CTL responses to the parasite are ‘turned off’ rather than absent. CTL activity follows a normal (bell-shaped) distribution in the immune animal, whereas the instant high level of activity following drug treatment suggests that the response is blocked until the parasite is removed.
Although it is possible that parasitized cells are directly inhibiting CD2 transcription or surface expression of the whole molecule, it seems more likely that the epitope to which the anti-CD2 antibody binds is altered. Changes in CD2 epitope expression have been previously linked to the failure of T cell responses [27]. Specifically, activated T cells can become anergized without proper costimulation. This is characterized by the loss of epitopes on CD2 resulting in a failure to bind LFA-3. Restoration of CD2 epitopes through exposure to IL-2 induces binding to LFA-3, restoring T cell function [27].
The series of events leading to the failure of CTL responses in cattle acutely infected with T. annulata, and their subsequent restoration, closely follows those described above, i.e. coincident with the loss of IL-2 responses, by day 10, > 60% of CD8+ cells have lost the epitope recognized by anti-CD2 MoAb and are not functional. When the parasite is eliminated, ELL instantly re-acquire the ability to respond to exogenous IL-2, accompanied by a restoration of CD2, and immediate CTL activity, presumably as CD8+ cells can bind to targets. The restoration of CD2 in this in vivo system (24–48 h) is quicker than in the in vitro model (up to 7 days), but the in vitro anergized cells may represent an artificially extreme case.
In conclusion, this study has shown that successful immune responses to T. annulata are characterized by extremely efficient responses to IL-2, accompanied by a strong CTL killing of macroschizont-infected cells. In contrast, despite activation of large numbers of CD8+ T cells, CTL responses are not effective during acute T. annulata infection. This is associated with alteration of CD2 expression by the CD8+ T cells. This anergy of CTL correlates with cessation of IL-2 production when parasite-activated CD4+ T cells leave the lymph node. Thus, the initial activation of CD4+ T cells by parasite-infected cells altering the IL-2 production in the draining lymph node is the likely cause of the failure of CTL responses.
Acknowledgments
This work was funded by the European Union R&D programme on Science and Technology TS3-CT92-0143, and the Overseas Development Administration, UK. A.K.N. was in receipt of a Commonwealth Scholarship. The authors would like to thank Professor C. G. D. Brown (Centre for Topical Veterinary Medicine, University of Edinburgh) for providing T. annulata sporozoites. We are also most grateful to Dr J. Hopkins (Veterinary Pathology, University of Edinburgh); Dr C. Howard, I.A.H. Compton and ILRI, Kenya, for the gift of MoAbs.
REFERENCES
- 1.Preston PM, Brown CGD, Bell-Sakyi L, Richardson W, Sanderson A. Tropical theileriosis in Bos taurus and Bos taurus cross Bos indicus calves: response to infection with graded doses of sporozoites of Theileria annulata. Res Vet Sci. 1992;53:230–43. doi: 10.1016/0034-5288(92)90115-i. [DOI] [PubMed] [Google Scholar]
- 2.Samantaray SN, Bhattacharyulu Y, Gill BS. Immunisation of calves against bovine tropical theileriosis (Theileria annulata) with graded doses of sporozoites and irradiated sporozoites. Int J Parasitol. 1980;10:355–8. doi: 10.1016/0020-7519(80)90035-1. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JDM, Brown DJ, Glass EJ, Hall FR, Spooner RL. Theileria annulata sporozoite targets. Parasite Immunol. 1994;16:501–5. doi: 10.1111/j.1365-3024.1994.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 4.Glass EJ, Innes EA, Spooner RL, Brown CGD. Infection of bovine monocyte/macrophage populations with Theileria annulata and Theileria parva. Vet Immunol Immunopathol. 1989;22:355–68. doi: 10.1016/0165-2427(89)90171-2. [DOI] [PubMed] [Google Scholar]
- 5.Spooner RL, Innes EA, Glass EJ, Brown CGD. Theileria annulata and T. parva infect and transform different mononuclear cells. Immunol. 1989;66:284–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Hooshmand-Rad P. The pathogenesis of anaemia in Theileria annulata infection. Res Vet Sci. 1976;20:324–9. [PubMed] [Google Scholar]
- 7.Pipano E, Tsur I. Experimental immunization against Theileria annulata with a tissue culture vaccine. Laboratory trials. Refuah Vet. 1966;23:186–94. [Google Scholar]
- 8.Innes EA, Millar P, Brown CGD, Spooner RL. The development and specificity of cytotoxic cells in cattle immunized with autologous or allogeneic Theileria annulata infected lymphoblastoid cell lines. Parasite Immunol. 1989;11:57–68. doi: 10.1111/j.1365-3024.1989.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Preston PM, Brown CGD, Spooner RL. Cell-mediated cytotoxicity in Theileria annulata infection of cattle with evidence for BoLA restriction. Clin Exp Immunol. 1983;53:88–100. [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell JDM, Howie SEM, Odling KA, Glass EJ. Theileria annulata induces aberrant T cell activation in vitro and in vivo. Clin Exp Immunol. 1995;99:203–10. doi: 10.1111/j.1365-2249.1995.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JDM, Brown DJ, Nichani AK, Howie SEM, Spooner RL, Glass EJ. A non-protective T helper 1 response against the intra-macrophage protozoan Theileria annulata. Clin Exp Immunol. 1997;108:463–70. doi: 10.1046/j.1365-2249.1997.3861290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass EJ, Spooner RL. Cellular Immunity in cattle. In: Weir D, editor; Herzenberg L, editor; Herzenberg L, editor. The handbook of experimental immunology. 5. Cambridge, MA: Blackwell Scientific Publications; 1997. [Google Scholar]
- 13.Ouhelli H. Recherche sur la Biologie des vecteurs (Hyalomma spp.) et sur les interactions hote–parasite. I.N.P. Toulouse; 1985. Theileriose Bovine a Theileria annulata (Dschunkowsky and Luhs, 1904) PhD Thesis. [Google Scholar]
- 14.McHardy N, Wekesa LS, Hudson AT, Randall AW. Antitheilerial activity of BW720C (buparvaquone): a comparison with parvaquone. Res Vet Sci. 1985;39:29–33. [PubMed] [Google Scholar]
- 15.Glass EJ, Spooner RL. Requirement for MHC class II positive accessory cells in an antigen specific bovine T cell response. Res Vet Sci. 1989;46:196–201. [PubMed] [Google Scholar]
- 16.Baldwin CL, MacHugh ND, Ellis JA, Naessens J, Newson J, Morrison WI. Monoclonal antibodies which react with bovine T-lymphocyte antigens and induce blastogenesis: tissue distribution and functional characteristics of the target antigens. Immunol. 1988;63:439–46. [PMC free article] [PubMed] [Google Scholar]
- 17.Howard CJ, Sopp P, Parsons KR, McKeever DJ, Taracha ELN, Jones BV, MacHugh ND, Morrison WI. Distinction of naive and memory BoCD4 lymphocytes in calves with a monoclonal antibody, CC76, to a restricted determinant of the bovine leukocyte-common antigen, CD45. Eur J Immunol. 1991;21:2219–26. doi: 10.1002/eji.1830210933. [DOI] [PubMed] [Google Scholar]
- 18.Maddox JF, Mackay CR, Brandon MR. Surface antigens, SBU-14 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunol. 1985;55:739–48. [PMC free article] [PubMed] [Google Scholar]
- 19.Clevers H, MacHugh ND, Bensaid A, et al. Identification of a bovine surface antigen uniquely expressed on CD4−CD8− T cell receptor γ/δ+ T lymphocytes. Eur J Immunol. 1990;20:809–17. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- 20.Naessens J, Sileghem M, MacHugh N, Park YK, Davis WC, Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunol. 1992;76:305–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Dutia BM, MacCarthy-Morrogh L, Glass EJ, Knowles G, Spooner RL, Hopkins J. Discrimination between major histocompatibility complex class II DR and DQ products in cattle. Anim Genet. 1995;26:111–4. doi: 10.1111/j.1365-2052.1995.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JDM, Fraser DC, Howie SEM, Glass EJ. Early T cell activation in response to Theileria annulata. In: Spooner R, Campbell J, editors. Proceedings of the 3rd EU Co-Ordination Meeting on Tropical Theileriosis. Edinburgh: Roslin Institute; 1994. pp. 123–7. [Google Scholar]
- 23.Nichani AK, Thorpe BH, Brown CGD, Campbell JDM, Brown DJ, Ritchie M, Spooner RL. In vivo development of Theileria annulata: Major changes in efferent lymph following infection with sporozoites and allogeneic schizont-infected cells. Parasitology. 1999 doi: 10.1017/s0031182098003904. in press. [DOI] [PubMed] [Google Scholar]
- 24.Boulter NR, Glass EJ, Knight PA, Bell-Sakyi L, Brown CGD, Hall R. Theileria annulata sporozoite antigen fused to hepatitis-B core antigen used in a vaccination trial. Vaccine. 1995;13:1152–60. doi: 10.1016/0264-410x(95)00026-w. [DOI] [PubMed] [Google Scholar]
- 25.Brown WC, Davis WC, Dobbelaere DAE, Rice-Ficht AC. CD4+ T-cell clones obtained from cattle chronically infected with fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 profiles. Infect Immun. 1994;62:818–27. doi: 10.1128/iai.62.3.818-827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bierer BE, Burakoff SJ. Lymphocyte-T activation—the biology and function of CD2 and CD4. Immunol Rev. 1989;111:267–94. doi: 10.1111/j.1600-065x.1989.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 27.Boussiotis VA, Freeman GJ, Griffin JD, Gray GS, Gribben JG, Nadler LM. CD2 is involved in maintenance and reversal of human alloantigen-specific clonal anergy. J Exp Med. 1994;180:1665–73. doi: 10.1084/jem.180.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]


