Abstract
C4BP has a central role in regulating the classical complement (C′) pathway, but it is still uncertain whether or not it is consumed during in vivo complement activation. Attempts to demonstrate changes in C4BP plasma levels in systemic lupus erythematosus and essential mixed cryoglobulinaemia have failed, probably due to up-regulation of this protein during the inflammatory reaction. We have studied one patient with severe post-transfusion complement-mediated anaphylaxis (CMA), and 67 patients with hereditary C1 inhibitor deficiency (hereditary angioedema (HAE)). The first of these two conditions is characterized by the absence of systemic inflammatory reaction and the second by acute and chronic activation of the C′ classical pathway. C4BP, C4BP–C4b complex, and soluble terminal C′ complex (sC5b-9) were measured in the patients' plasmas by ELISA techniques and C3a and C4a by radioimmunoassays. In CMA, 15 min after the transfusion, there was a massive C′ activation, with increases in C4a, C3a, sC5b-9, C4BP–C4b complexes and decreases in C4, C3 and C4BP. All parameters reverted to preinfusion values within 24 h. Depletion of C4 was correlated with that of C4BP. In patients with HAE, the median value of C4BP (83% range 54–165) was significantly lower (P < 0.0001) than in normal controls (99% range 70–159), with no difference between patients in remission or during acute attacks. C4BP–C4b complexes could not be detected in HAE patients. The results of this study indicate that C4BP is consumed in vivo during acute, and possibly during chronic activation of the C′ classical pathway, and that this protein, after interaction with C4b, not longer circulates in plasma.
Keywords: complement activation, C4BP, C4BP–C4b complex, C4
INTRODUCTION
C4BP is a large multimeric glycoprotein, being composed of six to seven identical 70-kD chains (α-chain) and a small 45-kD chain (β-) which are connected through disulfide bonds [1–3]. In plasma the protein circulates in three isoforms with different subunit compositions; the major one is a complex of 7α- and 1β-chain, the other two are made of 6α- and no β-chain, or 6α- and 1β-chain [4–6]. Plasma concentration is age-related, increasing from 40 μg/ml in newborns to 200-250 μg/ml in adults [7,8]. Although there is no gender-related difference, the synthesis appears to be influenced by sex hormones [8], being slightly increased during pregnancy [9] or oral contraceptive treatment [10] and reduced during androgen treatment [11]. Increased plasma levels of C4BP have been found in vivo during inflammatory diseases [8,12–14], and in vitro, inflammatory cytokines have been shown to up-regulate expression of C4BP genes [15], and therefore this protein is considered to be an acute-phase reactant.
C4BP controls the activation of the classical complement (C′) pathway and of the coagulation system [15–17]. The α-chains bind C4b non-covalently, acting as a cofactor to the Factor I in C4b proteolysis and accelerating the decay of the C3 convertase (C4b–2a complex) [18–22]. The β-chain interacts with and inactivates the anticoagulant vitamin K-dependent protein S [16]. The interaction with protein S does not interfere with complement inhibitory activity [23].
Although the protein is essential in controlling classical pathway activation, very little is known about the effects of in vivo C′ activation on plasma C4BP levels. It has been shown that in systemic lupus erythematosus (SLE), C4BP concentrations are normal or increased, suggesting that the profound complement activation that characterizes SLE does not increase C4BP catabolism [13,24]. However, this conclusion could have been wrong because of up-regulation of C4BP synthesis caused by the coexisting inflammatory reaction.
To gain insight into the involvement of C4BP in C′ activation in vivo, we evaluated C4BP plasma levels in two conditions characterized by acute or chronic classical pathway activation but without any systemic inflammatory reaction.
PATIENTS AND METHODS
Patients
Complement-mediated post-transfusion anaphylaxis was studied in a 24-year-old female with genetically determined severe von Willebrand factor (vWF) deficiency and high plasma levels of IgG alloantibodies to vWF. Plasma samples were obtained before and during severe complement-mediated anaphylaxis (CMA) triggered by replacement therapy with vWF concentrate (for details see [25]).
Sixty-seven patients with hereditary C1 inhibitor deficiency (hereditary angioedema (HAE)) were studied. The diagnosis of HAE was based on personal and family history of angioedema, and on the demonstration of low plasma levels of C1-INH activity and of C4 [26]. Plasma samples were obtained from the 67 patients when they were symptom-free and from seven patients also during acute attack of angioedema. At the time of sampling no subject was on therapy, nor there was evidence of associated diseases.
Forty healthy volunteers (20 male and 20 female) served as the normal control group.
Blood was collected into EDTA- and Na citrate-containing tubes, immediately centrifuged at 2500 g for 10 min, and plasma aliquots were stored at −80°C until tested. Each plasma sample was used only once. EDTA plasma was used for complement assays and Na citrate plasma for coagulation protein S assays.
Complement assays
C1q, C4, C3 and C1-INH antigen were measured in plasma by single radial immunodiffusion (Nor-Partigen; Beheringwerke AG, Marburg, Germany). Functional C1-INH was measured by chromogenic assay (Immuno, Vienna, Austria). C4BP was measured by ELISA as previously described [27]. sC5b-9 was measured by ELISA (Quidel, San Diego, CA), and C4a and C3a by competitive equilibrium radioimmunoassay (Amersham, Aylesbury, UK).
C4BP–C4b complexes were detected in plasma samples by the Ito method [28] with minor changes. Briefly, wells were coated with anti-C4BP MoAb diluted in 100 mm bicarbonate buffer pH 9.6. Rabbit anti-human C4c and peroxidase-conjugated goat anti-rabbit IgG were used to detect C4b bound to C4BP. The reference curve was obtained for serum, activated with heat-aggregated IgG, serially diluted in normal EDTA plasma, diluted 1:5 in PBS containing PMSF (0.1 mm). The results were expressed in arbitrary units (AU), assuming that a 1:2 dilution of activated serum contained 100 AU.
Coagulation protein S assays
Total protein S (free and C4BP-bound) and free protein S concentrations were measured as previously described [29] and the values expressed as percentages of the amounts in pooled normal citrated plasma.
Other measurements
Albumin, transferrin and IgG plasma levels were measured in plasma by single radial immunodiffusion (Nor-Partigen; Beheringwerke AG).
Statistical analysis
Non-parametric analysis was performed by the Mann–Whitney test. Correlation between plasma C4BP and C4 levels was assessed by least-square linear regression.
RESULTS
Complement measurements
The patient with congenital severe vWF deficiency had normal C1q, C1-INH, C4, C3, complement activation products (C4a, C3a, sC5b-9) and C4BP before replacement therapy with vWF concentrate. Fifteen minutes after the infusion there was a massive C′ activation, shown by generation of large amounts of C4a, C3a and sC5b-9, which was associated with profound reductions of plasma C1q, C4, C3, C1-INH (antigen and function) and C4BP levels (Table 1). As shown in Fig. 1, plasma C4BP levels were closely correlated with those of C4. All complement parameters had returned toward basal values at 24 h.
Table 1.
Changes in plasma complement parameters before and during acute activation of the classical complement pathway
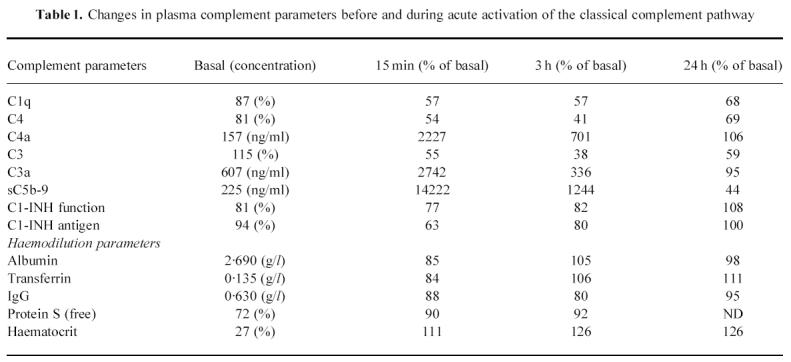
Fig. 1.
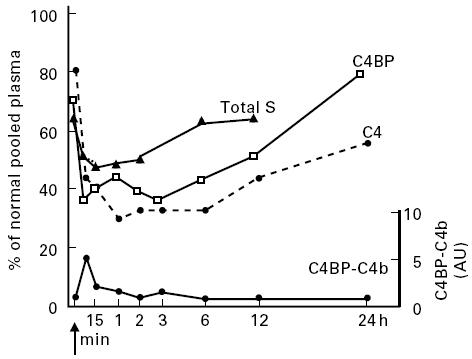
In vivo acute activation of the classical complement pathway resulted in parallel decreases of concentrations of C4, C4BP and C4BP–S complexes and in rapid clearance from the circulation of C4BP–C4b complexes. Arrow, Infusion of von Willebrand factor (vWF) concentrate; %, percentage of contents of normal pooled plasma; AU, arbitrary units.
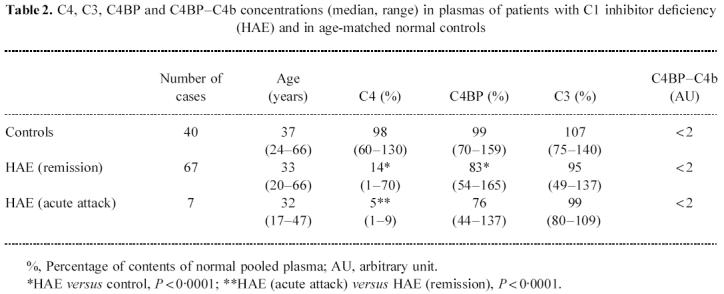
In plasma of patients with hereditary C1-INH deficiency, the C4BP concentration was significantly lower than in controls (P < 0.0001), without significant differences between patients in remission or during acute attacks of angioedema (Table 2). As expected, the C4 concentration was significantly lower in HAE than in controls (P < 0.0001), with a further decrease during acute attacks. There was no statistically significant correlation between the levels of C4BP and those of C4.
Table 2.
C4, C3, C4BP and C4BP–C4b concentrations (median, range) in plasmas of patients with C1 inhibitor deficiency (HAE) and in age-matched normal controls
C4BP–C4b complexes
Undiluted normal serum was incubated at 37°C with heat-aggregated IgG, samples were removed from the reaction mixture at different times and assayed for C4BP–C4b complexes by ELISA. In agreement with a previous report [28], we found that in vitro the amounts of the complexes generated by C′ activation decreased rapidly during incubation at 37°C (Fig. 2). In vivo these complexes were not detectable in controls, nor in HAE patients (Table 2), but they could be measured in the vWF-deficient patient in the early phase of acute C′ activation during C′-mediated anaphylaxis (Fig. 1).
Fig. 2.
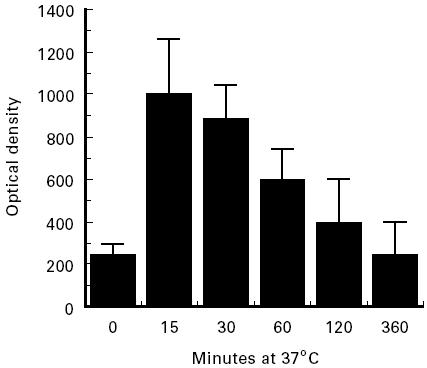
Kinetics of the dissociation of C4BP–C4b complexes generated in vitro by incubating normal human serum with heat-aggregated IgG at 37°C. Serum (100 μl) was removed from reaction mixture at different times, mixed with equal volumes of a 1:5 dilution of normal EDTA-PMSF plasma and assayed for C4BP–C4b complexes by ELISA. The results are expressed as mean ± s.d. of three experiments in duplicate.
Other measurements
To evaluate the effects of haemodilution and of protein loss due to changes in vascular permeability during CMA, we measured haematocrit and plasma levels of non-C′-related proteins of different sizes. As shown in Table 1, haematocrit increased and albumin, transferrin, protein S and IgG decreased, suggesting plasma extravasation. However, these changes were markedly lower than those of the C′ parameters. The concentration of total protein S (free and bound to C4BP) correlated with that of C4BP (Fig. 1).
DISCUSSION
The results of this study indicate that acute, and possibly chronic activation of classical C′ pathway in vivo lead to consumption of C4BP, as suggested by the reduction in its plasma levels. It is unlikely that such a reduction could be due to defective recognition of C4BP, which undergoes conformational changes following C′ activation, or to the increased vascular permeability induced by C′ anaphylatoxins. In vitro studies have demonstrated that binding to C4b did not affect C4BP antigenicity [28], and in CMA the change in plasma levels of the non-C′-related proteins was minimal compared with the reductions in C4BP and C′ parameters and the increases in C′ activation products (C4a, C3a, sC5b-9) (Table 1). Previous attempts to demonstrate consumption of C4BP during chronic activation of classical pathway have been done in patients with SLE and essential mixed cryoglobulinaemia (EMC) [13,24]. Both these conditions are characterized by intense systemic inflammatory reactions, and up-regulation of C4BP synthesis could have masked C4BP consumption. Therefore, we are confident in concluding that the reductions of the levels of this protein in plasma of our patients were truly the result of its increased catabolism.
Patients with hereditary C1-INH deficiency (HAE) represent a unique model of profound chronic activation of the classical C′ pathway in the absence of immune inflammatory events which can influence acute reactant proteins. The large series of HAE patients that we have presented here clearly demonstrated that C4BP levels were significantly lower than in normal subjects (Table 2). The absence of a significant difference between remission and attacks suggests that the change in C4 catabolism during the attacks of angioedema is not quantitatively relevant, probably because of the chronically reduced levels of C4 in these patients.
In HAE the classical C′ pathway is activated efficiently but without formation of a stable C3 convertase, as shown by the normal levels of C3 in these patients. In vitro experiments demonstrated that the activation of classical C′ pathway in C4BP-depleted serum caused C3 consumption, which was instead balanced by reconstitution with C4BP [30,31]. Therefore, it is likely that in HAE patients, in order to block the excess formation of C3 convertase, C4BP is actively used, this then resulting in a relative deficiency. Nevertheless, SLE patients, in whom activation of the classical C′ pathway continues through C3, suggest that the possibility of blocking the C3 convertase does not rely exclusively on the presence of C4BP. The demonstration that surface-bound C4b is more resistant to factor I-mediated inactivation than fluid-phase C4b [32] suggests that the different conditions in which the C3 convertase is formed (on immune complexes in SLE and in the fluid phase due to autoactivation of C1r and C1s in HAE) probably influence its susceptibility to inactivation by C4BP. This hypothesis is consistent with the finding that in our patient with CMA, in whom acute C′ activation was mediated by immune complexes, C4BP was unable to control massive activation of C3 (Table 1).
As could have been predicted, based on the theoretical interaction between C4 and C4BP, Fig. 1 demonstrates that the consumption of the two proteins was tightly correlated during acute C′ activation. However, in chronic C′ activation (HAE), we found that even with profound C4 depletion, C4BP was only moderately reduced (Table 2) and the levels of the two proteins were not significantly correlated. Although no data are available on the turnover of C4BP, the finding that in the patient with CMA C4BP returned to basal values faster than C4 (Fig. 1) seems to indicate that different synthesis rates for the two proteins could have been responsible for the lack of correlation in the situation of chronic C′ activation. Alternatively, in a condition of chronic C4 deficiency, like that of HAE patients, only limited amounts of C4b can be released upon activation of C1, and thus less C4BP is required for its degradation.
The observation that both acute and chronic C′ activation were associated with decreased plasma levels of C4BP might indicate that this protein, after interaction with C4b, no longer circulates in plasma. That this might be the case is also supported by the finding that C4BP–C4b complexes could be detected for only a few minutes after triggering C′ activation and that the decrease in the C4BP–C4b plasma level was not associated with a concomitant increase in C4BP concentration. Furthermore, in the CMA patient there was a rapid reduction in total protein S concentration (Fig. 1), without any significant change in the plasma level of the free molecule (Table 1). Since about 60% of protein S circulates in plasma bound to C4BP, our finding supports the hypothesis that there is increased catabolism of this protein during acute C′ activation. Whereas in the CMA patient the consumption of C4BP could be due to its incorporation into the immune complexes [19], in HAE it is also possible that, after the binding to C4b, C4BP is removed from circulation through interaction with C′ receptor (CR). However, we can not exclude that the chronically reduced levels of C4 could lead to a down-regulation of C4BP synthesis.
In conclusion, this study provides evidence that acute, and possibly chronic activation of the classical C′ pathway in vivo can lead to the consumption of C4BP, and that C4BP–C4b complexes are rapidly removed from the circulation. Despite the fact that the levels of C4BP in HAE patients were lower than in normal subjects, the high C4BP/C4 ratio may contribute to preventing C3 activation in this disease.
Acknowledgments
This paper was supported by Telethon Project no. EC469.
REFERENCES
- 1.Scharfstein J, Ferreira A, Gigli I, et al. Human C4-binding protein. Isolation and characterization. J Exp Med. 1978;148:207–22. doi: 10.1084/jem.148.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villiers MB, Reboul A, Thielens NM, et al. Purification and characterization of C4-binding protein from human serum. FEBS Letters. 1983;132:49–53. doi: 10.1016/0014-5793(81)80425-5. [DOI] [PubMed] [Google Scholar]
- 3.Dahlback B. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem J. 1983;209:847–56. doi: 10.1042/bj2090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sànchez-Corral P, Garcia OC, Rodrı&;grave;guez de Còrdoba S. Isoforms of human C4b-binding protein. Molecular basis for the C4BP isoform pattern and its variations in human plasma. J Immunol. 1995;155:4030–6. [PubMed] [Google Scholar]
- 5.Hessing M, Kanters D, Heijnen HFG, et al. Structure-function studies on human C4b-binding protein using monoclonal antibodies. Eur J Immunol. 1991;21:2077–85. doi: 10.1002/eji.1830210916. [DOI] [PubMed] [Google Scholar]
- 6.Hessing M, Kanters D, Hackeng TM, et al. Identification of different forms of human C4b-binding protein lacking β-chain and protein S binding ability. Thromb Haem. 1990;64:245–50. [PubMed] [Google Scholar]
- 7.Melissari E, Nicolaides KH, Scully MF, et al. Protein S and C4b-binding protein in fetal and neonatal blood. Br J Haematol. 1988;70:199–203. doi: 10.1111/j.1365-2141.1988.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 8.Marcovina SN, Zoppo A, Viganò-D'Angelo S, et al. Determination of serum levels of complement component C4b-binding protein: influence of age and inflammation. Int J Clin Lab Res. 1991;21:171–5. doi: 10.1007/BF02591638. [DOI] [PubMed] [Google Scholar]
- 9.Comp PC, Thurnau GR, Welsh J, et al. Functional and immunologic protein S levels are decreased during pregnancy. Blood. 1991;68:881–5. [PubMed] [Google Scholar]
- 10.Melissari E, Kakkar VV. The effects of oestrogen administration on the plasma free protein S and C4b-binding protein. Thromb Res. 1988;49:489–95. doi: 10.1016/s0049-3848(98)90006-8. [DOI] [PubMed] [Google Scholar]
- 11.Thorisdottir H, Evans JA, Schwartz HJ, et al. Some clotting factors in plasma during danazol therapy: free and total protein S, but not C4b-binding protein, are elevated by Danazol therapy. J Lab Clin Med. 1992;119:698–701. [PubMed] [Google Scholar]
- 12.Saeki T, Hirose S, Nukatsuka M, et al. Evidence that C4b-binding protein is an acute phase protein. Biochem Biophys Res Commun. 1989;164:1446–51. doi: 10.1016/0006-291x(89)91832-9. [DOI] [PubMed] [Google Scholar]
- 13.Barnum SR, Dahlback B. C4b-binding protein, a regulatory component of the classical pathway of complement, is an acute-phase protein and is elevated in systemic lupus erythematosus. Complement Inflamm. 1990;7:71–77. doi: 10.1159/000463131. [DOI] [PubMed] [Google Scholar]
- 14.de Frutos PG, Mohammed Alim RIH, Hardig Y, et al. Differential regulation of α and β chains of C4b-binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant protein S. Blood. 1994;84:815–22. [PubMed] [Google Scholar]
- 15.Garcia OC, Sànchez-Corral P, Rodrı&;grave;uez de Còrdoba S. Isoforms of human C4b-binding protein. Differential modulation on the C4BPA and C4BPB genes by acute phase cytokines. J Immunol. 1995;155:4037–43. [PubMed] [Google Scholar]
- 16.Barnum SR. C4b-binding protein, a regulatory protein of complement. Immunol Res. 1991;10:28–42. doi: 10.1007/BF02918165. [DOI] [PubMed] [Google Scholar]
- 17.Hessing M. The interaction between complement component C4b-binding protein and the vitamin K-dependent protein S forms a link between blood coagulation and the complement system. Biochem J. 1991;277:581–92. doi: 10.1042/bj2770581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziccardi RJ, Dahlback B, Muller-Eberhard HJ. Characterization of the interaction of human C4b-binding protein with physiological ligands. J Biol Chem. 1984;259:13674–9. [PubMed] [Google Scholar]
- 19.Scharfstein J, Correa EB, Gallo GR, et al. Human C4-binding protein. Association with immune complexes in vitro and in vivo. J Clin Invest. 1979;63:437–42. doi: 10.1172/JCI109320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welch TR, Forristal J, Beischel L. Relationship between the component and regulatory proteins of the classical pathway C3 convertase. J Lab Clin Med. 1986;107:529–33. [PubMed] [Google Scholar]
- 21.Hessing M, Van't Veer C, Bouma BN. The binding site of human C4b-binding protein on complement C4 is localized in the α-chain. J Immunol. 1990;144:2632–7. [PubMed] [Google Scholar]
- 22.Seya T, Nakamura K, Masaki T, et al. Human factor H and C4b-binding protein serve as Factor I-cofactors both encompassing inactivation of C3b and C4b. Mol Immunol. 1995;32:355–60. doi: 10.1016/0161-5890(94)00157-v. [DOI] [PubMed] [Google Scholar]
- 23.Dahlback B, Hildebrand B. Degradation of human complement component C4b in the presence of the C4b-binding protein–protein S complex. Biochem J. 1983;209:857–63. doi: 10.1042/bj2090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schifferli JA, Bakkaloglu A, Amos N, et al. C4-binding protein in sera of patients with systemic lupus erythematosus and mixed essential cryoglobulinemia. Complement. 1984;1:81–86. doi: 10.1159/000467819. [DOI] [PubMed] [Google Scholar]
- 25.Bergamaschini L, Mannucci PM, Federici AB, et al. Posttransfusion anaphylactic reaction in a patient with severe von Willebrand disease: role of complement and alloantibodies to von Willebrand factor. J Lab Clin Med. 1994;125:348–55. [PubMed] [Google Scholar]
- 26.Davis AE. C1 inhibitor and hereditary angioneurotic edema. Ann Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- 27.Coppola R, Tombesi S, Cristilli P, et al. Comparison of two immunoassays for the complement protein C4b-binding protein in health and disease. Int J Clin Lab Res. 1995;25:88–92. doi: 10.1007/BF02592363. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Fujita T, Tamura N. Determination of C4b–C4bp complex formed by the activation of classical complement pathway using an enzyme-linked immunosorbent assay. J Immunol Methods. 1987;105:145–50. doi: 10.1016/0022-1759(87)90425-x. [DOI] [PubMed] [Google Scholar]
- 29.Faioni EN, Valsecchi C, Palla A, et al. Free protein S deficiency is a risk factor for venous thrombosis. Thromb Haemost. 1997;78:1343–6. [PubMed] [Google Scholar]
- 30.Gigli I, Fukita T, Nessenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins, C4 binding protein and C3b inactivator. Proc Natl Acad Sci USA. 1979;76:6596–600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gronski P, Bodenbender L, Kanzy E-J, et al. C4-binding protein prevents spontaneous cleavage of C3 in sera of patients with hereditary angioedema. Complement. 1988;5:1–12. doi: 10.1159/000463026. [DOI] [PubMed] [Google Scholar]
- 32.Fujita T, Tamura N. Interaction of C4-binding protein with cell-bound C4b: a quantitative analysis of binding and the role of C4-binding protein in proteolysis of cell-bound C4b. J Exp Med. 1983;157:1239–51. doi: 10.1084/jem.157.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]


