Abstract
Development of an immunological tool to detect infection with Mycobacterium leprae would greatly benefit leprosy control programmes, as demonstrated by the contribution of the tuberculin test to tuberculosis control. In a new approach to develop a ‘tuberculin-like’ reagent for use in leprosy, two new fractions of M. leprae depleted of cross-reactive and immunomodulatory lipids— MLSA-LAM (cytosol-derived) and MLCwA (cell wall-derived)—have been produced in a form suitable for use as skin test reagents. T cell responses (interferon-gamma (IFN-γ) and lymphoproliferation) to these two new fractions were evaluated in a leprosy-endemic area of Nepal using a simple in vitro whole blood test. The two fractions were shown to be highly potent T cell antigens in subjects exposed to M. leprae—paucibacillary leprosy patients and household contacts. Responses to the fractions decreased towards the lepromatous pole of leprosy. Endemic control subjects also showed high responses to the fractions, indicating high exposure to M. leprae, or cross-reactive mycobacterial antigens, in this Nepali population. The new fractions, depleted of lipids and lipoarabinomannan (LAM) gave enhanced responses compared with a standard M. leprae sonicate. The cell wall fraction appeared a more potent antigen than the cytosol fraction, which may be due to the predominance of the 65-kD GroEL antigen in the cell wall. The whole blood assay proved a robust field tool and a useful way of evaluating such reagents prior to clinical trials.
Keywords: leprosy, diagnosis, whole blood, interferon-gamma, antigen
INTRODUCTION
The number of active cases of leprosy is currently estimated to be 1.26 million worldwide [1], mainly concentrated in leprosy-endemic areas of Africa, S. America and Asia, including Nepal. Despite a marked reduction in the prevalence of leprosy since the implementation of Multi Drug Therapy (MDT) in 1982, the detection rate of new cases has not shown a similar decline. Achievement of the WHO goal for global elimination of leprosy as a public health problem is hindered by the lack of information on the presence of Mycobacterium leprae infection within a given population. Control programmes are guided by treatment of cases of leprosy, and surveillance of their close contacts who have been shown to suffer from a higher incidence of leprosy than the general population [2,3], but by no means account for all new cases of leprosy. It has been suggested that in an area of high leprosy endemicity there may be no difference in incidence between contacts and the general population [4]. It is not known whether a constant proportion of subclinically infected people go on to develop disease [5]. A specific test for M. leprae infection could be used to answer some of these fundamental questions in leprosy epidemiology, and also to plan future requirements for leprosy control programmes, including targeted vaccination programmes with current or future vaccines. A sensitive and specific method to identify subclinical M. leprae infection is therefore a priority [6]. Approaches to date, including serology and mycobacterial gene amplification, have not been able to achieve sufficient specificity and sensitivity while being robust and simple enough for use in rural leprosy-endemic areas [7–9].
In such locations a skin test to detect exposure would be ideal, as demonstrated by the wide use of tuberculin (autoclaved fractionated culture fluid from M. tuberculosis grown in culture) in tuberculosis control programmes. A DTH reaction to the tuberculin skin test, measured at 48 h, reflects infection with M. tuberculosis, although exposure to cross-reactive mycobacteria (including bacille Calmette–Guérin (BCG) vaccination) may also be detected [10]. A directly equivalent preparation for use in leprosy control has not been available, due to the inability to grow M. leprae in culture. Skin testing with Lepromin A (an autoclaved homogenized preparation of M. leprae) resulting in a Mitsuda DTH skin response 4 weeks after administration reflects the host immune response to infection with, rather than prior exposure to, M. leprae. A partially purified fraction of the soluble protein components of homogenized bacteria, M. leprae soluble antigen (‘Rees-type’ MLSA), has been shown to provoke a more ‘tuberculin-like’ early skin reaction. Responses to MLSA have been evaluated in Malawi and South India [11], but are cross-reactive with M. tuberculosis [12] and possibly other mycobacterial species, and therefore not a specific indicator of infection with M. leprae. A new initiative is underway to extend the fractionation approach to derive a more M. leprae-specific skin test. This approach may prove more productive than the alternative ‘reductionist approach’ of identification and synthesis of peptides encoded by M. leprae-specific epitopes, recognition of which is HLA-restricted and may therefore result in recognition of different peptides by different populations as demonstrated for peptides of the 70-kD M. leprae antigen [13]. In addition, isolated peptides may be insufficiently immunogenic to provoke skin responses, as previously reported for peptides of M. tuberculosis ([14]; S. Jurcevic, personal communication); this may also be a problem for the use of individual mycobacterial proteins for skin testing, as reported for the M. tuberculosis MPT-64 and MPT-59 proteins [15].
Two new fractions have been prepared from M. leprae as candidate skin test reagents: a modified ‘Rees-type’ MLSA (termed MLSA-LAM), and a cell wall-derived fraction (MLCwA). Both fractions have undergone an extraction step to exclude lipids (predominantly lipoarabinomannan (LAM), lipomannan (LM), phosphatidyl inositol mannosides (PIM), and other lipids), which have previously been demonstrated to be immunosuppressive [16,17]. MLSA-LAM and MLCwA have already shown high potency as skin test antigens in guinea pigs (P. Brennan, unpublished data; [18]), and induced interferon-gamma (IFN-γ) responses in peripheral blood mononuclear cells from tuberculoid leprosy patients in Pakistan [19].
This study evaluated T cell responses to these new fractions in leprosy patients, leprosy household contacts and control subjects in a leprosy-endemic community in Nepal, prior to Phase 1 clinical trials. A simple in vitro whole blood assay was used as a non-invasive method of screening responses to these new fractions, that did not require approval for in vivo use and avoided the complexities of large scale field trials. A ‘type 1’ T cell response, involving lymphoproliferation and the production of IFN-γ, appears to correlate with protection in leprosy [20–22], and these two parameters were measured in the blood cultures as a marker of a memory T cell response to the test antigens. Large scale production of skin test reagents requires that they can be easily produced in sterile form while maintaining their antigenic potency, and the optimal method to achieve this was identified. This study assessed the potential of these new fractions as in vitro indicators of previous infection to M. leprae, prior to their use in vivo.
MATERIALS AND METHODS
Subjects
Leprosy patients were recruited from Anandaban Leprosy Hospital (Kathmandu, Nepal) who were receiving MDT (median nine doses, range 0–37), and were not in reaction at the time of testing. The patients were grouped as polar/borderline tuberculoid (TT/BT; n = 30, median age 30 years), borderline lepromatous (BL; n = 18, median age 35 years), or polar lepromatous (LL; n = 11, median age 39 years) according to clinical symptoms, bacterial index of skin slit smear samples, lepromin skin test result (measured at 4 weeks after administration of Lepromin A), and skin biopsy report where available. Contacts of leprosy patients were recruited from among relatives#x002F;spouses of patients attending Anandaban Hospital and from a hostel for children of leprosy patients, and showed no signs of leprosy at the time of testing (n = 28, median age 15 years). Endemic control subjects were leprosy-free subjects, who had not knowingly had any contact with a leprosy case apart from living in a leprosy-endemic area, who were attending Anandaban Hospital for some other reason (n = 19, median age 28 years). Non-endemic control subjects in the UK were non-BCG vaccinated subjects from Europe#x002F;USA who had not visited a leprosy-endemic area (n = 8, median age 33 years). All subjects gave their informed consent prior to venepuncture. Ethical approval for this study was granted by LSH & TM, Anandaban Leprosy Hospital and the Nepal Health Research Council.
Whole blood assay
Blood (4 ml) was taken by venepuncture from each subject, mixed with heparin at 20 U/ml and put into culture within 6 h, following our previous method [23]. The blood was diluted 1:10 with sterile RPMI 1640 tissue culture medium (Gibco BRL, Paisley, UK) containing 100 U/ml penicillin#x002F;100 μg/ml streptomycin (Gibco) and 2 mml-glutamine (Gibco). Antigens, mitogens (phytohaemagglutinin (PHA), positive control) or medium alone (negative control) were added to the wells of 96-well round-bottomed tissue culture plates (Nunc, Roskilde, Denmark) in a volume of 20 μl, to which 200 μl of diluted blood were added. The antigens were tested at a concentration of 10 μg/ml (unless otherwise stated), and PHA at 5 μg/ml. Tests were carried out in triplicate. After 6 days' incubation at 37°C#x002F;5% CO2, 150 μl supernatant were removed from each culture well and pooled for each test, frozen at −20°C, and subsequently tested for IFN-γ by ELISA [24]. This culture period has previously been shown to be optimal for both IFN-γ and proliferation responses to M. leprae antigens ([23] and unpublished data). Fresh growth medium (100 μl) and 1 μCi of methyl-3H-thymidine (3H-TdR; TRA.120; Amersham Int. plc, Aylesbury, UK) were added to each culture well, the cultures were harvested after 18 h further incubation and 3H-TdR incorporation was measured by scintillation counting. IFN-γ responses are given as mean units of IFN-γ (U/ml) of duplicate ELISA wells after subtraction of any non-specific IFN-γ production in non-stimulated cultures (which were predominantly negative). The positive response threshold was a response above the detection limit of the IFN-γ ELISA (1 U/ml). Proliferation responses are given as mean disintegrations per minute measurements of triplicate culture wells after subtraction of non-specific proliferation measured in non-stimulated cultures (dDPM; average proliferation in non-stimulated cultures from 137 subjects was 361 dpm).
Antigens
MLSA-LAM and MLCwA (batches 9 and 10) were prepared from M. leprae derived from infected armadillos as described [18]; briefly, M. leprae isolated from infected armadillo tissue were sonicated, and separated by centrifugation into supernatant (mainly cytosol) (MLSA) and pellet (cell wall and membrane), which was extracted with SDS. Both fractions were further extracted with Triton X-114 to yield the lipid and carbohydrate-free derivatives, MLSA-LAM and MLCwA, in PBS–0.00005% Tween 80 buffer at a concentration of 100 μg/ml. Mycobacterium leprae sonicate (batch CD212) and whole M. leprae (batch CD177; used at a concentration of 108 acid-fast bacilli/ml) were provided by Dr R. Rees (NIMR, Mill Hill, London, UK). Tuberculin purified protein derivative (PPD; batch RT47, for in vitro use) was supplied by Statens Seruminstitut (Copenhagen, Denmark). Mycobacterium leprae recombinant antigens were supplied by the listed laboratories: 65 kD, 28 kD, 18 kD, 10 kD (Dr J. van Embden, Bilthoven, The Netherlands, under the auspices of the IMMLEP programme of the WHO TDR); 70 kD, 35 kD (Dr W. Britton, University of Sydney, Australia); 45 kD, 30#x002F;31 kD (Dr B. Wieles, University of Leiden, The Netherlands). PHA was supplied by Sigma (Poole, UK). Lepromin A (lot C6) was supplied by NHDC (Carville, LA).
Statistical analysis
Inter-group comparisons were carried out using the Wilcoxon rank sum test. Use of other methods is indicated where appropriate.
RESULTS
In vitro T cell responses to the new fractions
Blood cultures from four tuberculoid leprosy patients made dose-dependent IFN-γ and lymphoproliferative responses to both MLSA-LAM and MLCwA, with greater responses at 10 μg/ml than 1 μg/ml. In subsequent experiments the fractions were tested at 10 μg/ml.
Effect of different sterilization methods on antigenicity of the fractions
To investigate whether these fractions could be prepared in the sterile form required for an in vivo skin test reagent, responses were compared for filter-sterilized and autoclaved preparations of the fractions in whole blood cultures from leprosy patients and contacts. The fractions gave high T cell responses even after autoclaving. IFN-γ responses to the autoclaved fractions were higher than to the filtered preparations for both MLSA-LAM (median response in 32 subjects: autoclaved 13 U/ml, filtered 0 U/ml; P = 0.04) and MLCwA (median response in 46 subjects: autoclaved 8 U/ml, filtered 6 U/ml; P = 0.05), possibly because the fractions may be depleted of key antigens as a result of filter sterilization. Lymphocyte proliferation measurements in the same cultures showed that autoclaved MLCwA gave a higher median response (5824 dpm) than filtered MLCwA (3478 dpm; P < 0.01), but responses for both autoclaved and filtered MLSA-LAM were not significantly different. Autoclave sterilization of these fractions for in vivo use, which is convenient for bulk production, did not appear to reduce their antigenic potency.
Potential of new fractions to identify infection with M. leprae
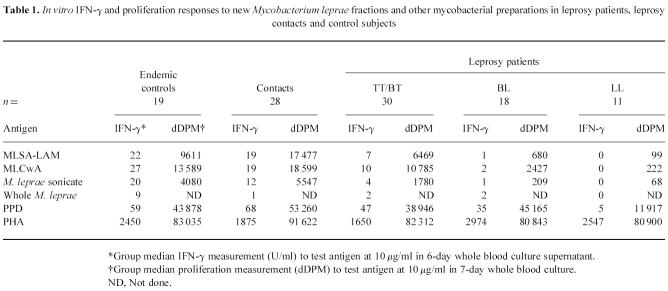
To assess T cell responses to the new fractions in a leprosy-endemic community in Nepal, IFN-γ and proliferation responses were measured to MLSA-LAM and MLCwA in whole blood cultures from endemic control subjects, household contacts of leprosy cases (who would be anticipated to have had higher exposure to M. leprae) and leprosy patients with varying severity of disease. Responses in each subject were also measured to whole M. leprae and M. leprae sonicate, to assess the effect of fractionation and lipid depletion on the antigenicity of M. leprae proteins. Mycobacterium tuberculosis PPD (or tuberculin) was also tested, to compare responses to a soluble protein preparation from a different species of mycobacteria; responses to PHA were measured to determine T cell viability. Results are presented in Table 1, and IFN-γ responses in individual subjects illustrated in Fig. 1. Proliferation responses generally paralleled IFN-γ responses to the antigens in individual subjects.
Table 1.
In vitro IFN-γ and proliferation responses to new Mycobacterium leprae fractions and other mycobacterial preparations in leprosy patients, leprosy contacts and control subjects
Fig. 1.
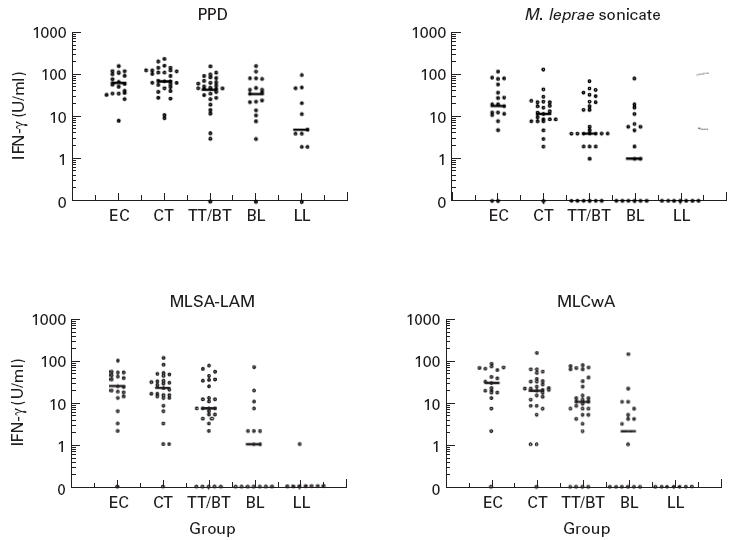
In vitro whole blood IFN-γ responses to prototype skin test antigen fractions, and purified protein derivative (PPD) and M. leprae sonicate, in different subject groups in Nepal (EC, endemic controls; CT, contacts; TT/BT, BL, LL, leprosy patients by classification). Each point represents mean IFN-γ measurement in day 6 culture supernatant for one subject; group median response is shown by a bar.
All cultures made a positive response to PHA and were therefore viable; there was no difference in response to PHA between the groups. This contrasts with earlier findings of reduced responses to PHA in peripheral blood leucocytes from untreated LL leprosy patients [25]; however, this may be due to our LL patient group including both treated and untreated patients. A decrease in magnitude of response to PPD was seen across the spectrum of leprosy patients, but there was no statistically significant difference between the groups. In contrast, responses to M. leprae disappeared with increasing severity of leprosy. This supports previous findings [26] of a selective lack of response to M. leprae, but not to other mycobacteria, in peripheral blood lymphocytes from lepromatous leprosy patients. The same pattern of response across the spectrum of leprosy was also seen to the new M. leprae fractions, but the magnitude of both IFN-γ and proliferation responses was greater to the fractions than to M. leprae sonicate. This demonstrates that MLSA-LAM and MLCwA are more antigenic in vitro than M. leprae sonicate, which may be due to the removal of immunosuppressive lipid/carbohydrate components during the fractionation procedure. This is supported by the observation that responses to whole M. leprae (in which the protein components are contained within the glycolipid cell wall, and require greater processing prior to presentation) were much lower than to M. leprae sonicate (or either of the fractions). However, there was no evidence that removal of the lipid components affects the specific lack of response to the M. leprae fractions in LL leprosy patients—the LL patients made significantly lower responses to the new fractions than the other groups (P < 0.01), including the BL group.
A significant association was observed between the in vitro IFN-γ response to both fractions, and the in vivo skin test response to Lepromin A in recently tested patients. The significance of this relationship should be elucidated by comparison of in vivo responses to the new fractions with both in vitro results, and responses to Lepromin A in the same individuals.
The highest T cell responses to MLSA-LAM and MLCwA were observed in the endemic control and contact groups. There was no significant difference in response between these two groups even though the household contacts should have had more exposure to M. leprae. Only two subjects in these groups did not make a response to the fractions, which suggests that the fractions may be recognized by most HLA types in this population.
Relative antigenicity of MLSA-LAM and MLCwA
Comparison (analysis by paired t-test) of responses in all subjects in the study showed that MLCwA stimulated significantly higher proliferation (P < 0.001) and IFN-γ responses (P = 0.005) than MLSA-LAM in the whole blood cultures (n = 106). There did not appear to be any group-specific effect to suggest that recognition of one fraction was associated with a particular group of subjects. MLCwA appears to be a more potent antigen for detecting exposure to M. leprae than MLSA-LAM
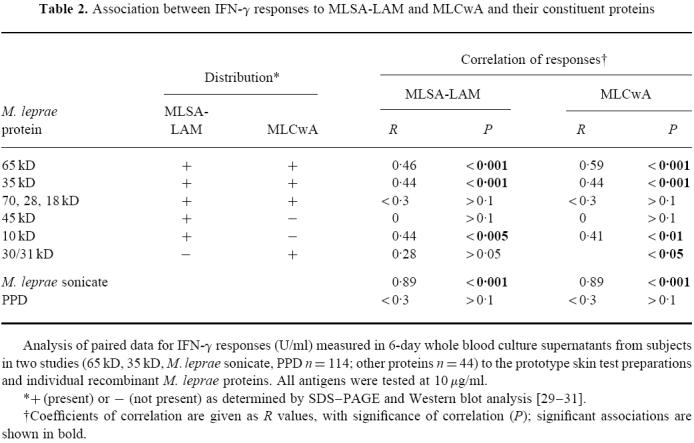
Protein composition of MLSA-LAM and MLCwA
SDS–PAGE gel electrophoresis and Western blotting using antibodies of known specificity [27] were used to identify the major proteins of MLSA-LAM and MLCwA ([28–30]; C. Pessolani, unpublished data). Each fraction was found to contain a wide array of proteins. An outline of protein distribution is shown in Table 2. The two fractions were found to share a number of proteins, although relative amounts of each protein differed, but some proteins were restricted to only one of the fractions. MLCwA is particularly rich in the 65-kD GroEL protein, while the 10-kD GroES protein is a major component of MLSA-LAM, which also contains other proteins including 22-kD and 38-kD proteins. In order to investigate which proteins may be contributing to the in vitro responses observed to each fraction, IFN-γ responses in the same subjects were compared to the two fractions and to a range of the identified individual M. leprae proteins. All antigens were tested at 10 μg/ml. Paired responses to MLSA-LAM, and MLCwA, and each individual protein, were analysed for correlation; a t-test was used to establish whether the derived R values were significantly different from zero (Table 2). The 65-kD (GroEL) and 35-kD (MMP-1) proteins showed greatest association with responses to MLSA-LAM and MLCwA, which is consistent with their prominence in these fractions. Of all the individual proteins tested, the 65-kD protein consistently induced the highest IFN-γ and proliferation responses. The dominant presence of 65 kD in the MLCwA fraction may explain the higher T cell responses to this fraction compared with MLSA-LAM.
Table 2.
Association between IFN-γ responses to MLSA-LAM and MLCwA and their constituent proteins
The similarity of responses in individual subjects to both of the new fractions, and to M. leprae sonicate from which they were derived, may be expected from the large number of common proteins present in each preparation. Several of these proteins are known to be present in other species of mycobacteria. In vitro responses to MLSA-LAM and MLCwA were also tested in whole blood cultures from eight healthy control subjects in the UK who had not been exposed to M. leprae. These subjects made in vitro IFN-γ and proliferation responses to both fractions (as well as to M. leprae sonicate and tuberculin PPD), with higher responses to MLCwA as observed in Nepal (data not shown). These results indicate the presence of cross-reactive antigens in the M. leprae fractions. However, measurement of responses to the new fractions in vivo may reveal relative exposure to M. leprae—this will become clear in forthcoming Phase I/II trials.
DISCUSSION
These results represent the first large scale testing of in vitro human T cell responses to new candidate skin test antigens derived from M. leprae—MLSA-LAM and MLCwA. T cell responses were measured in whole blood cultures by lymphocyte proliferation and production of IFN-γ, and were compared with responses to crude preparations of M. leprae (whole bacteria and a soluble sonicate), as well as to defined protein antigens of M. leprae. The new fractions were shown to be antigenic even after sterilization by autoclaving, which is the method of choice for skin test reagents.
These studies found that both MLSA-LAM and MLCwA could induce IFN-γ production and lymphocyte proliferation responses in the majority of tuberculoid leprosy patients. Responses were reduced in the BL patients, and absent in all but one of the LL leprosy patients. Responding subjects made higher responses to the cell wall fraction (MLCwA) than to the cytosolic fraction (MLSA-LAM). These fractions, which were depleted of LAM and other lipids, were more potent T cell antigens than M. leprae sonicate. This is consistent with previous research comparing in vitro responses to Dharmendra lepromin and delipidified M. leprae sonicate [31], and confirms and extends preliminary work on these fractions [19]. However, the removal of these potentially immunosuppressive components did not affect the lack of response to M. leprae antigens observed in LL leprosy patients, who consistently did not respond to MLSA-LAM or MLCwA. This was an M. leprae-specific effect, which was not observed with M. tuberculosis PPD. It remains to be determined whether individuals progressing to multibacillary disease may go through a stage of skin test positivity (indicative of subclinical infection) before the onset of clinical leprosy. Identification of the antigens recognized at this stage is central to the discrimination between subclinical infection and disease in leprosy.
The household contacts made higher responses to both of the new fractions than the tuberculoid leprosy patients, indicative of a high type 1 memory T cell response to these M. leprae antigens. However, the endemic control group in Nepal showed higher or equivalent responses to MLSA-LAM and MLCwA compared with the leprosy contact group. This is in agreement with previous studies of skin test responses to the original MLSA in leprosy-endemic areas [32,33], and may reflect exposure of the general population to M. leprae in this area of Nepal, or it may be due to cross-reactive responses following exposure to high levels of other (environmental) mycobacteria. Positive responses in non-M. leprae-exposed individuals tested in the UK confirmed that cross-reactive responses are being made to MLSA-LAM and MLCwA. These in vitro results suggest that further fractionation will be required to give preparations for use in a highly specific test of exposure to M. leprae. Comparison of T cell responses to the fractions with individual M. leprae proteins showed that the 65-kD and 35-kD proteins may be immunodominant antigens in these fractions, which is supported by the abundance of these two proteins in such preparations [30]. These and other proteins present in these fractions have previously been demonstrated to provoke type 1 responses in paucibacillary leprosy patients and healthy contacts: 10 kD [34], 18 kD [35], 28 kD [36], 30–31 kD (the Antigen 85 complex; [37]), 35 kD [38], 45-kD protein [39,40] and 65 kD [41]. IFN-γ responses to the ribosomal protein L7/L12 also showed significant association with responses to both MLSA-LAM and MLCwA, although the magnitude of responses to this protein were much lower than to the 65-kD or 35-kD proteins (Weir & Silbaq, unpublished data). Although species-specific epitopes have been demonstrated in some of the proteins that are found in the fractions [42], homology between complete proteins in different species of mycobacteria may be a limitation to the isolation of a truly M. leprae-specific skin test reagent in this way.
If skin test responses to the new fractions also indicate that non-leprosy-exposed individuals respond to the new fractions, an alternative approach could compare responses between equivalent fractions derived from different mycobacterial species. This approach has already been successfully employed for differentiating between infection with M. avium, M. tuberculosis and M. bovis [43,44]. Initial results comparing responses to equivalent fractions from killed sonicated M. tuberculosis bacteria showed that tuberculosis patients reacted preferentially to the M. tuberculosis cell wall fraction, while leprosy patients made responses of similar magnitude to CwA from both species (H. Dockrell, unpublished data). Interpretation of these responses is complicated by the difficulty of preparing directly comparable fractions from M. tuberculosis (grown in culture) and M. leprae (isolated from armadillo tissue). However, this comparative approach to identification of mycobacterial infection merits further investigation.
As the new fractions have not yet been used in vivo, it is not known whether skin tests or in vitro tests will be more sensitive and informative indicators of infection. The degree of exposure/subclinical infection with M. leprae that is required to generate skin test positivity needs to be defined. There is conflicting evidence for the relationship between in vitro and in vivo responses to the tuberculin skin test reagent, with poor correlation observed by some groups [45,46], although a recent study has shown significant correlation between IFN-γ production in whole blood culture and skin test responses to tuberculin [47]. This encourages the use of the whole blood assay as an indication of subsequent skin test responses.
The results presented in this study demonstrate the value of in vitro whole blood assays as an initial screen of T cell responses to new immunological reagents, such as skin test antigen candidates, in large groups of subjects, prior to clinical trials. Both the M. leprae cytosolic, and in particular the cell wall, fractions were shown to be highly antigenic in M. leprae-infected subjects, even after autoclaving. In vivo studies are now required to establish the potential of these antigens as diagnostic reagents for M. leprae infection.
Acknowledgments
Work conducted in Nepal and the UK was supported by the Hospital and Homes of St Giles, UK. Work conducted in the USA was supported by Contract AI 55262 from NIAID, NIH. The authors would like to thank the staff of the Mycobacterial Research Laboratories and Anandaban Hospital for their assistance with study subject recruitment and technical support for these studies; the Leprosy Mission International for their co-operation; Dr R.K. Shrestha, Dr G. Paudel and the Nepal Leprosy Relief Association for assisting with recruitment of study subjects; Dr K. B. Bohara and the Animal Health Research Division, Kathmandu, for helping with data collection; and the Nepal Health Research Council of HMG Nepal for permission to carry out this research.
REFERENCES
- 1.World Health Organisation. Weekly Epidemiological Record. 1996.
- 2.Sundar Rao PSS, Jesudasan K, Mani K, Christian M. Impact of MDT on incidence rates of leprosy among household contacts. Part 1. Baseline data. Int J Lepr. 1989;57:647–51. [PubMed] [Google Scholar]
- 3.Fine PEM, Sterne JAC, Ponnighaus JM, Bliss L, Saul J, Chihana A, Munthali M, Warndorff DK. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am J Epidemiol. 1997;146:91–102. doi: 10.1093/oxfordjournals.aje.a009195. [DOI] [PubMed] [Google Scholar]
- 4.Jesudasan K, Bradley D, Smith PG, Christian M. Time trends in the analysis of incidence rate of leprosy among household contacts. Ind J Lepr. 1984;56:792–806. [PubMed] [Google Scholar]
- 5.Noordeen SK. Leprosy 1962–1992. 4. Epidemiology and control of leprosy—a review of progress over the last 30 years. Trans R Soc Trop Med Hyg. 1993;87:515–7. doi: 10.1016/0035-9203(93)90068-2. [DOI] [PubMed] [Google Scholar]
- 6.Noordeen SK. The epidemiology of leprosy. In: Hastings RC, editor. Churchill Livingstone. Leprosy: Edinburgh; 1994. pp. 29–45. [Google Scholar]
- 7.Smith PG. The serodiagnosis of leprosy. Lepr Rev. 1992;63:97–100. doi: 10.5935/0305-7518.19920012. [DOI] [PubMed] [Google Scholar]
- 8.Baumgart KW, Britton WJ, Mullins RJ, Basten A, Barnetson RSC. Subclinical infection with Mycobacterium leprae— a problem for leprosy control strategies. Trans R Soc Trop Med Hyg. 1993;87:412–5. doi: 10.1016/0035-9203(93)90016-j. [DOI] [PubMed] [Google Scholar]
- 9.Van Beers SM, Izumi S, Madjid B, Maeda Y, Day R, Klatser PR. An epidemiological study of leprosy infection by serology and polymerase chain reaction. Int J Lepr. 1994;62:1–9. [PubMed] [Google Scholar]
- 10.Comstock GW, Daniel TM, Snider DE, Edwards PQ, Hopewell PC, Vandiviere HM. The tuberculin skin test. Am Rev Resp Dis. 1981;124:356–63. [Google Scholar]
- 11.Fine PEM, Sterne JAC, Ponnighaus JM, Rees RW. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet. 1994;344:1245–9. doi: 10.1016/s0140-6736(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 12.Mehra V, Bloom BR. Induction of cell-mediated immunity to Mycobacterium leprae in guinea pigs. Infect Immun. 1979;23:787–94. doi: 10.1128/iai.23.3.787-794.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams E, Britton WJ, Morgan AR, Sergeantson S, Basten A. Individuals from different populations identify multiple and diverse T-cell determinants on mycobacterial HSP 70. Scand J Immunol. 1994;39:588–96. doi: 10.1111/j.1365-3083.1994.tb03417.x. [DOI] [PubMed] [Google Scholar]
- 14.Mackall JC, Bai GH, Rouse DA, Armoa GRG, Chuidian F, Nair J, Morris SL. A comparison of the T cell delayed-type hypersensitivity epitopes of the 19-kD antigens from Mycobacterium tuberculosis and Mycobacterium intracellulare using overlapping synthetic peptides. Clin Exp Immunol. 1993;93:172–7. doi: 10.1111/j.1365-2249.1993.tb07961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcke JTR, Nybo Jensen B, Ravn P, Andersen AB, Haslov K. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tuber Lung Dis. 1996;77:250–6. doi: 10.1016/s0962-8479(96)90009-x. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan G, Gandhi RR, Weinstein DE, Levis WR, Patarroyo ME, Brennan PJ, Cohn ZA. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987;138:3028–34. [PubMed] [Google Scholar]
- 17.Moura ACN, Mariano M. Lipids from Mycobacterium leprae cell wall suppress T-cell activation in vivo and in vitro. Immunology. 1997;92:429–36. doi: 10.1046/j.1365-2567.1997.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan PJ, Cho S-N, Klatser PR. Immunodiagnostics, including skin tests. Int J Lepr. 1996;64:S58–S62. [PubMed] [Google Scholar]
- 19.Dockrell HM, Young SK, Britton K, et al. Induction of Th1 cytokine responses by mycobacterial antigens in leprosy. Infect Immun. 1996;64:4385–9. doi: 10.1128/iai.64.10.4385-4389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann SHE. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;1:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 21.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 22.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 23.Weir RE, Morgan AR, Britton WJ, Butlin CR, Dockrell HM. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immunoepidemiological field studies of leprosy. J Immunol Methods. 1994;176:93–101. doi: 10.1016/0022-1759(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 24.Holland MJ, Bailey RL, Hayes RJ, Whittle HC, Mabey DCW. Conjunctival scarring in trachoma is associated with depressed cell-mediated immune responses to chlamydial antigens. J Infect Dis. 1993;168:1528–31. doi: 10.1093/infdis/168.6.1528. [DOI] [PubMed] [Google Scholar]
- 25.Mehra VL, Talwar GP, Balakrishnan K, Bhutani LK. Influence of chemotherapy and serum factors on the mitogenic response of peripheral leucocytes of leprosy patients to phytohaemagglutinin. Clin Exp Immunol. 1972;12:205–13. [PMC free article] [PubMed] [Google Scholar]
- 26.Noguiera N, Kaplan G, Levy E, et al. Defective gamma-interferon production in leprosy. J Exp Med. 1983;158:2165–70. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanolkar-Young S, Kolk AHJ, Andersen AB, et al. Results of the Third Immunology of Leprosy/Immunology of Tuberculosis Antimycobacterial Monoclonal Antibody Workshop. Infect Immun. 1992;60:3925–7. doi: 10.1128/iai.60.9.3925-3927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter SW, Rivoire B, Mehra V, Bloom BR, Brennan PJ. The major native proteins of the leprosy bacillus. J Biol Chem. 1990;265:14065–8. [PubMed] [Google Scholar]
- 29.Pessolani MCV, Brennan PJ. Molecular definition and identification of new proteins of Mycobacterium leprae. Infect Immun. 1996;64:5425–7. doi: 10.1128/iai.64.12.5425-5427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marques MAM, Chitale S, Brennan PJ, Pessolani MCV. Mapping and identification of the major cell wall-associated proteins of Mycobacterium leprae. Infect Immun. 1998;66:2625–31. doi: 10.1128/iai.66.6.2625-2631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilangumaran S, Robinson P, Shankernarayan NP, Ramu G, Mahadevan PR, Muthukkaruppan VR. T lymphocyte reactivity of leprosy patients and healthy contacts from a leprosy-endemic population to delipidified cell components of Mycobacterium leprae. Lepr Rev. 1994;65:34–44. doi: 10.5935/0305-7518.19940003. [DOI] [PubMed] [Google Scholar]
- 32.Gupte MD, Anantharaman DS, Nagaraju B, Kannan S, Vallishayee RS. Experiences with Mycobacterium leprae soluble antigens in a leprosy endemic population. Lepr Rev. 1990;61:132–44. [PubMed] [Google Scholar]
- 33.Krishnamurthy P, Rao PS, Subramanian M, Suresh Kumar SK, Neelan PN. Mycobacterium leprae soluble antigen (Rees) skin test. Responses in an endemic population in India. Ind J Lepr. 1993;65:49–57. [PubMed] [Google Scholar]
- 34.Launois P, Niang N'Diaye M, Cartel JL, Mane I, Drowart A, van Vooren JP, Sarthou JL, Huygen K. Fibronectin-binding antigen 85 and the 10-kilodalton GroES-related heat shock protein are the predominant TH-1 response inducers in leprosy contacts. Infect Immun. 1995;63:88–93. doi: 10.1128/iai.63.1.88-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dockrell HM, Stoker NG, Lee SP, et al. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989;57:1979–83. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thangaraj HS, Lamb FI, Davis EO, Jenner PJ, Jeyakumar LH, Colston MJ. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990;58:1937–42. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Launois P, Niang N'Diaye M, Sarthou JL, Drowart A, van Vooren JP, Cartel JL, Huygen K. T cell reactivity against antigen 85 but not against the 18- and 65-kD heat shock proteins in the early stages of acquired immunity against Mycobacterium leprae. Clin Exp Immunol. 1994;96:86–90. doi: 10.1111/j.1365-2249.1994.tb06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triccas JA, Roche PW, Winter N, Feng CG, Butlin CR, Britton WJ. A 35 kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64:5171–7. doi: 10.1128/iai.64.12.5171-5177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vega-Lopez F, Brooks LA, Dockrell HM, De Smet KAL, Thompson JK, Hussain R, Stoker NG. Sequence and immunological characterization of a serine rich antigen from Mycobacterium leprae. Infect Immun. 1993;61:2145–53. doi: 10.1128/iai.61.5.2145-2153.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinke de Wit TE, Clark-Curtiss JE, Abebe F, Kolk AHJ, Janson AAM, van Agterveld M, Thole JER. A Mycobacterium leprae-specific gene encoding an immunologically recognized 45kDa protein. Mol Microbiol. 1993;10:829–38. doi: 10.1111/j.1365-2958.1993.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 41.Ilangumaran A, Shanker Narayan NP, Ramu G, Muthukkaruppan VR. Cellular and humoral immune responses to recombinant 65-kD antigen of Mycobacterium leprae in leprosy patients and healthy controls. Clin Exp Immunol. 1994;96:79–85. doi: 10.1111/j.1365-2249.1994.tb06234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams E, Basten A, Rodda S, Britton WJ. Human T-cell clones to the 70-kilodalton heat shock protein of Mycobacterium leprae define mycobacterium-specific epitopes rather than shared epitopes. Infect Immun. 1997;65:1061–79. doi: 10.1128/iai.65.3.1061-1070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Reyn CF, Green PA, McCormick D, Huitt GA, Marsh BJ, Magnusson M, Barber TW. Dual skin testing with Mycobacterium avium sensitin and purified protein derivative: an open study of patients with M. avium complex infection of tuberculosis. Clin Infect Dis. 1994;19:15–20. doi: 10.1093/clinids/19.1.15. [DOI] [PubMed] [Google Scholar]
- 44.Streeton JA, Desem N, Jones SL. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int J Tuberc Lung Dis. 1998;2:443–50. [PubMed] [Google Scholar]
- 45.Wilkinson RJ, Haslov K, Rappuoli R, et al. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J Clin Microbiol. 1997;35:553–7. doi: 10.1128/jcm.35.3.553-557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas JW, Clements D, Grzybowski S. In vitro lymphocyte responses and skin test reactivity following BCG vaccination. Clin Exp Immunol. 1971;9:611–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Converse PJ, Jones SL, Astemborski J, Vlahov D, Graham NMH. Comparison of a tuberculin interferon-γ assay with the tuberculin skin test in high-risk adults: effect of human immunodeficiency virus infection. J Infect Dis. 1997;176:144–50. doi: 10.1086/514016. [DOI] [PubMed] [Google Scholar]