Abstract
Excess nitric oxide formation caused by the activity of the inducible nitric oxide synthase has been implicated as a toxic effector molecule in the pathogenesis of experimental colitis and inflammatory bowel disease. It was therefore investigated whether inhibition of this synthase or the cytokines TNF and IFN-γ, inducers of nitric oxide synthase, had effects on chronic colitis in mice. Chronic colitis was induced in mice by repeated feeding of DSS. Cytokines were neutralized by treatment with MoAbs and nitric oxide synthase was inhibited by aminoguanidine. The degree of colonic inflammation was assessed by a histological score and colon length. Aminoguanidine treatment reduced nitric oxide activity by 60% (P = 0.0004), the histological score by 31% (P = 0.005) and increased colon length by 1.4 cm (P = 0.002). Neutralization of TNF and IFN-γ resulted in increased colon length (0.7 cm, P = 0.07 and 0.8 cm, P = 0.03), improved histological score (19%, P = 0.045 and 25%, P = 0.013), and reduced nitric oxide activity (31%, P = 0.07 and 54%, P = 0.004) compared with controls. The combination of anti-cytokine treatments had additive effects. TNF and IFN-γ are involved in perpetuation of chronic DSS-induced colitis, and induction of excessive nitric oxide activity could be their common effector mechanism.
Keywords: experimental colitis, dextran sulphate sodium, tumour necrosis factor, interferon-gamma, nitric oxide
INTRODUCTION
The pathogenesis of chronic inflammatory bowel disease (IBD) is still unknown. Its aetiology is complex and seems to be multifactorial. There is increasing evidence that the immune system plays a critical role in the development and perpetuation of ulcerative colitis (UC) and Crohn's disease (CD). The available data suggest that a disturbed balance of pro- and anti-inflammatory cytokines is an important mechanism of chronic inflammation in IBD.
Elevated levels of proinflammatory cytokines such as IL-1 [1–3], IL-2 [4], IL-6 [3,4], IL-8 [4–7], IFN-γ [8] and TNF [3,4,8] and impaired responses to anti-inflammatory cytokines such as IL-4 [9,10] have been reported in biopsies from patients. A disturbed balance between IL-1 and its antagonist, IL-1 receptor antagonist (IL-1Ra), has also been found [11,12]. The relevance of cytokines in IBD has been underlined by several animal models (reviewed in [13]). Both IL-2- and IL-10-deficient mice develop chronic colitis [14,15]. Colitis in IL-10-deficient mice could be abrogated by treatment with IL-10 [14]. Neutralization of TNF and IFN-γ after transfer of CD45RBhigh CD4+ T cells to severe combined immunodeficient (SCID) mice prevented the development of colitis [16]. Neutralization of IL-12, an important inducer of IFN-γ production [17], after induction of TNBS-colitis has been shown to reduce inflammation [18]. We have shown in a model of DSS-induced chronic colitis that treatment with neutralizing anti-TNF antibodies led to an improvement of the colitis score [19]. IFN-γ expression has been reported in biopsies from patients with IBD [20–26] and in some animal models of intestinal inflammation [16,18].
There is evidence that the inflammatory cytokines IL-1, TNF and IFN-γ are important inducers of nitric oxide (NO) generation in macrophages, intestinal epithelial and other cells [27–31].
An increased production of the inducible nitric oxide synthase (iNOS) has been found in IBD [32–38] and in models of intestinal inflammation [39–41]. Increased luminal activities of NO have also been detected in UC [42] and in the colonic lavage fluid in different animal models of IBD [43,44]. Excessive production of NO in chronic colitis may be detrimental to the integrity of the colonic mucosa [45]. In accordance with this information, inhibition of iNOS has shown positive effects in some animal models of intestinal inflammation [40,46–48] and autoimmune disease [49]. In another rat model, colonic inflammation can be induced by feeding peroxynitrite, a reaction product of the radical NO [50]. Recent results, however, make the role of NO in IBD again controversial (reviewed in [51]), and McCafferty et al. described even a protective role for NO in acetic acid-induced colitis using iNOS-deficient mice for experiments [52].
Collectively, these data suggested a role for NO in colitis and the possibility that IFN-γ and TNF exert their effects in colitis via induction of excessive amounts of iNOS. Therefore, we used our model of chronic and acute DSS-induced colitis in mice to study the effect of NO in both and the relationship between NO and TNF/IFN-γin vivo.
MATERIALS AND METHODS
Mice
Female BALB/c mice weighing 20–22 g (Charles River, Sulyfeld, Germany) were used for the experiments. Except for the periods of induction of colitis they had food and water ad libidum.
Reagents and cell culture conditions
Hybridoma cells were cultured in RPMI 1640 supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco BRL, Eggenstein, Germany) and 10% HC-3 (PAN Systems, Nürnberg, Germany). Rat anti-mouse TNF MoAb V1q (IgD, κ) [53] was purified from hybridoma culture medium by ammonium sulphate precipitation. The purified material contained > 96% antibody as judged by silver-stained SDS–PAGE analysis. Purified V1q (2 ng) neutralized 0.5 ng TNF in vitro in the L929 cytotoxicity assay. The purified material was extensively dialysed against PBS, diluted to a concentration of 1 mg/ml in PBS and stored at −20°C until use. The final material contained 2 pg/ml lipopolysaccharide (LPS) as measured by the limulus assay.
Rat anti-mouse IFN-γ MoAb R4-6A2 (IgG1) [54] was purified from hybridoma culture medium by ammonium sulphate precipitation. The purified material contained > 98% antibody as judged by silver-stained SDS–PAGE. Purified R4-6A2 (1 μg) neutralized 16 U/ml of IFN-γin vitro as measured in a bioassay. In this assay, R4-6A2 antibodies inhibited the protective function of IFN-γ for L929-fibrosarcoma cells against infection with vesicular stomatitis virus. The antibodies were stored at a final concentration of 0.7 mg/ml in PBS at −20°C.
DSS was purchased from ICN (cat. no. 160110, mol. wt 40 000; Eschwege, Germany), rat IgG and aminoguanidine hemisulphate were purchased from Sigma (Deisenhofen, Germany).
Induction of acute and chronic colitis
For induction of acute colitis mice received 5% DSS in drinking water for 7 days. For induction of chronic colitis mice received four cycles of DSS treatment as described [19,55]. Each cycle consisted of 5% DSS in drinking water for 7 days, followed by a 7–10-day interval with normal drinking water. Mice were used for the experimental treatment 4–6 weeks after completion of the last cycle. Mice used for experiments were age-matched and had received DSS treatment simultaneously.
Treatments
Anti-cytokine antibodies were administered intraperitoneally for 5 consecutive days. For neutralization of TNF 100 μg of V1q were injected. As little as 20 μg per mouse had been found to effectively neutralize TNF in a mouse model of experimental peritonitis [53]. For neutralization of IFN-γ antibody R4-6A2 was used at doses of 4 μg, 20 μg and 100 μg per mouse per day. Control groups received 100 μg of rat IgG intraperitoneally in 100 μl of PBS per mouse per day.
For inhibition of iNOS in chronic colitis 1 mg or 10 mg aminoguanidine (AG) was injected intraperitoneally in a volume of 100 μl PBS per mouse per day for 5 days. Control groups in these experiments received 100 μl of PBS per mouse per day.
In the acute form of colitis iNOS was inhibited during the induction phase of colitis from day 0 to day 7 by AG at 10 mg per mouse per day intraperitoneally. Control groups received again 100 μl of PBS.
In acute colitis mice were killed on day 8, in chronic colitis mice were killed on day 6 of the experiment.
Determination of nitrite/nitrate in colonic biopsies
For determination of nitrite/nitrate content 1 cm of the last third of the mechanically cleaned colon was stored in 200 μl of distilled water at −20°C. After thawing, the tissue samples were removed. To 100 μl of the supernatant 14 mU of nitrate reductase (Aspergillus niger; Sigma) plus 10 μl of NADPH/H+ (40 μm; Sigma) were added. The reduction of nitrate was stopped after 5 min by 50 μl of water and 10 μl of diaminonaphthalene (0.05 mg/ml; Sigma) were added. After another 10 min of incubation in the dark at room temperature the reaction was stopped with 5 μl of 2.8 m NaOH. Finally the volume was adjusted to 400 μl with water for evaluation. Fluorescence was measured with a Fluorescence-Spectrophotometer (F-2000; Hitachi, Unterhaching, Germany; excitation 365 nm, emission 405 nm). Nitrite concentrations were calculated from a standard curve using NO2− concentrations in a range from 10 μm to 250 nm [56]. To confirm the validity of this method either one, two or three colonic specimens (each in triplicate) were stored in 200 μl of distilled water, followed by the procedure described above. It was found that the means of the measured nitrite/nitrate concentrations correlated with the number of colonic specimens: (‘number of biopsies in 200 μl’→‘nitrate/nitrite ± s.e.m.’: 3 → 1.066 ± 0.092 μm; 2 → 0.657 ± 0.077 μm; 1 → 0.276 ± 0.064 μm; r2 = 0.995). Furthermore, we did not find quantitative differences between measurement immediately after thawing and measurement after another 30 min of incubation, indicating that the diffusion was complete after thawing the samples.
Determination of histological score and colonic length
Since we found a significant reduction of colonic length [19] it was used as a parameter for colonic inflammation, as also described by others [57]. The colon was removed, mechanically cleaned, and was measured to 0.5 cm precision. The distal third of the colon was then cut longitudinally and laid on a filter paper and fixed in 10% formalin overnight. Sections of the paraffin-embedded material were made longitudinally. Three 5-μm sections were cut serially at a distance of 20 μm. The next three sections were cut at a distance of 100 μm. A third set of sections was cut after another 100 μm. The sections were stained with haematoxylin–eosin. Histological analysis was performed in a blinded fashion. Three sections obtained from each of three sites at 100 μm distance were evaluated. Mice were scored individually. Each score represented the mean of nine sections.
Histology was scored as follows: Epithelium (E): 0, normal morphology; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; 4, loss of crypts in large areas. Infiltration (I): 0, no infiltrate; 1, infiltrate around crypt basis; 2, infiltrate reaching to L. muscularis mucosae; 3, extensive infiltration reaching the L. muscularis mucosae and thickening of the mucosa with abundant oedema; 4, infiltration of the L. submucosa. The total histological score represents the sum of the epithelium and infiltration score (total score = E + I).
Statistical analysis
Statistical analysis was performed by Students-t test (nitrite values, body weight) or Mann–Whitney rank sum test (histological score, length of colon). Correlations (r2) were derived from linear regression analysis.
RESULTS
Characteristics of acute and chronic colitis
Acute colitis induced by a single cycle of DSS feeding was characterized by loose stools from day 3 on and bloody stools beginning on day 4 continuing to days 8–9, and severe weight loss being regained on days 9–12 (not shown). Histological examination on day 8 showed goblet cell and crypt loss and severe inflammatory infiltrate mainly consisting of granulocytes.
The majority of mice with chronic colitis induced by four cycles of DSS feeding had loose stools (60%), a minority had diarrhoea (10%), and bloody stools could hardly be detected macroscopically. These symptoms disappeared gradually after DSS treatment and usually subsided within the first 8 weeks after completion of the induction cycles. Histologically, colitis could still be detected 4 months after completion of the last cycle. The colons of mice with chronic colitis were reduced in length to about 7–8 cm compared with about 11–12 cm of those from healthy mice. Histologically, ulcers were rarely detected, whereas the main effects were epithelial damage and inflammatory cell infiltration, usually restricted to the mucosa and mainly consisting of lymphocytes and macrophages and to a lesser degree (< 15%) of granulocytes [19].
Effects of AG treatment on parameters of acute and chronic colitis
For inhibition of iNOS AG was used because it has a relative specificity for iNOS compared with cNOS [58–62]. Treatment of mice with chronic colitis for 5 days with graded doses of AG led to a significant dose-dependent decrease in nitrite/nitrate concentration of colonic biopsies (Fig. 1). In parallel, as shown in Fig. 2a, AG treatment led to a significant dose-dependent increase in colon length (P = 0.002). The histological score improved significantly in the 10 mg/day AG-treated group (P = 0.005), but no significant effect was seen at 1 mg/day AG (Fig. 2b). This correlation of nitrite/nitrate content with severity of colitis suggests a causative role of NO in chronic intestinal inflammation. In contrast, AG treatment (10 mg/day) during the acute phase of colitis induction (day 0 to day 7) aggravated colitis compared with PBS-treated control mice. The histological score was altered by 15.1% without reaching statistical significance (Fig. 2b) and in accordance with this finding of aggravation, average weight gain on day 8, 1 day after the end of DSS feeding, was significantly reduced in AG-treated mice compared with the weight gain of PBS-treated control mice on day 8 (Fig. 3).
Fig. 1.
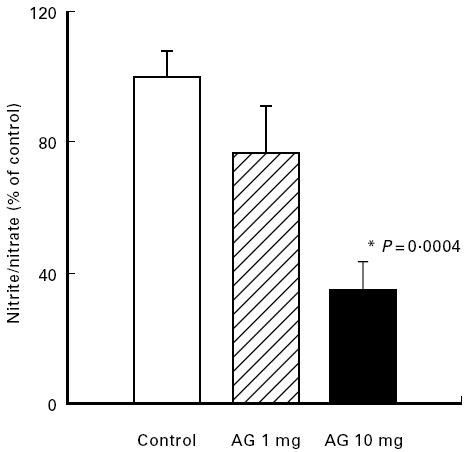
Effect of aminoguanidine (AG) on colonic NO activity. Mice with chronic DSS-induced colitis received either PBS (control), 1 mg or 10 mg per mouse AG from day 1 to day 5 of the experiment. Nitrite/nitrate contents were determined as described in Materials and Methods. Each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control group.
Fig. 2.
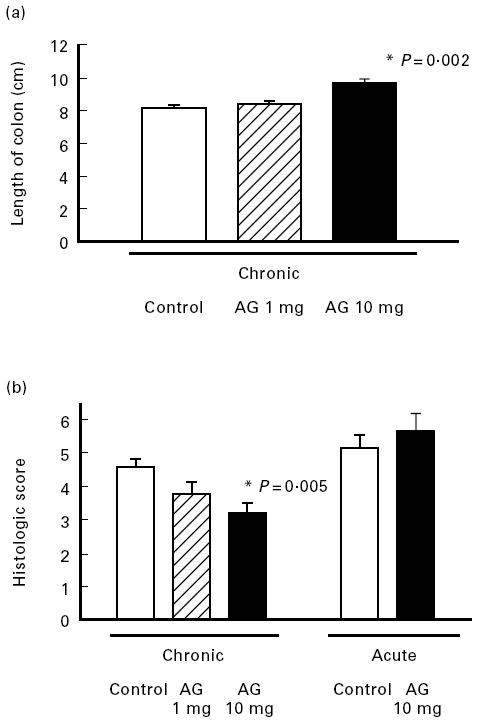
Effect of aminoguanidine (AG) on inflammatory parameters in chronic and acute colitis. Mice with chronic DSS-induced colitis received either PBS (control), 1 mg or 10 mg per mouse AG from day 1 to day 5 of the experiment or in acute colitis 10 mg AG per mouse per day or PBS (control) from day 0–7 of the induction phase. Colonic length (a) and histological score (b) were determined as described in Materials and Methods. In experiments each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups.
Fig. 3.
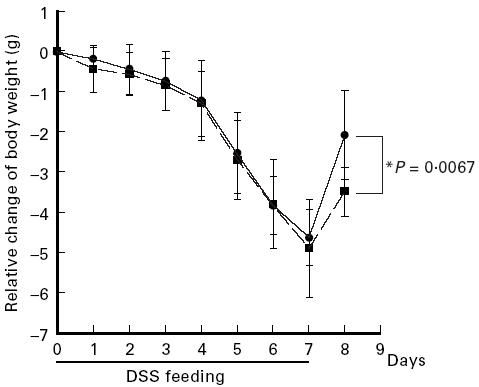
Kinetics of body weight of mice with acute colitis. During induction of acute colitis (days 0–7) mice were treated daily with aminoguanidine (AG) at 10 mg per mouse (▪) or with PBS (control) (•). Body weight of individual mice was monitored daily. In experiments each group consisted of five mice. The result is representative of three separate experiments. Data points represent mean values ± s.d.
The cytokines IL-1, TNF and IFN-γ are known to be strong inducers of NO generation. We therefore tested whether neutralization of these cytokines would improve the parameters of chronic colitis and decrease NO activity in colonic biopsies.
Effects of anti-IFN-γ treatment on parameters of chronic colitis
Neutralization of IL-1 activity with antibodies to the IL-1 receptor type I or IL-1Ra had no effect on histological score, as shown before [19], and also no change was found for mucosal nitrite/nitrate concentration or colonic length (data not shown).
Neutralization of IFN-γ with antibodies, however, significantly increased the length of the colon (P = 0.028) (Fig. 4a) and improved the colitis score (P = 0.013) (Fig. 4b) in a dose-dependent fashion compared with treatment with rat IgG. As shown in Fig. 5, these effects were paralleled by a dose-dependent decrease in nitrite/nitrate concentrations in the colon which was significant (P = 0.004) at a dose of 100 μg/mouse.
Fig. 4.
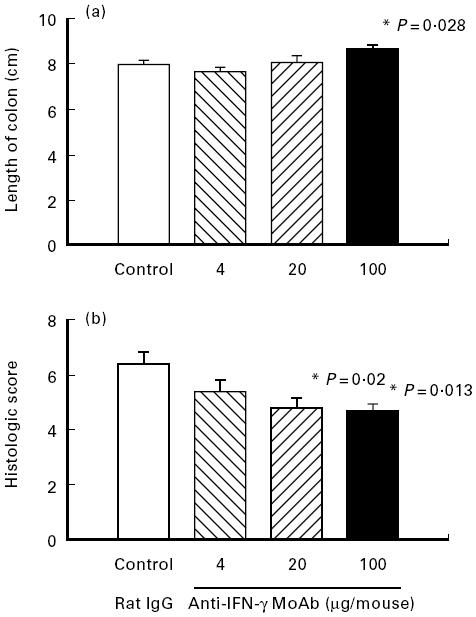
Effect of IFN-γ neutralization on inflammatory parameters in chronic colitis. Mice with chronic DSS-induced colitis received either rat IgG (control), 4 μg, 20 μg or 100 μg of neutralizing IFN-γ MoAb from day 1 to day 5 of the experiment. Colonic length (a) and histological score (b) were determined as described in Materials and Methods. Each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups.
Fig. 5.
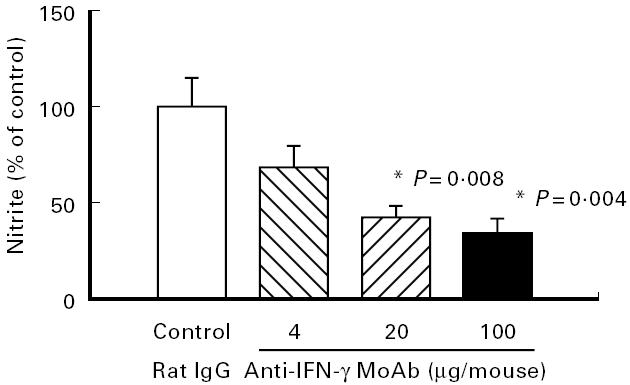
Effect of neutralization of IFN-γ on colonic nitric oxide (NO) activity. Mice with chronic DSS-induced colitis received either rat IgG (control), 4 μg, 20 μg or 100 μg of neutralizing IFN-γ MoAb from day 1 to day 5 of the experiment. Nitrite/nitrate concentrations were determined as described in Materials and Methods. Each group consisted of 9–10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control group.
Effects of combined anti-IFN-γ and anti-TNF treatment on parameters of chronic colitis
We had shown in earlier studies that neutralization of TNF led to improvement of the histological score in chronic DSS-induced colitis [19]. Therefore the effects on colonic length and nitrite/nitrate concentrations were also investigated. When mice were treated with neutralizing anti-TNF antibodies, the histological score was significantly improved (Fig. 6b), but colonic length (Fig. 6a) and nitrite/nitrate contents (Fig. 7) only showed a trend to increase or decrease, respectively, without being significantly different.
Fig. 6.
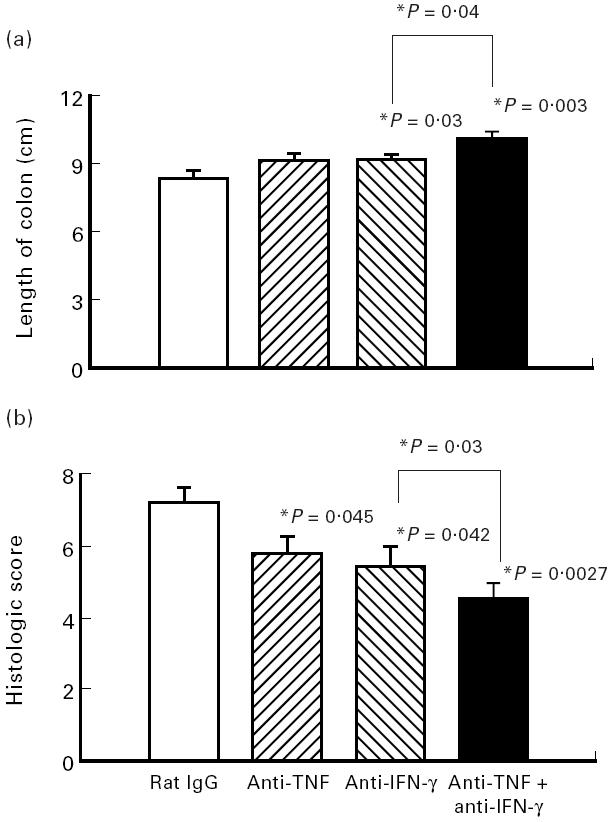
Effect of neutralization of TNF and IFN-γ in combination on parameters of colitis. Mice with chronic DSS-induced colitis received either rat IgG (control), 100 μg of neutralizing IFN-γ MoAb, 100 μg of neutralizing TNF MoAb or a combination of both MoAbs from day 1 to day 5 of the experiment. Colonic length (a) and histological score (b) were determined as described in Materials and Methods. Each group consisted of 10 mice. The result is representative of three separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups unless otherwise indicated.
Fig. 7.
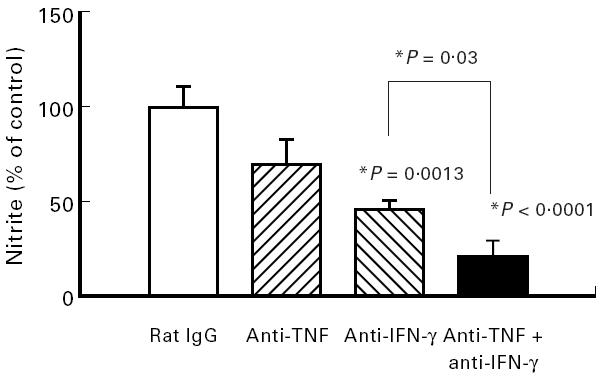
Effect of neutralization of TNF and IFN-γ in combination on nitric oxide (NO) activity. Mice with chronic DSS-induced colitis received either rat IgG (control), 100 μg of neutralizing IFN-γ MoAb, 100 μg of neutralizing TNF MoAb or a combination of both MoAbs from day 1 to day 5 of the experiment. Nitrite/nitrate concentrations were determined as described in Materials and Methods. Each group consisted of 10 mice. The result is representative of two separate experiments. Bars represent mean ± s.e.m. *Significantly different from control groups unless otherwise indicated.
When both cytokines, TNF and IFN-γ, were neutralized in combination with 100 μg anti-IFN-γ antibody plus 100 μg anti-TNF antibody, colon length increased significantly (P = 0.03) and additively (Fig. 6a). The same effect was found for the histological score (Fig. 6b). The most prominent effect of the combined treatment was found for nitrite/nitrate contents in colonic biopsies, which were significantly reduced by 80% (P < 0.0001) (Fig. 7). As shown in Fig. 8, severity of disease as expressed by the histological score and the measured nitrite/nitrate contents of colonic tissue correlated strongly (r2 = 0.985 and 0.980, respectively).
Fig. 8.
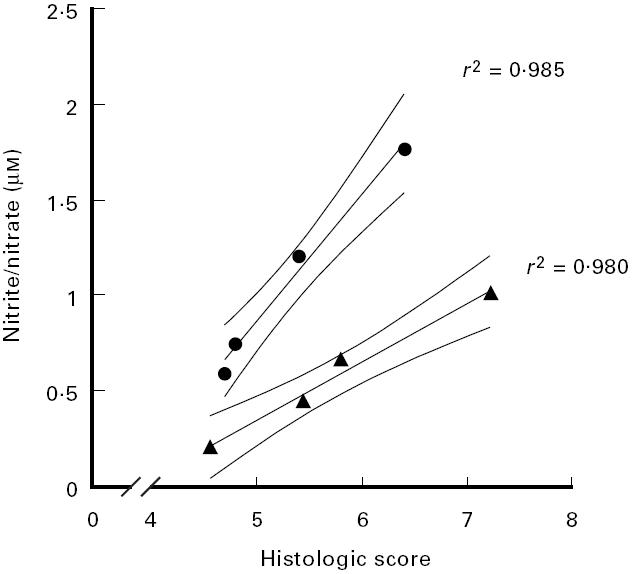
Correlation of the histological score and nitrite/nitrate concentrations of colonic tissue. The histological scores (abscissa) and nitrite/nitrate (ordinate) concentrations measured in the anti-IFN-γ dose response experiment (•) and in the anti-IFN-γ/anti-TNF combination experiment (▴) were correlated. Results were taken from experiments presented in Fig. 4b and Fig. 5 (anti-IFN-γ dose response) and in Fig. 6b and Fig. 7 (anti-IFN-γ/anti-TNF combination). A confidence interval of 95% is given. r2 were derived from linear regression analysis.
DISCUSSION
We show in this study that decreased NO generation after inhibition of iNOS aggravated acute colitis but improved the condition of chronic colitis in the model of DSS-induced experimental colitis. We also show that inhibition of endogenous TNF and/or IFN-γ by antibodies ameliorated the chronic stage of colitis and concomitantly decreased NO generation, demonstrating clear correlation. We conclude therefore that in chronic colitis NO contributes to inflammation and tissue damage and that IFN-γ and TNF exert their detrimental role at least partly via the induction of iNOS and the toxic effector molecule NO.
Nitric oxide formation has been implicated in the pathogenesis of several inflammatory diseases including IBD. Evidence that NO is a potent toxic mediator in chronic inflammation was initially demonstrated in animal models of adjuvant-induced arthritis [63] and diabetes [64]. Increased iNOS activity has also been measured in TNBS-induced experimental colitis [65] and in a granulomatous model of chronic colitis, where inhibition of iNOS reduced colonic tissue damage [66,67]. NO activities have been found increased in peripheral blood [34,38], and in the colon of patients with IBD [33,36,42]. Recent results, however, make the role of NO in IBD again controversial. Kubes et al. questioned the hypothesis that ‘large’ amounts of NO cause tissue damage in the gut by demonstrating that NO donors had a protective effect after induction of gut inflammation by platelet-activating factor in cats [68]. When iNOS-deficient mice were used to study the role of NO in colonic inflammation, NO again revealed its protective qualities. After instillation of acetic acid (3%) intrarectally iNOS-deficient mice showed macroscopically a two-fold stronger inflammatory response than wild-type mice after 24 h [52]. Interestingly, these two reports describe the role of NO in an acute phase of inflammation.
Our data provide evidence that in acute inflammation NO indeed tends to be protective, as was previously shown for the cytokines TNF and IL-1 by us [19] and was evidenced in the two reports mentioned above [52,68]. In this line is our result that antibiotics improve acute DSS-induced colitis (Hans et al., submitted), arguing for the hypothesis that a competent immune response is necessary to form a barrier against bacteria infiltrating the damaged mucosa during the acute phase.
In the chronic phase of colitis, however, we demonstrate that iNOS blocking significantly ameliorated inflammation and in parallel decreased colonic NO levels, whereas antibiotics (Hans et al., submitted) showed no benefit, suggesting an ongoing and dysregulated immune response leading to destruction of the mucosa. These results reveal the different quality of acute and chronic inflammation and help to interpret the contradictory results (reviewed in [51]) concerning the role of NO in intestinal inflammation.
In vitro induction of iNOS and NO formation in macrophages, in a colonic epithelial cell line and other cells by IL-1, TNF and IFN-γ has been demonstrated by several authors [31,69–75]. In addition, a correlation of NO production and expression of TNF and IFN-γ in the course of disease has been demonstrated in a model of cerebral toxoplasmosis [76] and in a model of experimental allergic encephalitis [77,78].
As cited in the Introduction, there is ample evidence in human IBD that the inflammatory cytokines IL-1, TNF, and IFN-γ are over-expressed, and this finding correlates with reports of excessive amounts of NO produced by activated iNOS in lamina propria mononuclear cells and colon epithelial cells [79,80]. This prompted us to investigate whether it was possible in our chronic colitis model to manipulate NO activities by cytokine inhibition and thus decrease mucosal damage.
Neutralization of IFN-γ or TNF was effective in amelioration of chronic DSS-induced colitis and in decreasing colonic NO activity, showing strong correlation. This indicates that IFN-γ is involved in the perpetuation of chronic colitis in this model and suggests the possibility that at least in part excessive NO generation is responsible for the deterioration of the mucosa.
Neutralization of IFN-γ was more effective than neutralization of TNF. Both cytokines had an additive effect both on histological score and on colonic NO concentrations, also arguing for a common mechanism of tissue destruction induced by TNF and IFN-γ. Using the hypothesis that NO generation induced by cytokines is the cause for mucosal damage, the differential effectiveness is supported by the fact that IFN-γ is the most potent inducer of iNOS in macrophages [72] and is essential for iNOS induction in colon epithelial cells [31].
It would be interesting to investigate the effect of long-lasting antibody treatment. In our experiments treatment was not extended beyond 5 days to avoid complications due to development of an anti-rat immunoglobulin response. Chronic colitis in this model is a months-long established disease which can be improved by 30–40% by a 5-day treatment and we think that we are only looking at the beginning of a repair and healing process. Receptor antagonists or genetically engineered antibodies are unfortunately not available for these cytokines.
We provide the first in vivo evidence that NO concentrations can be down-regulated via neutralization of IFN-γ and TNF in a model of chronic colitis, resulting in a significant amelioration of disease. In conclusion, NO tends to be protective in acute intestinal inflammation, whereas chronic colitis in this model is characterized by the destructive effect of inadequate NO generation induced by a dysregulated TNF and IFN-γ production.
Acknowledgments
The excellent technical expertise of Kirstin Grube, who supplied the purified antibodies, is gratefully acknowledged.
REFERENCES
- 1.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease—enhanced production during active disease. Gut. 1990;31:686–9. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989;30:835–8. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata Y, Ishiguro Y, Itoh J, Munakata A, Yoshida Y. The role of proinflammatory and immunoregulatory cytokines in the pathogenesis of ulcerative colitis. J Gastroenterol. 1995;30(Suppl. 8):56–60. [PubMed] [Google Scholar]
- 5.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased IL-8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–22. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsuyama K, Toyonaga A, Sasaki E, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1994;96:432–6. doi: 10.1111/j.1365-2249.1994.tb06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaufelberger HD, Uhr MR, McGuckin C, Logan RP, Misiewicz JJ, Gordon Smith EC, Beglinger C. Platelets in ulcerative colitis and Crohn's disease express functional interleukin-1 and interleukin-8 receptors. Eur J Clin Invest. 1994;24:656–63. doi: 10.1111/j.1365-2362.1994.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;1:301–5. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rückert Y, Schindler U, Heinig T, Nickolaus S, Raedler A, Schreiber S. IL-4 signaling mechanisms in inflammatory bowel disease mononuclear phagocytes. Infl Bowel Dis. 1996;2:244–52. [PubMed] [Google Scholar]
- 10.West GA, Matsuura T, Levine AD, Klein JS, Fiocchi C. Interleukin-4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–95.. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- 11.Andus T, Daig R, Lock G, Scholmerich J, Falk W, Gross V. Balance between pro- and antiinflammatory cytokines in the colonic mucosa in inflammatory bowel disease. Gastroenterology. 1995;108:A770. [Google Scholar]
- 12.Casini Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–40. [PubMed] [Google Scholar]
- 13.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 15.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 16.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 17.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183:2559–69. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Schölmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breese E, Braegger CP, Corrigan CJ, Walker Smith JA, MacDonald TT. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78:127–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Deem RL, Shanahan F, Targan SR. Triggered human mucosal T cells release tumour necrosis factor-alpha and interferon-gamma which kill human colonic epithelial cells. Clin Exp Immunol. 1991;83:79–84. doi: 10.1111/j.1365-2249.1991.tb05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsasser Beile U, von Kleist S, Gerlach S, Gallati H, Monting JS. Cytokine production in whole blood cell cultures of patients with Crohn's disease and ulcerative colitis. J Clin Lab Anal. 1994;8:447–51. doi: 10.1002/jcla.1860080618. [DOI] [PubMed] [Google Scholar]
- 23.Kusugami K, Fukatsu A, Tanimoto M, et al. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995;40:949–59. doi: 10.1007/BF02064182. [DOI] [PubMed] [Google Scholar]
- 24.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguchi M, Hiwatashi N, Liu Z, Toyota T. Enhanced interferon-gamma production and B7-2 expression in isolated intestinal mononuclear cells from patients with Crohn's disease. J Gastroenterol. 1995;30(Suppl. 8):52–55. [PubMed] [Google Scholar]
- 26.Watanabe M, Takaishi H, Hosoda Y, et al. CD4+ intestinal mucosal lymphocytes in the pathogenesis of Crohn's disease. J Gastroenterol. 1995;30(Suppl. 8):73–75. [PubMed] [Google Scholar]
- 27.Chenais B, Tenu JP. Involvement of nitric oxide synthase in antiproliferative activity of macrophages: induction of the enzyme requires two different kinds of signal acting synergistically. Int J Immunopharmacol. 1994;16:401–6. doi: 10.1016/0192-0561(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 28.Eizirik DL, Cagliero E, Bjorklund A, Welsh N. Interleukin-1 beta induces the expression of an isoform of nitric oxide synthase in insulin-producing cells, which is similar to that observed in activated macrophages. FEBS Letters. 1992;308:249–52. doi: 10.1016/0014-5793(92)81285-t. [DOI] [PubMed] [Google Scholar]
- 29.Saito S, Nakano M. Nitric oxide production by peritoneal macrophages of Mycobacterium bovis BCG-infected or non-infected mice: regulatory role of T lymphocytes and cytokines. J Leuk Biol. 1996;59:908–15. doi: 10.1002/jlb.59.6.908. [DOI] [PubMed] [Google Scholar]
- 30.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 31.Kolios G, Brown Z, Robson RL, Robertson DA, Westwick J. Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT-29. Br J Pharmacol. 1995;116:2866–72. doi: 10.1111/j.1476-5381.1995.tb15938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boughton Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338–40. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- 33.Grisham MB, Yamada T. Neutrophils, nitrogen oxides, and inflammatory bowel disease. Ann NY Acad Sci. 1992;664:103–15. doi: 10.1111/j.1749-6632.1992.tb39753.x. [DOI] [PubMed] [Google Scholar]
- 34.Oudkerk Pool M, Bouma G, Visser JJ, Kolkman JJ, Tran DD, Meuwissen SG, Pena AS. Serum nitrate levels in ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1995;30:784–8. doi: 10.3109/00365529509096328. [DOI] [PubMed] [Google Scholar]
- 35.Iwashita E, Miyahara T, Hino K, Tokunaga T, Wakisaka H, Sawazaki Y. High nitric oxide synthase activity in endothelial cells in ulcerative colitis. J Gastroenterol. 1995;30:551–4. doi: 10.1007/BF02347578. [DOI] [PubMed] [Google Scholar]
- 36.Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–23. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roediger WE, Lawson MJ, Radcliffe BC. Nitrite from inflammatory cells—a cancer risk factor in ulcerative colitis? Dis Colon Rectum. 1990;33:1034–6. doi: 10.1007/BF02139219. [DOI] [PubMed] [Google Scholar]
- 38.Tran DD, Visser JJ, Pool MO, Cuesta MA, Hoekman K, Pena AS, Meuwissen SG. Enhanced systemic nitric oxide production in inflammatory bowel disease. Lancet. 1993;341:1150. doi: 10.1016/0140-6736(93)93166-x. [DOI] [PubMed] [Google Scholar]
- 39.Miller MJ, Thompson JH, Zhang XJ, et al. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;109:1475–83. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- 40.Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–55. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribbons KA, Zhang XJ, Thompson JH, et al. Potential role of nitric oxide in a model of chronic colitis in rhesus macaques. Gastroenterology. 1995;108:705–11. doi: 10.1016/0016-5085(95)90442-5. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Hellstrom PM, Lundberg JM, Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994;344:1673–4. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 43.Gunawardana SC, Jergens AE, Ahrens FA, Niyo Y. Colonic nitrite and immunoglobulin G concentrations in dogs with inflammatory bowel disease. J Am Vet Med Assoc. 1997;211:318–21. [PubMed] [Google Scholar]
- 44.Ferretti M, Gionchetti P, Rizzello F, et al. Intracolonic release of nitric oxide during trinitrobenzene sulfonic acid rat colitis. Dig Dis Sci. 1997;42:2606–11. doi: 10.1023/a:1018897519880. [DOI] [PubMed] [Google Scholar]
- 45.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–41. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogaboam CM, Jacobson K, Collins SM, Blennerhassett MG. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol. 1995;268:G673–84. doi: 10.1152/ajpgi.1995.268.4.G673. [DOI] [PubMed] [Google Scholar]
- 47.Miller MJ, Sadowska Krowicka H, Chotinaruemol S, Kakkis JL, Clark DA. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993;264:11–16. [PubMed] [Google Scholar]
- 48.Neilly PJ, Gardiner KR, Rowlands BJ. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1996;38:475–475. doi: 10.1136/gut.38.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross AH, Misko TP, Lin RF, Hickey WF, Trotter JL, Tilton RG. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93:2684–90. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rachmilewitz D, Stamler JS, Karmeli F, Mullins ME, Singel DJ, Loscalzo J, Xavier RJ, Podolsky DK. Peroxynitrite-induced rat colitis—a new model of colonic inflammation. Gastroenterology. 1993;105:1681–8. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- 51.Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996;270:G225–37. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- 52.McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–7. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- 53.Echtenacher B, Falk W, Mannel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–6. [PubMed] [Google Scholar]
- 54.Havell EA. Purification and further characterization of an anti-murine interferon-gamma monoclonal neutralizing antibody. J Interferon Res. 1986;6:489–97. doi: 10.1089/jir.1986.6.489. [DOI] [PubMed] [Google Scholar]
- 55.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 56.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 57.Axelsson LG, Landstrom E, Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther. 1998;12:925–34. doi: 10.1046/j.1365-2036.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 58.Misko TP, Moore WM, Kasten TP, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 59.Leib SL, Kim YS, Black SM, Tureen JH, Tauber MG. Inducible nitric oxide synthase and the effect of aminoguanidine in experimental neonatal meningitis. J Infect Dis. 1998;177:692–700. doi: 10.1086/514226. [DOI] [PubMed] [Google Scholar]
- 60.Mattson DL, Maeda CY, Bachman TD, Cowley AWJ. Inducible nitric oxide synthase and blood pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- 61.Okuda Y, Sakoda S, Fujimura H, Yanagihara T. Aminoguanidine, a selective inhibitor of the inducible nitric oxide synthase, has different effects on experimental allergic encephalomyelitis in the induction and progression phase. J Neuroimmunol. 1998;81:201–10. doi: 10.1016/s0165-5728(97)00180-x. [DOI] [PubMed] [Google Scholar]
- 62.Laszlo F, Evans SM, Whittle BJ. Aminoguanidine inhibits both constitutive and inducible nitric oxide synthase isoforms in rat intestinal microvasculature in vivo. Eur J Pharmacol. 1995;272:169–75. doi: 10.1016/0014-2999(94)00637-m. [DOI] [PubMed] [Google Scholar]
- 63.Connor JR, Manning PT, Settle SL, Moore WM, Jerome GM, Webber RK, Tjoeng FS, Currie MG. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur J Pharmacol. 1995;273:15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- 64.Corbett JA, Mikhael A, Shimizu J, Frederick K, Misko TP, McDaniel ML, Kanagawa O, Unanue ER. Nitric oxide production in islets from nonobese diabetic mice: aminoguanidine-sensitive and -resistant stages in the immunological diabetic process. Proc Natl Acad Sci USA. 1993;90:8992–5. doi: 10.1073/pnas.90.19.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo HG, Takata I, Nakamura M, Tatsumi H, Suzuki K, Fujii J, Taniguchi N. Induction of nitric oxide synthase and concomitant suppression of superoxide dismutases in experimental colitis in rats. Arch Biochem Biophys. 1995;324:41–47. doi: 10.1006/abbi.1995.9932. [DOI] [PubMed] [Google Scholar]
- 66.Grisham MB, Specian RD, Zimmerman TE. Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. J Pharmacol Exp Ther. 1994;271:1114–21. [PubMed] [Google Scholar]
- 67.Yamada T, Sartor RB, Marshall S, Specian RD, Grisham MB. Mucosal injury and inflammation in a model of chronic granulomatous colitis in rats. Gastroenterology. 1993;104:759–71. doi: 10.1016/0016-5085(93)91011-6. [DOI] [PubMed] [Google Scholar]
- 68.Kubes P, Reinhardt PH, Payne D, Woodman RC. Excess nitric oxide does not cause cellular, vascular, or mucosal dysfunction in the cat small intestine. Am J Physiol. 1995;269:G34–41. doi: 10.1152/ajpgi.1995.269.1.G34. [DOI] [PubMed] [Google Scholar]
- 69.Chesrown SE, Monnier J, Visner G, Nick HS. Regulation of inducible nitric oxide synthase mRNA levels by LPS, INF-gamma, TGF-beta, and IL-10 in murine macrophage cell lines and rat peritoneal macrophages. Biochem Biophys Res Comm. 1994;200:126–34. doi: 10.1006/bbrc.1994.1424. [DOI] [PubMed] [Google Scholar]
- 70.Hausmann EH, Hao SY, Pace JL, Parmely MJ. Transforming growth factor beta 1 and gamma interferon provide opposing signals to lipopolysaccharide-activated mouse macrophages. Infect Immun. 1994;62:3625–32. doi: 10.1128/iai.62.9.3625-3632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lau KS, Nakashima O, Aalund GR, Hogarth L, Ujiie K, Yuen J, Star RA. TNF-alpha and IFN-gamma induce expression of nitric oxide synthase in cultured rat medullary interstitial cells. Am J Physiol. 1995;269:F212–7. doi: 10.1152/ajprenal.1995.269.2.F212. [DOI] [PubMed] [Google Scholar]
- 72.Liew FY. Nitric oxide in infectious and autoimmune diseases. Ciba Found Symp. 1995;195:234–9. [PubMed] [Google Scholar]
- 73.Misko TP, Trotter JL, Cross AH. Mediation of inflammation by encephalitogenic cells: interferon gamma induction of nitric oxide synthase and cyclooxygenase 2. J Neuroimmunol. 1995;61:195–204. doi: 10.1016/0165-5728(95)00091-f. [DOI] [PubMed] [Google Scholar]
- 74.Roach TI, Barton CH, Chatterjee D, Liew FY, Blackwell JM. Opposing effects of interferon-gamma on iNOS and interleukin-10 expression in lipopolysaccharide- and mycobacterial lipoarabinomannan-stimulated macrophages. Immunology. 1995;85:106–13. [PMC free article] [PubMed] [Google Scholar]
- 75.Weisz A, Cicatiello L, Esumi H. Regulation of the mouse inducible-type nitric oxide synthase gene promoter by interferon-gamma, bacterial lipopolysaccharide and NG-monomethyl-l-arginine. Biochem J. 1996;316:209–15. doi: 10.1042/bj3160209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–81. [PubMed] [Google Scholar]
- 77.Waldburger KE, Hastings RC, Schaub RG, Goldman SJ, Leonard JP. Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms. Am J Pathol. 1996;148:375–82. [PMC free article] [PubMed] [Google Scholar]
- 78.Cross AH, Keeling RM, Goorha S, San M, Rodi C, Wyatt PS, Manning PT, Misko TP. Inducible nitric oxide synthase gene expression and enzyme activity correlate with disease activity in murine experimental autoimmune encephalomyelitis. J Neuroimmunol. 1996;71:145–53. doi: 10.1016/s0165-5728(96)00147-6. [DOI] [PubMed] [Google Scholar]
- 79.Godkin AJ, De Belder AJ, Villa L, Wong A, Beesley JE, Kane SP, Martin JF. Expression of nitric oxide synthase in ulcerative colitis. Eur J Clin Invest. 1996;26:867–72. doi: 10.1111/j.1365-2362.1996.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 80.Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–85. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]


