Abstract
Idiopathic CD4 T lymphocytopenia (ICL) is an unusual immune defect in which there is an unexplained deficit of CD4 T cells, leading to fungal, parasitic or other serious opportunistic infections. Current treatment efforts are directed at eliminating infections. Here we describe the use of a novel treatment, subcutaneous polyethylene glycol (PEG)–IL-2 injections, in a woman with this disorder, who had chronic severe mycobacterial disease which led to repeated hospitalizations, and advancing respiratory insufficiency. For this patient, PEG–IL-2, 50 000 U/m2, has been given by weekly subcutaneous injections for 5.5 years. This treatment has resulted in marked (and still continuing) long-term immunological improvement with normalized T cell functions and increased CD4 cell numbers. She has had substantial clinical improvement with clearing of mycobacterial disease, reducing hospitalizations and improved lung functions. The improvement seen in this patient suggests that low-dose IL-2 is a safe and practical therapy, which might be useful in other subjects with this potentially serious immune defect.
Keywords: lymphopenia, T cell, IL-2 treatment, Mycobacterium chelonei, Mycobacterium avium
INTRODUCTION
In the past 10 years there have been a number of reports describing persons with idiopathic CD4 T lymphocytopenia (ICL), an immune defect that may lead to fungal, parasitic or other serious opportunistic infections [1–5]. A working definition of ICL includes an absolute CD4 count of < 300 cells/cm, or < 20% of T cells on more than one occasion, lack of HIV infection, and absence of known immune deficiency disease or therapy associated with lymphopenia. ICL has been associated with Pneumocystis carinii, mycobacterial and cryptococcal infections, mucosal candidiasis, verrucae, chronic inflammatory skin diseases, toxoplasmosis, cytomegalovirus retinitis, and a number of fungal infections. Human retroviruses, HIV-1, HIV-2, HTLV-1, HTLV-2, and other transmissible agents have not been isolated, and there appear to be no familial components. This disease may be associated with substantial morbidity; in one series, two of 12 died of acute complications of immune deficiency [3] and in another, three of five died [1]. A similarly high death rate was found by the Italian Study group [6]. Current treatment efforts are directed at preventing or eliminating opportunistic infections. In this study we describe a case of ICL treated with low doses of subcutaneously injected IL-2 for 5 years, producing long-term increases in CD4 cell numbers, improved T cell functions, and substantial clinical improvement.
METHODS
Case report
The patient is a now 67-year-old woman, whose pulmonary disease started in January 1985, when at the age of 54 she developed a chronic cough and dyspnoea on exertion. She was diagnosed with pneumonia in February 1985 and was put on antibiotics. A chest computed tomography (CT) showed bronchiectasis; the forced expiratory volume in 1 s (FEV1) was reduced at 1.75 l. In January 1986, because of unresolved chest disease, she had a bronchoscopy, which demonstrated granulomata; cultures showed Mycobacterium avium (MAI). She was started on isoniazid, ethambutol and rifampin, then cycloserine and ethionamide. She became intolerant of ethionamide; a trial of clofazimine led to cataract formation, and pyrazinamide increased uric acid levels. These were discontinued. Sputum tests continued to be positive for MAI on a regimen of isoniazid, ethambutol and cycloserine. In August 1989 she was admitted to the National Jewish Medical Center where a heavy sputum growth of M. chelonei was found. An indwelling catheter was placed; she was started on cefoxitin, amikacin, and ansamycin. Liver function tests became abnormal on ansamycin; medications were changed back to ethambutol, isoniazid and cycloserine. CT of the chest demonstrated increased bronchiectasis throughout both lung fields. In October 1988, she had a catheter sepsis due to Pseudomonas aeruginosa; the catheter was removed. By August 1989 she had < 50% lung capacity, with an FEV1 of 1.1/l, and an FEV1/forced vital capacity (FVC) of 45%. In May 1991 M. chelonei persisted on sputum cultures, resistant to standard antibiotics. Clarithromycin was added. From July 1987 until April 1993 she had a total of 18 hospitalizations for ongoing mycobacterial disease resulting in respiratory insufficiency. Her past medical history included hypertension, and hypertrophic cardiomyopathy diagnosed in 1962.
The absolute number of CD4 cells in April 1986 was 428, but fell over the subsequent 7 years; the CD4/CD8 ratio dropped to 0.2 (Fig. 1). Antibody tests for HIV-1 and HIV-2 were repeatedly negative. She was given interferon-gamma (IFN-γ; 100 μg/m2) by subcutaneous injection, 5 days a week, from November 1989 to March 1990 and May 1990 to August 1990 with stabilization CD4 cell numbers (Fig. 1). By July 1992 the CD4 count was 29. In January 1993 she retired from work due to persistent cough, weight loss, and dyspnoea. Pulmonary function studies showed severe obstructive disease with FEV1 reduced to 0.57 l with a diffusing capacity 69% of expected. Sputum cultures were still positive for M. chelonei.
Fig. 1.

The absolute number of CD4 T cells and the ratio of CD4/CD8 cells during the period of study is given in the top two panels. The normal absolute number of CD4 cells would be 500–1700 cells/mm3 or greater, since April 1994 the absolute number of CD4 cells has been > 100/mm3, as indicated. In the lower panel the number of B cells (CD19) is given at the indicated time points. Normal B cell numbers of would be 75–375 cells/mm3. Two short courses of IFN-γ were given in 1989 and 1990; since April 1993 the patient has received IL-2 as indicated.
In April 1993, lymphocyte function tests showed very poor responses to mitogens, and insignificant responses to candida or tetanus toxoid antigens (Fig. 2). The mixed lymphocyte culture reaction was absent. Because of poor T cell functions and deteriorating clinical condition, subcutaneous injections of polyethylene glycol-conjugated recombinant IL-2 (PEG–IL-2) were started in April 1993 with Institutional Review Board consent. PEG–IL-2 (Chiron Corp., Emeryville, CA) is recombinant IL-2, covalently bonded to PEG 5000, which extends the half-life 10-fold without loss of biologic activity [7]. Treatment has been given continuously since this time, using weekly injections of 50 000 U/m2.
Fig. 2.
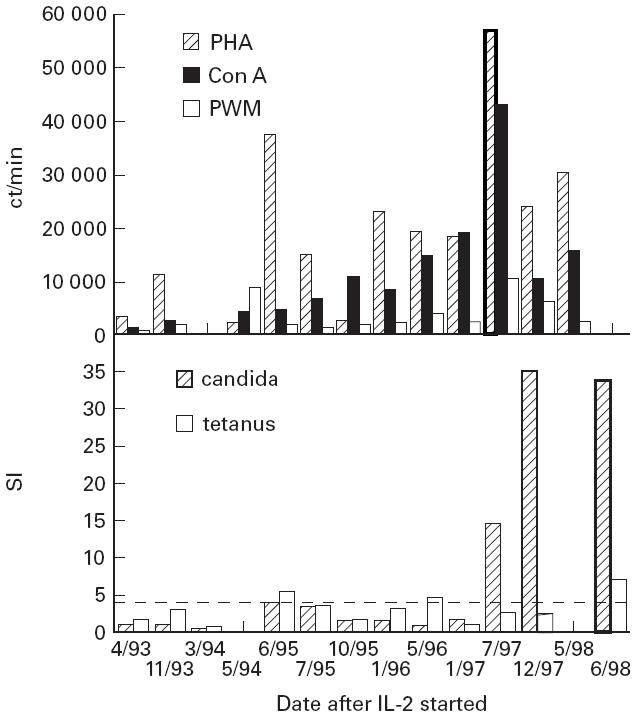
Lymphocyte proliferative responses to the mitogens phytohaemagglutinin (PHA), concanavalin A (Con A) and pokeweed mitogen (PWM) were determined at intervals after starting IL-2. The results are given as ct/min of 3H-thymidine incorporation (top panel). For normal subjects, lymphocyte proliferation to PHA would be 20 000–90 000 ct/min, for Con A 7500–45 000 ct/min, and for PWM it would be 2500–30 000 ct/min. Similarly, lymphocytes were tested for proliferative responses to the antigens candida and tetanus toxoid (lower panel). These results are expressed as the stimulation index (SI), which is the number of counts for the test wells containing the antigen/the number of counts in test wells that contained no antigen. Normal subjects with positive lymphocyte responses would have SIs of ≥ 4.0 (as shown in the figure).
Lymphocyte populations and proliferation
The proliferative responses of peripheral blood lymphocytes to phytohaemagglutinin (PHA), tetanus toxoid, Candida albicans and allogeneic cells were determined at intervals [8]. Lymphocytes were analysed with a fluorescence-activated cell sorter (FACS/IV; Becton Dickinson, Mountain View, CA) with the fluorescent labelled MoAbs to CD3, CD8, CD4, CD20, CD56 and CD45RA (naive) and CD29 (CD45RO, memory) antigens (Becton Dickinson).
RESULTS
After 1 year of IL-2 treatment, CD4 cell counts increased to 100/mm3 or more, and have been between 100 and 200 cells/mm3 since this time (Fig. 1). Before IL-2 treatment the patient had a profound loss of CD4+CD45+RA+ cells (CD4+CD45+RA+/CD4 ratio 0.18) rather than CD4+CD45+RA+RO cells (ratio 0.8). After 5 years of IL-2, the proportion of naive cells increased (CD4+CD45+RA+/CD4 ratio 0.23 and the proportion of CD4+CD45+RO+ (memory) cells decreased (ratio 0.55). While the overall numbers of CD3, CD8 and CD56 cells did not change, IL-2 treatment was also associated with a steady increase in B cell numbers (Fig. 1). Lymphocyte responses to mitogens PHA, concanavalin A, and pokeweed mitogen slowly improved; lymphocyte proliferation responses to candida normalized after 4 years of IL-2 treatment (Fig. 2). T cell proliferation to tetanus toxoid remains reduced, but no tetanus booster has been given.
Sputum cultures have been negative for M. chelonei since October 1993; anti-mycobacterial agents were discontinued. Pulmonary functions have improved; the FEV1 increased from its nadir of 0.5 l in 1993 to 0.9 l in January 1998. The patient has been hospitalized only twice in the 5.5 years since starting IL-2, for a subdural haematoma due to trauma in August 1993, and in April 1996 for a bronchial Pseudomonas infection. Minor adverse reactions to IL-2 occurred in the first year of treatment, consisting of local skin reactions, fatigue and frontal headache.
DISCUSSION
IL-2 has been used in varying doses to enhance T cell immunity in congenital [8–11] and acquired immunodeficiency disease [12–16]. IL-2 in low doses can reverse skin test anergy to recall antigens and may be a useful immunopotentiator in HIV-infected patients [14–16]. How IL-2 treatment enhanced the immune functions of our patient is uncertain. IL-2 stimulates the growth of progenitor T cells from primary lymphoid organs, accelerates the expansion of peripheral T cells, and is essential for the development of antigen-specific and non-specific immune responses [17]. IL-2 also plays a role in protecting antigen-stimulated T cells from programmed cell death [18], an activity that could protect against the apoptotic T cell loss previously documented in ICL [19]. Whether or not the patient had accelerated CD4 cell death prior to treatment with IL-2 is unknown, although she had a disproportionate loss of CD4+CD45RA cells (ratio 0.18), suggesting a more selective loss of naive CD4+ cells; an increase in CD4 CD45RA T cells occurred after IL-2 treatment.
What is curious about our patient is the protracted course of improvement; while IL-2 was started in April 1993, enhanced T cell proliferation to mitogens was not evident until 1 year later, and to candida antigen after 4 years of treatment. The delayed enhancement of proliferation is difficult to explain unless new clonal T cell populations were permitted to emerge and expand. B cell numbers increased after 12 months of treatment, suggesting that proliferation of this cell population also occurred, a biological effect which has previously been ascribed to IL-2 [20]. Although expansion of peripheral natural killer numbers and eosinophilia is common in IL-2-treated subjects with HIV infection, the patient had no overall increase in either cell population. Whether IL-2 might arrest or reverse CD4 cell loss in other subjects with ICL is unknown, but the lack of significant adverse affects suggests that it might provide a useful treatment for subjects in whom significant similar cellular dysfunction and opportunistic disease develop.
Acknowledgments
The authors thank Sarah Martin, Monica Reiter-Wong and Vivian Badami, who provided excellent nursing care, and Lydia Lopez for superb secretarial assistance. This work was supported in part by grant FD-R 001162 from the Office of Orphan Products Development and grant CA 53341 from the National Cancer Institute.
REFERENCES
- 1.Laurence J, Siegal FP, Schattner E, Gelman IH, Morse S. Acquired immunodeficiency without evidence of infection with human immunodeficiency virus types 1 and 2. Lancet. 1992;340:273–4. doi: 10.1016/0140-6736(92)92359-n. [DOI] [PubMed] [Google Scholar]
- 2.Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T lymphopenia without HIV infection: an investigation of cases in the United States. N Engl J Med. 1993;328:373–9. doi: 10.1056/NEJM199302113280601. [DOI] [PubMed] [Google Scholar]
- 3.Ho DD, Cao Y, Zhu T, Farthing C, Wang N, Gu G, Schooley RT, Daar ES. Idiopathic CD4+ T lymphopenia (ICL): immunodeficiency without evidence of human immunodeficiency virus infection. N Engl J Med. 1993;328:380–6. doi: 10.1056/NEJM199302113280602. [DOI] [PubMed] [Google Scholar]
- 4.Spira TJ, Jones BM, Nicholson JKA, et al. Idiopathic CD4+ T lymphocytopenia—an analysis of five patients with unexplained opportunistic infections. N Engl J Med. 1993;328:86–92. doi: 10.1056/NEJM199302113280603. [DOI] [PubMed] [Google Scholar]
- 5.Duncan RA, Von Reyn CF, Alliegro GM, Toossi Z, Sugar AM, Levitz SM. Idiopathic CD4+ T lymphocytopenia—four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393–8. doi: 10.1056/NEJM199302113280604. [DOI] [PubMed] [Google Scholar]
- 6.Reza G, Pezzofti P, Aiuti F. Acquired immunodeficiency without HIV infection: epidemiology and clinical outcome in Italy. Br Med J. 1995;311:785–6. doi: 10.1136/bmj.311.7008.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katre NV, Knauf JM, Laird WJ. Chemical modification of recombinant interleukin-2 by polyethylene glycol increases in potency in the murine Meth A sarcoma model. Proc Natl Acad Sci USA. 1987;84:1487–91. doi: 10.1073/pnas.84.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham-Rundles C, Mayer L, Sapira E, Mendelsohn L. Restoration of immunoglobulin secretion in vitro in common variable immunodeficiency by in vitro treatment with polyethylene glycol-conjugated human recombinant interleukin-2. Clin Immunol Imunopathol. 1992;64:46–56. doi: 10.1016/0090-1229(92)90058-v. [DOI] [PubMed] [Google Scholar]
- 9.Pahwa R, Paradise C, Pahwa S, et al. Recombinant IL-2 therapy in severe combined immunodeficiency disease (SCID) Proc Natl Acad Sci USA. 1989;86:5069–73. doi: 10.1073/pnas.86.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham-Rundles C, Kazbay K, Hassett J, Zhou Z, Mayer L. Enhanced humoral immunity in common variable immunodeficiency with polyethylene glycol-conjugated interleukin 2. New Engl J Med. 1994;331:918–21. doi: 10.1056/NEJM199410063311405. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham-Rundles C, Kazbay K, Mayer L, Zhou Z. Immunologic effects of low dose polyethylene glycol-conjugated human recombinant interleukin-2 in common immunodeficiency. Lymphokine Cytokine Res. 1995;15:269–76. doi: 10.1089/jir.1995.15.269. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med. 1995;332:567–75. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 13.Piscitelli SC, Wells MJ, Metcalf JA, Baseler M, Stevens R, Davey RT. Pharmacokinetics and pharmacodynamics of subcutaneous interleukin-2 in HIV-infected patients. Pharmacotherapy. 1996;16:754–9. [PubMed] [Google Scholar]
- 14.Teppler H, Kaplan G, Smith KA, Cameron P, Montana A, Meyn P, Cohn Z. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin-2 in patients with human immunodeficiency virus type 1 infection. J Exp Med. 1993;177:483–92. doi: 10.1084/jem.177.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci USA. 1996;93:10405–10. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinter A, Fauci AS. Interleukin-2 and human immunodeficiency virus infection: pathogenic mechanisms and potential for immunologic enhancement. Immunol Res. 1996;15:1–15. doi: 10.1007/BF02918280. [DOI] [PubMed] [Google Scholar]
- 17.Smith KA. Interleukin-2: inception, impact and implications. Sci. 1988;240:1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 18.Zamorano J, Wang HY, Wang R, Shi Y, Longmore GD, Keegan AD. Regulation of cell growth by IL-2: role of STAT5 in protection from apoptosis but not in cell cycle progression. J Immunol. 1998;160:3502–12. [PubMed] [Google Scholar]
- 19.Laurence J, Mitra D, Steiner M, Lynch DH, Siegal FP, Staiano CL. Apoptotic depletion of CD4+ T cells in idiopathic CD4+ T lymphocytopenia. J Clin Invest. 1996;97:672–80. doi: 10.1172/JCI118464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagoo A, Tseng CK, Sell S. Interleukin-2 produced by activated B lymphocytes acts as an autocrine proliferation-inducing lymphokine. Cytokine. 1990;2:272–9. doi: 10.1016/1043-4666(90)90028-r. [DOI] [PubMed] [Google Scholar]


