Abstract
The expression of αEβ7 integrin has been related to the selective retention of lymphocytes in mucosal tissues of gut, urogenital tract and lung. To identify potential disease-associated αEβ7 expression patterns on cells accounting for lymphocytic alveolitis in interstitial lung disease (ILD), αE expression on CD4+ and CD8+ T cell subsets was evaluated by dual-colour flow cytometry in peripheral blood and bronchoalveolar lavage fluid (BALF) of patients with idiopathic pulmonary fibrosis (IPF; n = 18), hypersensitivity pneumonitis (HP; n = 20) and sarcoidosis (n = 44) in comparison with healthy controls (n = 15). In both healthy individuals and all patient groups the proportion of αE-bearing T cells in peripheral blood was < 2%, whereas the vast majority of alveolar CD8+ T cells consistently co-expressed αE. Absolute alveolar CD8+αE+ cell numbers/ml were up to 30-fold increased in HP patients. Proportions of αE-bearing CD4+ cells in BALF were significantly elevated in IPF (74.0 ± 2.7%) and HP (70.0 ± 2.4%) compared with normals (30.0 ± 1.8%) (mean ± s.e.m.; P < 0.01). In sarcoidosis, the αE expression on BALF CD4+ cells displayed subgroup dependency: proportions significantly lower than normal were noted in chest radiographic stage I (14.3 ± 1.5%), but increased proportions in stages II (50.0 ± 3.8%) and III (64.0 ± 4.8%). Correlations between common markers of T cell activation or BALF transforming growth factor-beta (TGF-β) bioactivity and αE expression were not noted. We conclude that the vast majority of alveolar CD8+ T cells consistently express αEβ7 and that distinct patterns of αEβ7 expression on alveolar CD4+ lymphocytes in sarcoidosis are related to the diverse manifestations of the sarcoid inflammatory process in the lung.
Keywords: αEβ7 integrin, bronchoalveolar lavage, T lymphocytes, interstitial lung disease
INTRODUCTION
The integrin β7-chain associates with two α-chains, α4 (CD49d) and αE (CD103), to form a heterodimer involved in cell adhesion [1]. The expression of both members of the β7 family is mainly restricted to lymphocyte subsets: the α4β7 heterodimer is expressed on circulating T cells and mediates adhesion to a mucosal structure called MadCAM-1 which is likely to be important during lymphocyte homing to Peyer's patches [2]; in contrast, αEβ7 is found on the surface of intraepithelial and lamina propria lymphocytes in mucosal tissues with < 2% frequency on circulating peripheral blood lymphocytes [3,4]. The expression of the site-specific αEβ7 integrin, first described as human mucosal antigen-1 (HML-1), on T cells within or adjacent to the epithelium of the gut [5,6] has been related to the compartmentalization and retention of immune reactive cells to the gut mucosa [7,8]. Recently, E-cadherin expressed on the basolateral surface of epithelial cells has been identified as one counter-receptor for αEβ7 [9,10]. In addition, human αEβ7 and its rat and mouse homologues are expressed on intraepithelial lymphocytes in mucosal areas of the urogenital tract and the lung [11,12]. In particular, a significant proportion of lymphocytes, predominantly of the CD8+ subset, derived from the alveolar space of normal individuals was found to express the αEβ7 integrin [13]. In interstitial lung disease (ILD) selected T cell subsets are known to accumulate in the alveolar space associated with profound changes of alveolar cytokines [14] such as transforming growth factor-beta (TGF-β), which has been related to both αEβ7 induction on T cells [4] and progressive lung fibrosis [15,16]. We hypothesized that T cells accounting for the lymphocytic alveolitis in patients with ILD display distinct disease-associated αEβ7 expression patterns potentially related to alveolar TGF-β levels. Therefore a representative panel of patients with idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP) and sarcoidosis was evaluated for the expression of αEβ7 on T cells in paired samples of peripheral blood and lung epithelial lining fluid and for alveolar levels of TGF-β bioactivity.
PATIENTS AND METHODS
Study population
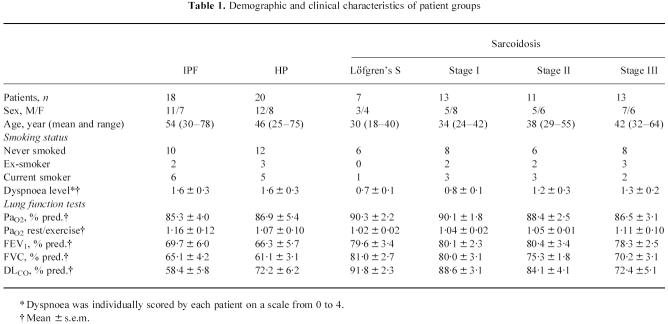
Eighty-two spontaneously breathing patients (age 18–78 years) with ILD, non-treated at the time of initial lavage, were recruited into the study over a period of 3 years. The patients were compared with 15 healthy control subjects (age 18–55 years; six female, nine male, 10 non-smokers, two ex-smokers, three current smokers) without a history of cardiac or pulmonary disease, normal chest radiographs and pulmonary function testing. The study protocol was approved by the local ethics committee and informed consent was obtained from each subject before entry into the study. Classification into IPF, HP and sarcoidosis of different chest radiographic stages was performed according to the following criteria: IPF (n = 18): unknown aetiology; restrictive pattern in pulmonary function in combination with reduced pulmonary-diffusing capacity for carbon monoxide (DLCO); typical findings in chest x-ray or computed tomography (CT/HRCT); increased neutrophil counts in bronchoalveolar lavage fluid (BALF), which may be accompanied by an elevated percentage of lymphocytes or eosinophils; interstitial pneumonitis with interstitial fibrosis with no evidence of granulomas, vasculitis, or inorganic material in lung biopsy; HP (n = 20): proof of an inhalative antigen (history of periodically recurring or permanent exposure-dependent complaints) in combination with the detection of precipitating antibodies; patchy interstitial infiltration of both lungs in chest x-ray or CT/HRCT; elevated BAL lymphocyte counts with mostly decreased CD4/CD8 ratio; typical histological finding in lung biopsy; sarcoidosis (n = 44): abnormal chest radiograph (bilateral hilar adenopathy, without (stage I, n = 20) or with (stage II, n = 11) pulmonary infiltrates such as coarse reticulonodules or fluffy cotton wool confluent shadows; infiltrates without hilar adenopathy (stage III, n = 13)); elevated BAL lymphocyte counts with mostly increased CD4/CD8 ratio; typical non-caseating epithelioid cell granuloma in transbronchial lung biopsy; stage I patients with Löfgren's syndrome (n = 7) were regarded as a separate subgroup. Characteristics of all study populations are outlined in Table 1.
Table 1.
Demographic and clinical characteristics of patient groups
Bronchoalveolar lavage
BAL was performed in spontaneously breathing subjects during routine flexible bronchoscopy as previously described [17]. The distal end of the bronchoscope was wedged into a segment bronchus of the right middle lobe or the lingula. Ten 20-ml portions of sterile 0.9% saline were instilled, aspirated, cooled to 4°C and pooled. The overall fluid recovery ranged between 55% and 70%. Lavage fluids were filtered through sterile gauze, immediately placed on ice and cells were separated by centrifugation at 200 g for 10 min at 4°C. The aliquoted supernatant was subsequently frozen and kept at −80°C until further use. Cells were counted with a haemocytometer, viability was assessed by trypan blue exclusion (0.5% w/v in PBS; Sigma, St Louis, MO), and differential cell counting was performed in Pappenheim-stained cytocentrifuge preparations (May–Grünwald and Giemsa solution; Merck, Darmstadt, Germany). In parallel to the BAL procedure, mononuclear cells were obtained from heparinized samples of peripheral venous blood on a Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) density gradient as described by Böyum [18].
Flow cytometry
The phenotype of lung and blood lymphocytes was determined by immunofluorescence and flow cytometry as previously described [17] using the FITC- or PE-conjugated mouse MoAbs b-ly7 (CD103) (Biermann, Bad Nauheim, Germany), FIB504 (integrin β7) (Pharmingen, San Diego, CA), Leu-3a (CD4), Leu-2a (CD8α), anti-HLe-1 (CD45), Leu-M3 (CD14), L243 (MHCII/DR), 2A3 (CD25), Leu-23 (CD69) (all purchased from Becton Dickinson, San Jose, CA), AC2 (CD39), 84H10 (ICAM-1, CD54), IgG1, IgG2a and IgG2b isotype controls (all from Dianova, Hamburg, Germany), Tue169 (MHCII/DQ) (Pharmingen). Mononuclear peripheral blood or BALF cells (2 × 105) were incubated in the presence of saturating amounts of fluorochrome-labelled MoAbs in flexible round-bottomed microtitre plates (Dynatech, Nuertingen, Germany) on ice for 30 min with occasional shaking. After two washes with PBS containing 0.1% bovine serum albumin (BSA; Behring, Marburg, Germany) and 0.02% NaN3 (PBS–BSA–NaN3), cells were analysed on a FACScan flow cytometer (Becton Dickinson). Daily calibration of fluorescence intensity was performed with standardized calibration beads and AutoCOMP software (Becton Dickinson). Lymphocytes were gated by dual light scatter characteristics with gate checking using CD45–FITC/CD14–PE (Leukogate; Becton Dickinson). Fluorescence distribution properties (using 4-decade logarithmic amplifiers) were recorded for 104 events, collected in list mode data files on FACScan research software and further analysed using the PC-Lysys research software (Becton Dickinson). Quadrant plots of fluorescence 1 versus fluorescence 2 were used to determine numbers of αE-bearing cells as percentages of CD4+ and CD8+ lymphocytes. Corresponding absolute numbers/ml of the respective T cell subsets in BALF were calculated from the total BALF leucocyte count per microlitre and the differential counts as described for peripheral blood [19].
Assay for TGF-β
To assess biologic activity of TGF-β in BALF, we used an assay based on the growth-inhibition of mink-lung epithelial cells (Mv 1 Lu, CCL-64; American Type Culture Collection, Rockville, MD) [20]. Briefly, subconfluent CCL-64 cells were trypsinized and plated at 1 × 104 cells in 0.1 ml/well in 96-well plates (Nunc, Nuertingen, Germany). Aliquots of BALF samples that had been acidified and subsequently neutralized were applied with or without addition of a polyclonal anti-TGF-β antibody (Biermann Ltd, Bad Nauheim, Germany). To quantify the percentage of TGF-β released in its active form, TGF-β activity was measured without preceding acidification in an additional series. After 22 h, inhibition of proliferation of CCL-64 cells was measured by MTT assay as described previously [21]. A standard curve of human recombinant TGF-β (R&D Systems, Minneapolis, MN) was included in each assay.
Statistical analysis
Data are expressed as mean ± s.e.m., median values are additionally displayed. Statistical evaluation of differences between healthy controls and patient groups was performed by applying the Kruskal–Wallis H-test and the Wilcoxon–Mann–Whitney test. For interpretation of multiple comparisons, the Bonferroni correction was considered. P < 0.05 was considered significant.
RESULTS
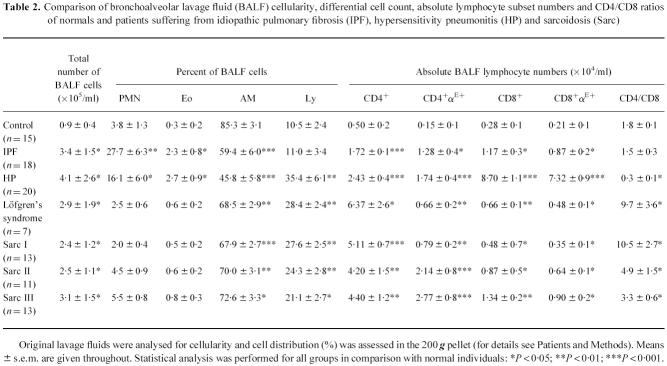
While the αE surface molecule, which was always found to be co-expressed with β7 in dual-colour staining experiments, was detectable on < 2% of peripheral blood T cells in both normal individuals and all patient groups (data not shown), distinct αE expression patterns could be identified on BALF CD4+ and CD8+ lymphocytes shown as representative cytograms for controls, IPF, HP and sarcoidosis patients in Fig. 1. The vast majority of CD8+ lymphocytes was stained by anti-αE antibodies without significant differences in mean and median CD8+αE+ cell proportions between normals (n = 15) and patients with IPF (n = 18), HP (n = 20), or sarcoidosis (n = 44) (Fig. 2a). Absolute numbers/ml of CD8+αE+ cells, however, were increased in all patient groups in comparison with healthy controls, most pronounced in HP with a > 30-fold alveolar accumulation of this subset (Table 2). In contrast to CD8+ cells, the proportion of alveolar CD4+ cells co-expressing αE showed significant differences between the various patient groups and controls (Fig. 2a). Thus, in IPF and HP the mean and median percentages of αE-bearing CD4+ cells were significantly higher than the levels found in normal volunteers (median values 74.5% (range 55–95%) and 67.1% (range 54.5–86.2%) versus 28.0% (range 20.8–26.5%), respectively; P < 0.01). In the mixed sarcoidosis group, including patients in all radiographic stages, the mean and median percentages of αE-expressing CD4+ BALF lymphocytes did not significantly differ from normal values. Subgroup analysis did, however, show that the frequency of αE expression on the expanded CD4+ BALF lymphocyte subset varied to a great extent. Lower frequencies of αE than normal were noted in sarcoidosis I with or without Löfgren's syndrome (median values 9.9% (range 6.3–19.7%) and 16.0% (range 5.9–27.0%), respectively), and significantly elevated frequencies compared with normals were observed in sarcoidosis II (50.0% (range 29–74%)) and in sarcoidosis III (64% (range 36.2–94%)) (Fig. 2b). When BALF T cell subsets of all study groups were examined for the expression of surface molecules described as early and late T cell activation markers like HLA-DR, HLA-DQ, CD25, CD39, CD54, and CD69, we did not detect a significant correlation of αE integrin expression to any of the other T cell activation markers analysed (data not shown).
Fig. 1.
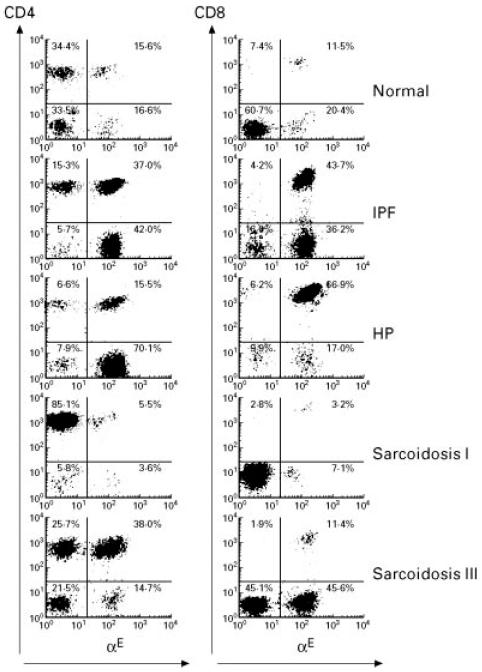
Representative flow cytometric profiles of αE expression on CD4+ and CD8+ bronchoalveolar lavage fluid (BALF) lymphocytes from normal individuals, idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP), sarcoidosis chest radiographic stages I and III. FACS-generated dot plot projections showing fluorescence intensity for gated BALF lymphocytes dual labelled by FITC-conjugated anti-αE (b-ly7) (abscissa, log scale) and PE-conjugated anti-CD4 (Leu-3a) or anti-CD8 (Leu-2a) antibodies (ordinate, log scale).
Fig. 2.
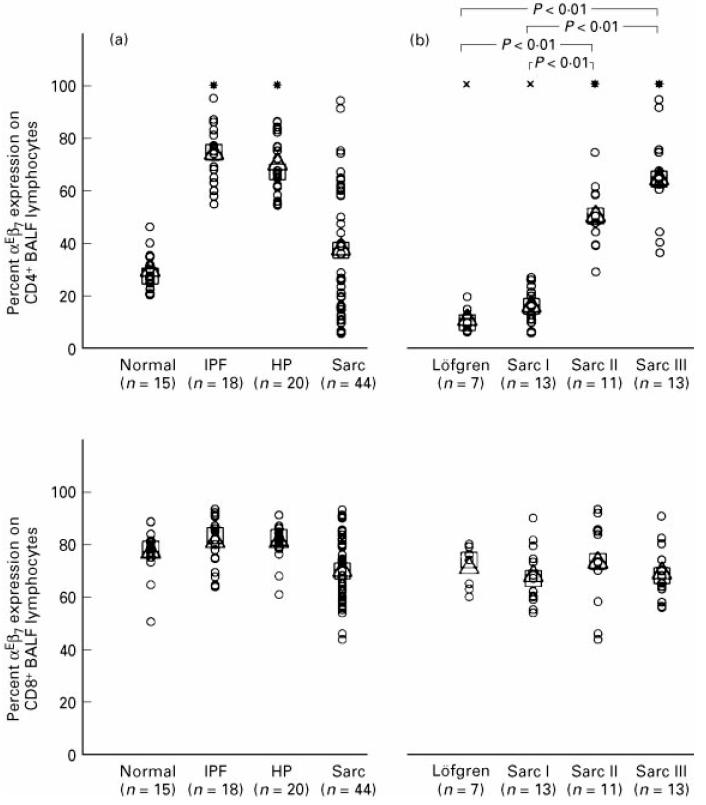
Proportion of αE-expressing CD4+ (upper part) and CD8+ (lower part) T lymphocytes in bronchoalveolar lavage fluid (BALF) determined by dual-colour immunofluorescence staining and flow cytometry. (a) Comparison of normal individuals and patients suffering form idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP), and sarcoidosis (Sarc). (b) Comparison of sarcoidosis patients with different radiographic stages (radiographic stages I–III, Löfgren's syndrome excluded from stage I as a separate subgroup). Results are expressed as percentage αE-expressing cells within the CD4+ and CD8+ T cell subset. Single values (○), mean (Δ) and median values (□) are given. Significantly increased (*) or decreased (×) mean and median values compared with normal individuals; P < 0.01.
Table 2.
Comparison of bronchoalveolar lavage fluid (BALF) cellularity, differential cell count, absolute lymphocyte subset numbers and CD4/CD8 ratios of normals and patients suffering from idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP) and sarcoidosis (Sarc)
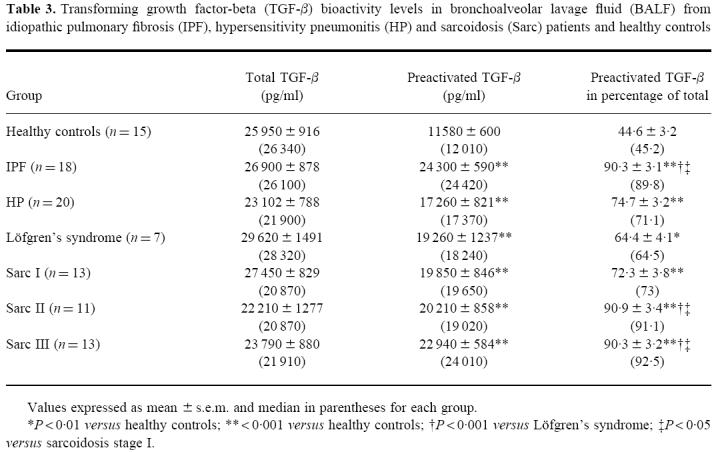
Since TGF-β is known to be a potent inducer of αE on peripheral blood T cells in vitro, TGF-β bioactivities in BALF of all study groups were determined by bioassay (Table 3). Mean and median values of total TGF-β bioactivity were not significantly different between normals and patient groups, arguing against a relationship of total BALF TGF-β bioactivity levels to the induction of αE on alveolar CD4+ cells. In contrast, mean and median proportions of in vivo activated TGF-β in relation to total TGF-β induced by in vitro acidification were significantly elevated in all patient groups compared with normals (median value 45.2% (range 33.7–65.2%)), and moreover to a significantly higher extent in IPF (median value 89.8% (range 70.2–100%)) as well as sarcoidosis stage II (median value 91.1% (range 63.2–100%)) and III (median value 92.5% (range 81.1–100%)) when compared with Löfgrens's syndrome (median value 64.5% (range 50.5–84.8%)) or sarcoidosis stage I (median value 73% (range 60.4–98.6%)). However, correlations between amount or proportion of activated TGF-β bioactivity and proportions or absolute numbers of αE-expressing lymphocyte subsets within groups did not reach statistical significance (data not shown).
Table 3.
Transforming growth factor-beta (TGF-β) bioactivity levels in bronchoalveolar lavage fluid (BALF) from idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP) and sarcoidosis (Sarc) patients and healthy controls
DISCUSSION
Evaluation of αE integrin expression on BALF-derived T lymphocytes from normal individuals and from patients with ILD revealed: (i) αE expression on a high and remarkably constant proportion of alveolar CD8+ cells in both normal individuals and all patient groups, but with an up to 30-fold increased alveolar accumulation of CD8+αE+ cells in HP patients; (ii) statistically significant differences in the proportion of αE-bearing CD4+ cells, with values significantly lower in sarcoidosis radiographic stage I including Löfgren's syndrome compared with controls and values significantly elevated in HP, IPF and sarcoidosis radiographic stage II/III; (iii) strictly compartmentalized αE+ expression without detectable re-entry of αE+ cells from the alveolar space to the circulating lymphocyte pool despite several-fold alveolar expansion of αE-expressing T cells in patients with ILD.
A narrow and highly specialized pattern of αE expression, being restricted largely to T cells, particularly CD8+ cells, within or immediately adjacent to mucosal epithelia, has been observed both in mouse and man [5,12], suggesting local induction of αE expression by factors of the mucosal microenvironment. This concept is further supported by data indicating a role of αE not in lymphocyte emigration [22] but in tissue-specific retention of lymphocytes in distinct mucosal compartments [7,8], probably being operated by E-cadherin which is expressed basolaterally on epithelial cells as one ligand [9,10].
Concerning the αE expression on BALF T cell subsets, the mechanisms responsible for its induction, its functional role and further potential ligands in the alveolar space remain to be defined. Our data indicate that special requirements for the induction of the αE integrin chain on T cell subsets seem to be mandatory, since we detected distinct disease-associated patterns on CD4+ T cells. Moreover, we did not find a correlation between early or late T cell activation markers such as HLA-DR, HLA-DQ, CD25, CD39, CD54, or CD69 and αE expression on peripheral blood or BALF T cells, confirming previous reports [23,24]. Since the vast majority of CD8+ BALF T cells co-express αE even in healthy controls, constitutive factors in the pulmonary microenvironment may be sufficient to induce αE on the majority of CD8+ cells. This observation is also true for the heavily expanded CD8+ T lymphocyte pool residing in the alveolar space in HP patients.
In contrast, the induction mechanisms of αE on CD4+ cells are apparently different and dependent on additional stimuli obviously present in HP, IPF and sarcoidosis II/III but not in normals or sarcoidosis stage I. TGF-β has been shown to play a key role in the induction of αEin vitro [4,22,25] and it has been suggested that a similar inductive effect operates in vivo in the mucosal microenvironment. Moreover, there is increasing evidence from experimental models and clinical settings to suggest that TGF-β also plays an important role in the pathogenesis of lung fibrosis [15,16]. When evaluated by bioassay in the present study, total TGF-β levels in BALF were found highest in normals and patients with Löfgren's syndrome, confirming data obtained by Yamauchi et al. [26] for healthy individuals and by Zissel et al. [27], who demonstrated an increased in vitro TGF-β production of alveolar macrophages from sarcoidosis patients with self-limiting disease. Although levels of TGF-β bioactivity in BALF detectable without activation by acidic treatment in vitro were found elevated in BALF derived from IPF, HP and sarcoidosis II/III, correlations between proportion or absolute counts of αE-expressing BALF lymphocytes and levels of preactivated BALF TGF-β within patient groups did not reach significance. This does not totally exclude a role of TGF-β in the induction of αE on BALF CD4+ T cells, since BALF levels of TGF-β may not properly reflect microenvironmental TGF-β bioactivity in the lung. TGF-β1 has been shown to be present in high amounts in alveolar epithelial cells in advanced pulmonary fibrosis [15]. However, there may be a role for additional factors with αE induction potency on CD4+ cells in the lung. Rihs et al. [24] have recently shown that T cell activation by anti-CD3 cross-linking in the presence of TGF-β is sufficient to induce αE on the majority of CD8+ peripheral blood T lymphocytes in vitro, whereas additional antibody-induced cross-linking of α4β1 integrin was necessary for αE induction on the majority of CD4+ cells. The relevance of this in vitro finding for the variety of β1-integrin–ligand interactions supposed to occur in vivo during lymphocyte transmigration remains to be evaluated.
In the present study the percentage of αE co-expression on CD4+ cells was able to discriminate not only between normal individuals and patients with ILD but, more importantly, among sarcoidosis patients presenting with hilar adenopathy alone (i.e. radiographic type I) and patients with pulmonary infiltrates (i.e. radiographic types II and III). Although this conventional x-ray categorization of sarcoidosis neither represents consecutive stages of the disease nor reflects the inflammatory process within the lower respiratory tract, it is still related to the extent of tissue damage in the lung parenchyma and, albeit indirectly, to the extent of pulmonary fibrosis [28]. This is reflected by significantly reduced DLCO values in patients with type III sarcoidosis in the present study. Since patients with type I x-rays, in particular patients with Löfgren's syndrome, tend to have acute or subacute, reversible forms of disease, while those with type II and III more often have chronic progressive manifestations, a prognostic significance of the amount of αE co-expression on CD4+ BALF cells may be assumed. Whether microenvironmental changes associated with the development of lung fibrosis play a role in the induction of αE on alveolar CD4+ cells in sarcoidosis, or whether alveolar αE+ T cells themselves are involved in the fibrotic process, remains to be elucidated.
In conclusion, the data presented lend further support for a role of αE integrin not only on mucosal intraepithelial and lamina propria lymphocytes but also on T cells in the alveolar space. The observed differences of αE co-expression on CD4+ cells between patient groups may reflect variations of the alveolar microenvironment cytokine profile or differences in dynamics and turnover of lymphocytes in ILD-associated alveolitis, which are complex and incompletely understood. In addition, the striking differences of αE co-expression on CD4+ T cells in sarcoidosis patients with different radiographic stages suggest that analysis of this integrin might be helpful to discriminate between acute self-limiting forms of alveolitis and chronic manifestations with the tendency towards fibrosis, and warrants further studies concerning the prognostic value of αE expression on BALF T cell subsets in sarcoidosis.
Acknowledgments
We thank Dr R. Snipes for proof reading the manuscript and G. Wahler for excellent technical assistance. Supported by the Deutsche Forschungsgemeinschaft (Klinische Forschergruppe ‘Respiratorische Insuffizienz’). The manuscript includes parts of the thesis of J.F.
REFERENCES
- 1.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Berlin C, Berg EL, Briskin MJ, et al. α4β7 integrin mediates lymphocyte homing to the mucosal vascular addressin MadCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 3.Cerf-Bensussan N, Begue B, Gagnon J, Meo T. The human intraepithelial lymphocyte marker HML-1 is an integrin consisting of a β7 subunit associated with a distinctive α chain. Eur J Immunol. 1992;22:273–7. doi: 10.1002/eji.1830220140. [DOI] [PubMed] [Google Scholar]
- 4.Parker CM, Cepek KL, Russell GJ, Shaw SK, Posnett DN, Schwarting R, Brenner MB. A family of β7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci USA. 1992;89:1924–8. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–85. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 6.Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–7. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- 7.Roberts K, Kilshaw PJ. The mucosal T cell integrin αM290β7 recognizes a ligand on mucosal epithelial cell lines. Eur J Immunol. 1993;23:1630–5. doi: 10.1002/eji.1830230735. [DOI] [PubMed] [Google Scholar]
- 8.Cepec KL, Parker CM, Madara JL, Brenner MB. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–70. [PubMed] [Google Scholar]
- 9.Cepec KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 10.Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin αM290β7 (αEβ7) Eur J Immunol. 1995;24:852–6. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 11.Pudney J, Anderson DJ. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–65. [PMC free article] [PubMed] [Google Scholar]
- 12.Kilshaw PJ, Baker KC. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Letters. 1988;18:149–54. doi: 10.1016/0165-2478(88)90056-9. [DOI] [PubMed] [Google Scholar]
- 13.Erle DJ, Brown T, Christian D, Aris R. Lung epithelial lining fluid T cell subsets defined by distinct patterns of β7 and β1 integrin expression. Am J Respir Cell Mol Biol. 1994;10:237–44. doi: 10.1165/ajrcmb.10.3.7509610. [DOI] [PubMed] [Google Scholar]
- 14.Agostini C, Chilosi M, Zambello R, Trentin L, Semenzato G. Pulmonary immune cells in health and disease: lymphocytes. Eur Respir J. 1993;6:1378–401. [PubMed] [Google Scholar]
- 15.Khalil N, O'Connor R, Unruh H, Warren P, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta (TGFβ) in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1990;5:155–62. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 16.Khalil N, O'Connor R, Flanders KC, Shing W, Whitman CI. Regulation of type-2 alveolar epithelial cell proliferation by TGF-β during bleomycin induced lung injury in rats. Am J Physiol. 1994;267:L498–L507. doi: 10.1152/ajplung.1994.267.5.L498. [DOI] [PubMed] [Google Scholar]
- 17.Lohmeyer J, Friedrich J, Rosseau S, Pralle H, Seeger W. Improved multiparameter flow cytometric analysis of bronchoalveolar lavage derived inflammatory cells. J Immunol Methods. 1994;172:59–70. doi: 10.1016/0022-1759(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 18.Böyum A. Separation of leucocytes from blood and bone marrow. Scand J Clin Lab Invest. 1968;21(Suppl. 97):77–89. [PubMed] [Google Scholar]
- 19.Nakata K, Sugie T, Nakano H, Sakai T, Aoki M. Gamma-delta T cells in sarcoidosis: correlation with clinical features. Am J Respir Crit Care Med. 1994;149:981–8. doi: 10.1164/ajrccm.149.4.8143064. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda T, Lioubin MN, Marquardt H. Human transforming growth factor type beta 2: production by a prostatic adenocarcinoma cell line, purification, and initial characterization. Biochem. 1987;26:2406–10. doi: 10.1021/bi00383a002. [DOI] [PubMed] [Google Scholar]
- 21.Schütte H, Lohmeyer J, Rosseau S, et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J. 1996;9:1858–67. doi: 10.1183/09031936.96.09091858. [DOI] [PubMed] [Google Scholar]
- 22.Austrup F, Rebstock S, Kilshaw PJ, Hamann A. Transforming growth factor-beta 1-induced expression of the mucosa-related integrin alpha E on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25:1487–91. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 23.Picker LJ, Martin RJ, Trumble A, Newman LS, Collins PA, Bergstresser PR, Leung DYM. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur J Immunol. 1994;24:1269–77. doi: 10.1002/eji.1830240605. [DOI] [PubMed] [Google Scholar]
- 24.Rihs S, Walker C, Virchow JC, Boer C, Kroegel C, Giri SN, Braun RK. Differential expression of αEβ7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by α4β1-integrin crosslinking and TGFβ. Am J Respir Cell Mol Biol. 1996;15:660–10. doi: 10.1165/ajrcmb.15.5.8918367. [DOI] [PubMed] [Google Scholar]
- 25.Cerwenka A, Bevec D, Majdic O, Knapp W, Holter W. TGF-β 1 is a potent inducer of human effector T cells. J Immunol. 1994;153:4367–77. [PubMed] [Google Scholar]
- 26.Yamauchi K, Martinet Y, Basset P, Fells GA. Crystal RG. High levels of transforming growth factor-β are present in the epithelial lining fluid of the normal respiratory tract. Am Rev Respir Dis. 1988;137:1360–63. doi: 10.1164/ajrccm/137.6.1360. [DOI] [PubMed] [Google Scholar]
- 27.Zissel G, Homolka J, Schlaak J, Schlaak M, Müller-Quernheim J. Anti-inflammatory cytokine release by alveolar macrophages in pulmonary sarcoidosis. Am J Respir Crit Care Med. 1996;154:713–9. doi: 10.1164/ajrccm.154.3.8810610. [DOI] [PubMed] [Google Scholar]
- 28.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]