Abstract
We have studied the effect of the probiotic strain Lactobacillus plantarum 299v on the immune functions of gnotobiotic rats. One group of germ-free rats was colonized with the type 1-fimbriated Escherichia coli O6:K13:H1 and another group with the same E. coli strain together with L. plantarum 299v. One and 5 weeks after colonization, bacterial numbers were determined in the contents of the small intestine, caecum and mesenteric lymph nodes. Small intestinal sections were examined for CD8+, CD4+, CD25+ (IL-2R α-chain), IgA+ and MHC class II+ cells and mitogen-induced spleen cell proliferation was determined. Immunoglobulin levels and E. coli-specific antibodies were measured in serum. Rats given L. plantarum in addition to E. coli showed lower counts of E. coli in the small intestine and caecum 1 week after colonization compared with the group colonized with E. coli alone, but similar levels after 5 weeks. Rats colonized with L. plantarum+ E. coli had significantly higher total serum IgA levels and marginally higher IgM and IgA antibody levels against E. coli than those colonized with E. coli alone. They also showed a significantly increased density of CD25+ cells in the lamina propria and displayed a decreased proliferative spleen cell response after stimulation with concanavalin A or E. coli 1 week after colonization. The results indicate that L. plantarum colonization competes with E. coli for intestinal colonization and can influence intestinal and systemic immunity.
Keywords: Escherichia coli, Lactobacillus plantarum, intestinal colonization, immunomodulation
INTRODUCTION
Lactobacilli are lactic acid-producing bacteria, which are members of the normal microbial flora of the small and large intestinal tract of man and animals [1–3]. Lactobacilli are mostly non-pathogenic and produce antibacterial substances such as bacteriocins and hydrogen peroxide [4] and have therefore been presumed, since the beginning of the 20th century, to compete with pathogenic bacteria and be beneficial to human health [3].
The species Lactobacillus plantarum is a major colonizer of the human intestine [1,4] and the strain L. plantarum 299v has been shown to establish in the human intestinal tract in experimental colonization trials [4]. This strain and related strains of L. plantarum express a mannose-specific adhesin by which it can adhere to human colonic cells [4,5]. Mannose-specific adhesins are also expressed by type 1 fimbriated Escherichia coli and bind to rat, mouse, guinea pig and human intestinal epithelial cells [6–10]. It is thus possible that L. plantarum could directly interfere with E. coli colonization.
Lactobacilli have been claimed to affect the immune system, but few controlled studies have been performed. Oral administration of L. casei to mice 2 days before oral challenge with Salmonella or E. coli decreased translocation and increased IgA antibodies to these bacteria [11]. Administration of the L. rhamnosus strain GG to infants with rotavirus diarrhoea increases the anti-viral secretory IgA response [12]. In studies of methotrexate-induced entercolitis in rats, the administration of L. plantarum strain 299v reduced translocation of gut commensal bacteria, increased colonic and ileal levels of SIgA, increased the numbers of CD8+ and CD4+ cells in the lamina propria and reduced mucosal inflammation [13,14].
In the present study, the ability of L. plantarum to establish in the intestine of germ-free rats was studied, as well as if such an establishment would influence colonization with a type 1-fimbriated E. coli strain. The effect of colonization on intestinal lymphocyte populations, spleen cell reactivity and antibody responses to E. coli was investigated.
MATERIALS AND METHODS
Rats
Male and female rats of the AGUS strain were reared in isolators under germ-free conditions [15]. The sterility of the rats and isolators prior to the experiment was checked as reported before [6].
Bacterial strains and colonization protocol
Escherichia coli O6:K13:H1 (strain no. 20561 CCUG (Culture Collection University of Göteborg)) was originally isolated from the urine of a patient with cystitis. The bacteria were cultivated three times in static Luria broth containing 0.1% CaCl2 to favour the production of type 1 fimbriae followed by an overnight culture on tryptic soy agar (TSA) plates before colonization.
Lactobacillus plantarum299v was originally isolated from human intestine [16]. The bacteria were cultured on Rogosa agar plates (Difco Labs, Detroit, MI).
Both bacteria were harvested in sterile saline solution, adjusted to approx. 108/ml by optical density (OD) measurement and poured into 10-ml glass ampoules which were sterilized on the outside with chromo-sulphuric acid and transferred into the germ-free isolators. One group of seven rats received E. coli alone, another group of seven rats received both E. coli and L. plantarum. Each rat was given 1 ml of the bacterial suspension perorally with a syringe and some drops were also spread onto the fur. The numbers of viable lactobacilli and E. coli given were found to be 7.8 × 108 and 6.8 × 108, respectively, as assessed by cultivating the mixture on appropriate media.
The animals were kept within the isolators until sacrifice at 1 week (3 + 3 rats) or 5 weeks (4 + 4 rats). The rats were anaesthetized with sodium penthobarbital (60 mg/kg; Apoteksbolaget, Umeå, Sweden) and exsanguinated by heart puncture. Two age-matched non-colonized rats served as germ-free controls (one for each colonization week).
Determination of bacterial numbers in the intestinal lumen and in association with the gut wall
A sample from the caecal and small intestinal contents was obtained using a calibrated loop, serially diluted in sterile saline and spread on TSA plates (for E. coli) or Rogosa agar plates (for lactobacilli). After overnight culture, the number of colonies was counted and the results were expressed as colony-forming units (CFU)/g of gut contents.
To assess the composition of the bacterial population closely associated with the mucosal surface, pieces of large intestine were excised, rinsed and homogenized. The homogenate was diluted and cultivated on agar plates as described above.
Determination of bacterial translocation
To determine translocation, the mesenteric lymph node chain was aseptically removed and homogenized with a syringe piston through a sterile mesh sieve. The homogenate was distributed on agar plates and viable counts were performed as described for the intestinal contents. The results were expressed as the number of CFU recovered from the mesenteric lymph nodes from each rat. In order to check the sterility of the procedure, the homogenates were also cultivated on blood agar plates to detect the possible presence of contaminants.
Bacterial adhesin expression
The type 1 fimbrial expression of E. coli was assessed by determining the mannose-sensitive haemagglutination of horse erythrocytes. A (3% v/v) suspension of horse erythrocytes was made either in PBS or in PBS with 2.5% methyl-α-d-mannoside and mixed with a loopful of bacteria from a selected colony suspended in PBS. After gentle tilting of the slide for 1–5 min, haemagglutination was read by the naked eye.
The mannose-specific adhesin of L. plantarum was detected using agglutination of yeast cells (Saccharomyces cerevisiae) which contain mannan as a major component of their cell wall. Individual lactobacilli colonies were, however, too small to yield enough bacteria for agglutination. Instead, a thick suspension of an agar culture of the bacteria was tested for agglutination of yeast cells. Two-fold dilutions of bacteria, starting with 2 × 109, were mixed in 96-well U-shaped microplates (Labora, Upplands Väsby, Sweden) with an equal amount of either α-methyl-mannoside or PBS, then twice this volume of a 1% solution (w/v) of baker's yeast was added. The plate was shaken and incubated overnight at 4°C. Agglutination was recorded using a microscope (× 250). The titre was determined as the reciprocal of the highest dilution of bacteria that resulted in visible agglutination.
Antibody measurement
Serum antibody titres against E. coli were measured by ELISA [17]. Dynatech 96-well Immunolon plates (Alexandria, VA) were coated overnight at room temperature with 100 μl heat-extracted E. coli antigen (mainly lipopolysaccharide (LPS)) [18] per well, which approximately corresponds to 2 × 109 bacteria/ml. The plates were washed three times with PBS containing 0.05% Tween 20 (Merck, Darmstadt, Germany) and 100 μl of appropriately diluted rat serum (five-fold beginning with 1:10) were added in duplicates and incubated for 1–3 h. After washing in PBS–Tween, biotin-conjugated monoclonal mouse anti-rat IgA or unconjugated rabbit anti-rat IgM or rabbit anti-rat IgG (Zymed Labs, San Francisco, CA; diluted 1:2000, 1:1000 and 1:10 000, respectively) was added for 1 h, followed by alkaline phosphatase-conjugated streptavidin (Dako, Glostrup, Denmark; 1:2000) or goat anti-rabbit IgG coupled to alkaline phosphatase (Boeheringer-Mannheim, Mannheim, Germany; 1:10 000) for 1 h. After washing, the substrate p-nitrophenyl phosphate diluted in diethanolamine buffer 1 m pH 9.8 was added. The absorbance at 405 nm was determined by spectroscopy (Multiskan Bichromatic; Labsystems, Helsinki, Finland). Antibody titres were determined as the reciprocal of the dilution giving an OD of 0.2 above the background level.
Absorption of cross-reactive antibodies
To determine the specificity of the anti-E. coli antibodies, rat sera were absorbed with E. coli, or with L. plantarum and then assayed for anti-E. coli antibodies as described above. Absorption was carried out by mixing 200 μl rat serum with 10 μl of packed bacteria and incubating with end-over-end rotation at 4°C for 1 h. The bacteria were spun down and discarded, and the procedure was repeated once.
Total immunoglobulin quantification
For measurement of total IgA, IgG and IgM concentrations in serum, 96-well plates (Dynatech Immunolon) were coated with affinity-purified goat anti-rat IgA (1:750; Saxon Biochemicals, Hanover, Germany), goat anti-rat IgG (F(ab′)2 fragment-specific; Jackson, Westgrove, PA; 1 μg/ml) or rabbit anti-rat IgM (1:750; Zymed). Rat IgA or IgM (purified, κ light chain isotype; PharMingen San Diego, CA) and rat IgG (2.5 μg/ml; Zymed) were used as standards. The samples were appropriately diluted; samples and standard were added to the plates and incubated for 1–3 h at room temperature. After washing, alkaline phosphatase-conjugated goat anti-rat IgG (Fcγ fragment-specific; Jackson; 1:4000), affinity-purified alkaline phosphatase-conjugated goat anti-rat IgA (Saxon; 1:750) or monoclonal biotin conjugated mouse anti-rat IgM (Serotec, Oxford, UK; 1:1000) were added to the plates and incubated for 1 h. Alkaline phosphatase-conjugated streptavidin (Dako; 1:2000) was added to the IgM plates and incubated for 1 h. The plates were washed and the same detection system was used as described above.
Cell proliferation assay
Cell suspensions were prepared by gently pressing the rat spleen through a mesh sieve using a syringe piston. Erythrocytes were lysed with distilled water. The cells were washed twice in ice-cold Iscove's (Gibco, Grand Island, NY) medium and adjusted to 2 × 106 cells/ml in Iscove's medium supplemented with 10% fetal calf serum (FCS; Integro B.V., Zaandham, Holland), 2 mm glutamine (Gibco BRL, Life Technologies) and 50 mg of gentamycin per ml (Sigma Chemical Co., St Louis, MO). Cultures with a final volume of 200 μl/well were set up in 96-well flat-bottomed microtitre plates (Nunc, Roskilde, Denmark) and stimulated with concanavalin A (Con A) at a final concentration of 10 μg/ml (Sigma), with 5 × 107 formalin-killed E. coli/ml (Histofix; Histolab, Göteborg, Sweden) or left unstimulated (background control level) and incubated at 37°C in a humidified atmosphere supplemented with 5% CO2. Proliferation was measured on days 1, 4 and 7 by incorporation of tritium-labelled thymidine (5 μCi/ml; Amersham, Aylesbury, UK) added during 6 h. The cells were harvested onto glass filters, which were analysed in a β-counter (Matrix 96; Canberra Packard, Uppsala, Sweden).
Immunohistochemistry
Pieces of jejunum were embedded in Tissue-Tek (Miles Inc., Elkhart, IN) in vinyl biopsy moulds (Miles), introduced into isopentane (Kebo Labs, Spånga, Sweden) and frozen immediately in liquid nitrogen and kept at −70°C. Sections of 5 μm thickness were obtained using a cryostat (1720 Digital; Leitz, Germany), fixed in acetone (50% for 0.5 min and then 100% for 10 min) and left to air dry. The slides were immersed in a 37°C glucose-oxidase solution (Sigma) for 20 min to eliminate endogenous peroxidases, washed in PBS, and to further block endogenous avidin-biotin-binding activity, the tissues were treated first with avidin (30 μl/section, undiluted solution (Vector Labs, Burlingame, CA) and then with biotin (30 μl/section, undiluted solution; Vector Labs). After washing with PBS–Tween (0.05%), the slides were incubated overnight at 4°C in a humid chamber with the specific mouse anti-rat MoAb appropriately diluted in PBS–Tween with 2% bovine serum albumin (BSA). The following antibodies were used: anti-MHC class II (1:400; MCA Serotec, clone MRC Ox-17), anti-CD4 (1:400; Serotec, clone W3/25), anti-CD8 (1:600; Serotec, clone MRC Ox-8), anti-IL-2R α-chain (CD25) (1:250, Serotec, clone NDS 61), or biotinylated anti-IgA α-chain (1:250; Serotec, clone Mara-biotin, batch 3431). As negative control antibodies, monoclonal mouse anti-human IgG4 (1:600; BioZac, Järfälla, Sweden) or anti-human CD4 (1:200; Dako M716) were used. As secondary antibody, biotinylated F(ab′)2 rabbit anti-mouse IgG diluted 1:200 in PBS–Tween with 2% BSA and 1% normal rat serum was applied during 1 h at room temperature, followed by avidin-conjugated peroxidase (ABC-complex; Dako) for 1 h in the dark. The colour was developed using amino-ethyl-carbazole (AEC; Sigma) for 8 min and the slides were counterstained with haematoxylin for 3–5 min. The slides were washed and fixed with Aquamount (BDH Lab. Supplies, Poole, UK).
The slides were analysed blindly. On each slide, 10–20 villi were examined in a microscope (Leitz DMRB) connected to a computerized image-analysis system (Leica Q500 MC; Cambridge, UK). For CD8+, CD25+ and IgA+ cells the number of cells/area of tissue was determined. For CD4+ and MHC class II+ cells the stained area in percentage of the total villus area was instead assessed, since individual cells were difficult to delineate.
Statistical analysis
Statistical differences between the groups were analysed using Student's t-test. Translocation data were analysed by Mann–Whitney U-test.
RESULTS
Bacterial colonization
The first group of rats received E. coli alone, while the second group of rats received E. coli and L. plantarum. Escherichia coli established in the caecum at 109–1010 CFU/g of contents, while the numbers in the small intestine were lower and more variable (Table 1). Lactobacillus plantarum reduced the E. coli levels, both in the caecum and in the small intestine. This difference was significant 1 week after colonization, but not after 5 weeks (Table 1). Lactobacillus plantarum colonized the small intestine and the caecum; the population levels increased between 1 and 5 weeks after colonization but did not reach as high levels as E. coli (Table 1). Escherichia coli and L. plantarum were present in homogenized mucosa in proportions similar to those in the intestinal contents (data not shown).
Table 1.
Bacterial levels in the caecum and small intestinal contents
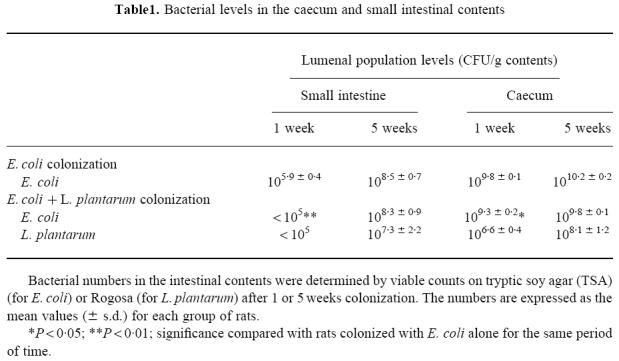
Type 1 fimbrial expression in E. coli
Twenty colonies of E. coli in the initial mixture were tested for the expression of type 1 fimbriae. All colonies expressed type 1 fimbriae as evidenced by a mannose-sensitive haemagglutination (MSHA) of horse erythrocytes. Haemagglutination was also performed on colonies obtained after culture of the intestinal contents. After 1 week's colonization, most of the E. coli still expressed type 1 fimbriae (34/37 colonies tested in the group colonized with E. coli alone and 51/56 colonies tested in the group colonized with E. coli and L. plantarum). After 5 weeks however, none of the 77 colonies tested from the rats colonized with E. coli alone and only 6/92 colonies tested from rats colonized with E. coli and L. plantarum expressed type 1 fimbriae.
Lactobacillus plantarum adhesin expression
The mannose-specific adhesin of L. plantarum was determined by agglutination of baker's yeast and the agglutination titre was recorded. The initial inoculum given to the rats agglutinated yeast cells with a titre of 32. Most of this agglutination was abolished in the presence of α-methyl mannoside. Lactobacilli obtained from the rats after 1 week of colonization showed an increased agglutination titre (107 ± 37). After 5 weeks, the yeast agglutination titre had decreased (27 ± 9) and the ability of the receptor analogue α-methyl mannoside to inhibit the agglutination was also reduced (data not shown).
Translocation
Translocation was assessed by cultivating bacteria from homogenates of the mesenteric lymph nodes. Escherichia coli translocated in all rats after 1 week of colonization. The numbers of translocating bacteria were slightly lower in the group colonized with E. coli and L. plantarum than in the group colonized with E. coli alone, but the difference was not significant (161 ± 150 versus 413 ± 221; P = 0.17). Translocation of E. coli could not be evaluated after 5 weeks, because the plates were contaminated.
Lactobacillus plantarum could not be detected in the mesenteric lymph nodes in any of the three rats evaluated after 1 week's colonization, but could be detected in two of the four rats analysed at 5 weeks (140 and 230 bacteria, respectively).
Immunoglobulin levels
The serum immunoglobulin levels of the two groups of rats are shown in Fig. 1. IgM levels were unaffected by bacterial colonization but the levels of IgG increased significantly after bacterial colonization in both groups of rats. Serum IgA concentrations increased to a significantly higher level in the rats colonized with E. coli and L. plantarum compared with the rats colonized with E. coli alone, which only displayed a slight increase (P < 0.05; Fig. 1c).
Fig. 1.
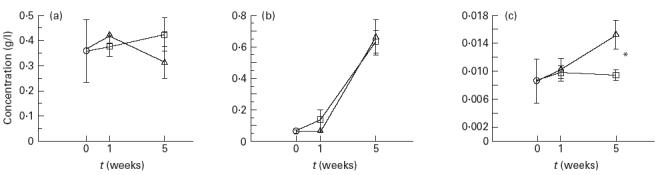
Total (a) IgM, (b) IgG and (c) IgA levels in serum of rats colonized by Escherichia coli (□) or E. coli and Lactobacillus plantarum (Δ). Two control rats were included, which were germ-free rats matched by age. Error bars represent s.e.m. ○, Control. *P < 0.05.
Antibodies against E. coli
Antibodies of the IgM isotype against E. coli heat-extracted antigen could be detected after colonization for 1 week, whereas antibodies of the IgG or IgA isotype were detectable after 5 weeks (Fig. 2). Rats colonized with E. coli and L. plantarum showed marginally higher levels of IgM and IgA anti-E. coli antibodies than rats colonized with E. coli alone, although the differences were not statistically significant (P = 0.19 and 0.13, respectively; Fig. 2a,c). The levels of IgG anti-E. coli were similar in the two groups (Fig. 2b).
Fig. 2.
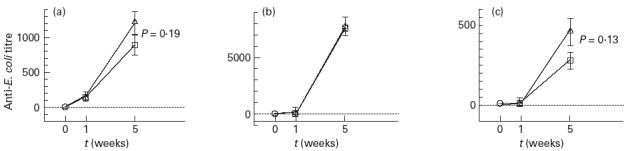
Serum antibody levels of the (a) IgM, (b) IgG and (c) IgA isotype against Escherichia coli heat-extracted antigen in rats colonized with E. coli alone (□) or with E. coli and Lactobacillus plantarum (Δ). Antibody titres were determined as the reciprocal of the dilution having an optical density of 0.2 above background level. The controls include germ-free rats matched by age. The error bars represent s.e.m. ○, Control.
Absorption of cross-reactive antibodies
Rat sera were absorbed with E. coli or L. plantarum to determine the specificity of the E. coli antibodies obtained from the rats. Cross-reactivity was found mainly for the IgG isotype, where 67 ± 13% (mean ± s.d.) of the anti-E. coli antibodies were eliminated by absorption with L. plantarum. IgM anti-E. coli antibodies, in contrast, showed little cross-reactivity with L. plantarum. Thus, 14 ± 11% of the anti-E. coli activity was absorbed with L. plantarum, compared with 85 ± 27% which was absorbed by E. coli. The IgA anti-E. coli antibodies were abolished by absorption with L. plantarum in 3/4 rats colonized with E. coli and L. plantarum. There was no activity remaining in the sera absorbed with E. coli (titre < 10 in 8/8 rats).
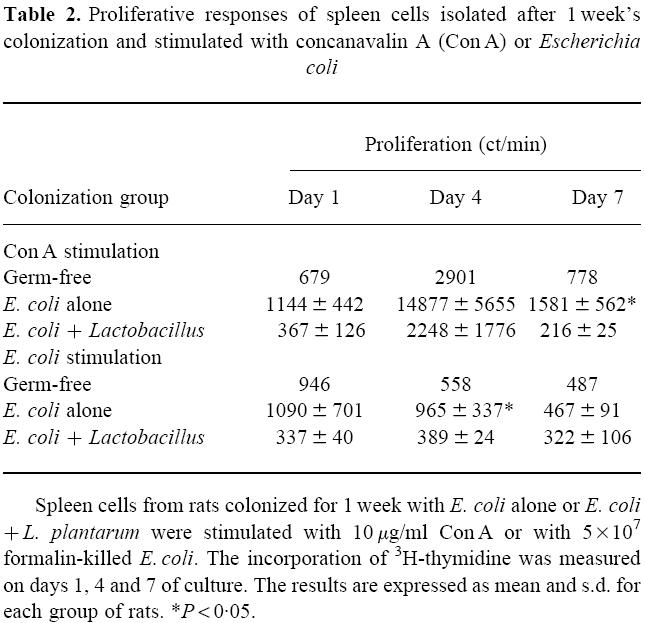
Proliferation of mononuclear cells after stimulation with Con A or E. coli
Spleen cells were stimulated with Con A or formalin-killed E. coli and proliferation was measured on days 1, 4 and 7. Spleen cells from rats colonized with E. coli and L. plantarum for 1 week showed a lower proliferative response to Con A or E. coli, compared with spleen cells from rats colonized with E. coli alone for the same period of time (Table 2).
Table 2.
Proliferative responses of spleen cells isolated after 1 week's colonization and stimulated with concanavalin A (Con A) or Escherichia coli
After 5 weeks of colonization, the proliferative response of spleen cells taken from rats colonized with E. coli alone did not differ from those colonized with E. coli and L. plantarum (data not shown).
Immunohistochemistry of jejunal villi
Immunohistochemistry was performed on small intestinal sections 1 and 5 weeks after colonization.
CD4+ cells were measured as the percentage of stained area, because it was in some cases very difficult to define individual cells. The area stained for CD4 increased with colonization time and was similar in both groups (Fig. 3a).
Fig. 3.
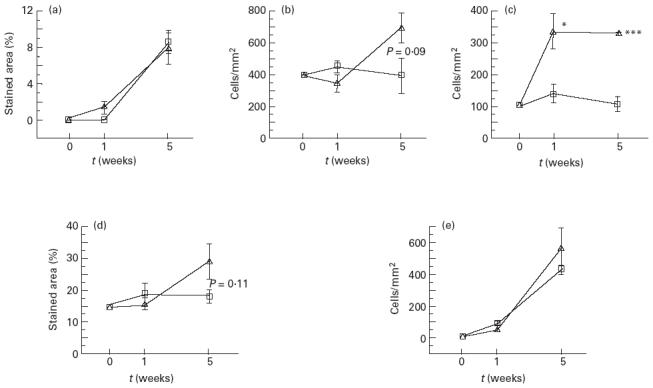
Immunohistochemistry data showing the distribution of cells expressing CD4 (a), CD8 (b), CD25 (c), MHC class II (d) or IgA (e) in jejunal villi. For CD8+, CD25+ and IgA+ cells, the results are expressed as the number of cells/mm2, while for CD4+ and MHC class II+ cells the stained area in percentage of total area is presented. Ten to 20 villi were analysed per sample. Error bars represent s.e.m. □, Escherichia coli; Δ, E. coli+L. plantarum. *P < 0.05; ***P < 0.001.
The density of CD8+ cells increased more in the rats colonized with E. coli and L. plantarum than in the rats colonized with E. coli alone, although the difference was not statistically significant (Fig. 3b). The CD8+ cells were located not only between epithelial cells, but also in the lamina propria of the villus core (data not shown).
The number of cells stained for CD25 (IL-2R α-chain) increased much more in the group colonized with E. coli and L. plantarum compared with the group colonized with E. coli alone, and the difference between the groups was significant at both time points (Fig. 3c). The CD25 cells were localized in the lamina propria of the villus core.
The area stained for MHC class II remained constant in the rats colonized with E. coli alone, while it increased in the group colonized with E. coli+ L. plantarum, although the difference between the groups was not significant (Fig. 3d).
The number of IgA+ cells increased strongly with time in both groups. No significant differences were obtained between both groups (Fig. 3e).
DISCUSSION
Lactobacilli are candidates for probiotic bacteria. One mechanism by which probiotic bacteria exert their effects is by competing with potentially pathogenic bacteria for ecological niches, thereby preventing their colonization. The data from the present study indicate that the mannose-binding L. plantarum strain 299v could establish in the rat small and large intestine and affect the colonization of the germ-free rats by a type 1-fimbriated E. coli. A reduction of E. coli colonization was especially clearly seen in the small intestine, which is a less suited niche for E. coli than the caecum and large intestine.
It is possible that the presence of the mannose-dependent adherence mechanism in L. plantarum was partly responsible for the competition with E. coli early after colonization. Another Lactobacillus species, L. acidophilus, used in a similar colonization experiment, did not reduce intestinal E. coli population levels, although it colonized at higher levels than L. plantarum 299v [6]. The L. acidophilus strain adhered to rat colonic cells, but not via a mannose-dependent mechanism [19].
The L. plantarum mannose-binding adhesin was expressed in vivo, especially in the early phase of colonization. At 5 weeks, the adhesin expression had decreased and the binding of the adhesin was not inhibited by α-methyl-mannoside, which implies a changed specificity of the adhesin. Similarly, the mannose-binding capacity of E. coli also decreased with increased colonization time, which has previously been noted with other type 1-fimbriated E. coli strains colonizing gnotobiotic rats [6,20]. Once the bacteria have stably colonized, expression of the mannose-specific adhesins may not be important for survival in the intestinal milieu.
The administration of lactobacilli has been shown to increase antibody responses to gut pathogens [11,12]. Accordingly, the antibody response of the IgM and IgA isotype against E. coli LPS was somewhat higher in the rats colonized with L. plantarum in addition to E. coli, compared with those colonized with E. coli alone. The IgA and IgG anti-E. coli antibodies seemed to cross-react with L. plantarum structures, since they could to a large extent be absorbed with L. plantarum antigen. As shown before [19], lactobacilli share antigenic determinants with E. coli. Cross-reactive antibodies may be responsible for the increased anti-rotavirus response seen after administration of L. rhamnosus [21].
The levels of serum IgM were unaffected by colonization, while serum IgG increased similarly in both groups. It is well documented that serum IgG is very low in germ-free animals and increases upon colonization with intestinal bacteria [22]. Five weeks after colonization an increased level of total serum IgA was noted in the rats given L. plantarum in addition to E. coli. Serum IgA has been shown to be anti-inflammatogenic by modulating the release of inflammatory mediators such as tumour necrosis factor-alpha (TNF-α), IL-6 and reactive oxygen burst in neutrophils and macrophages [23,24].
An important feature of certain normal intestinal bacteria is that they might modulate the handling of food antigens by the gut immune system, increasing the capacity to develop tolerance. Mice devoid of a normal intestinal microbiota develop oral tolerance to certain antigens like ovalbumin, but the suppression is weaker and lasts a shorter time [25,26]. The rats colonized with L. plantarum in addition to E. coli exhibited a marked and persistent increase in cells expressing the IL-2 receptor α-chain (CD25) in the lamina propria, presumably representing activated T cells. It has been suggested that CD25+ cells in the intestine play a role in the regulatory maintenance of homeostasis in the intestinal immune system [27]. Thus, CD25+ cells were found in the intestinal lamina propria of rats made tolerant to ovalbumin, but not in non-tolerized rats upon reintroduction of ovalbumin [28]. CD25+ cells have also been shown to be involved in tolerance or down-regulation of the immune response to self antigens and non-self antigens [29], and an absence of IL-2R α-chain is associated with autoimmune disorders in adult mice [30]. The increased level of CD25+ cells with potential regulatory function in the lamina propria of rats colonized with L. plantarum in addition to E. coli could be in part responsible for the reduced proliferative response of spleen cells in these rats by the secretion of anti-proliferative cytokines such as IL-10 or transforming growth factor-beta (TGF-β).
There was also an increase of MHC class II expression 5 weeks after colonization in the L. plantarum and E. coli colonized rats compared with rats colonized with E. coli alone. Such an up-regulation of MHC class II expression may be caused by interferon-gamma (IFN-γ) produced by activated T cells. In this context, it is interesting to note that the expression of MHC class II may be important for development of oral tolerance, since the capacity to generate active oral tolerance is temporally related to the expression of MHC class II on the epithelium [27,31–34].
Probiotic bacteria may influence the gut and systemic immune system after translocation of whole bacteria or bacterial products over the epithelium. Alternatively, or in addition, a close interaction with intestinal epithelial cells may trigger nervous or cytokine signals with the capacity to influence the gut immune system. Lactobacilli able to colonize the small intestine seem to be able to regulate intestinal immunity. Their capacity to improve oral tolerance to innocuous environmental antigens remains to be determined. The potential of down-regulating inflammatory conditions in the mucosa, e.g. in inflammatory bowel disease, may also be considered.
Acknowledgments
The efficient technical help of Eva Ågren is very much appreciated. The study was supported by the Swedish Council for Forestry and Agricultural Research.
REFERENCES
- 1.Johansson M-L. Lund, Sweden: Laboratory of Food Technology, Chemical Centre, Lund University; 1995. Systematics and starter culture selection of Lactobacillus for human intestine and Nigerian ogi, with special reference to Lactobacillus plantarum. PhD Thesis. [Google Scholar]
- 2.Finegold SM, Attebery HR, Sutter VL. Effect of diet on human faecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27:1546–69. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]
- 3.Kandler O, Weiss N. Regular, non-sporing, Gram positive rods. Bergey's Manual Syst Bacteriol. 1986;2:1208–34. [Google Scholar]
- 4.Johansson M-L, Molin G, Jeppsson B, et al. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environ Microbiol. 1993;59:15–20. doi: 10.1128/aem.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adlerberth I, Ahrné S, Johansson M-L, et al. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Env Microbiol. 1996;62:2244–51. doi: 10.1128/aem.62.7.2244-2251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herías MV, Midtvedt T, Hanson LÅ, et al. Role of Escherichia coli P fimbriae in intestinal colonization in gnotobiotic rats. Infect Immun. 1995;63:4781–9.. doi: 10.1128/iai.63.12.4781-4789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadolkowski EA, Laux DC, Cohen PS. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of adhesion to mucosal receptors. Infect Immun. 1988;56:1036–43. doi: 10.1128/iai.56.5.1036-1043.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duguid JP. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959;21:271–86. doi: 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- 9.Adlerberth I, Hanson LÅ, Svanborg C, et al. Adhesins of Escherichia coli associated with extra-intestinal pathogenicity confer binding to colonic epithelial cells. Microb Pathogen. 1995;18:373–85. doi: 10.1006/mpat.1995.0034. [DOI] [PubMed] [Google Scholar]
- 10.Wold AE, Thorssén M, Hull S, et al. Attachment of Escherichia coli via mannose- or Galα1–4Galβ-containing receptors to human colonic epithelial cells. Infect Immun. 1988;56:2531–7. doi: 10.1128/iai.56.10.2531-2537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perdigón G, Alvarez S, Pesce de Ruiz Holgado A. Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on the secretory immune response and protective capacity in intestinal infections. J Dairy Res. 1991;58:485–96. doi: 10.1017/s0022029900030090. [DOI] [PubMed] [Google Scholar]
- 12.Kaila M, Isolauri E, Soppi E, et al. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–4.. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mao Y, Nobaek S, Kasravi B, et al. The effects of Lactobacillus strains, and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterol. 1996;111:334–44. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Ljungh Å, Molin G, Jeppeson B. Intestinal immune response to oral administration of Lactobacillus reuteri R2LC, Lactobacillus–plantarum DSM 9843, pectin and oatbase on methotrexate-induced enterocolitis in rats. Microb Ecol Health Dis. 1996;9:261–70. [Google Scholar]
- 15.Gustafsson BE. Light weight stainless steel systems for rearing germfree animals. Ann N Y Acad Sci. 1959;78:17–28. doi: 10.1111/j.1749-6632.1959.tb53092.x. [DOI] [PubMed] [Google Scholar]
- 16.Molin G, Jeppsson B, Johansson M-L, et al. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J Appl Bacteriol. 1993;74:314–23. doi: 10.1111/j.1365-2672.1993.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 17.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, ELISA. III. Quantitation of specific antibodies by enzyme-labeled anti- immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–35. [PubMed] [Google Scholar]
- 18.Holmgren J. Studies of methods for quantitation of agglutinins and precipitins to Escherichia coli O and K antigens. Int Arch Allergy. 1970;37:380–94. doi: 10.1159/000230238. [DOI] [PubMed] [Google Scholar]
- 19.Herías MV, Midtvedt T, Hanson LÅ, et al. Increased antibody production against gut colonizing E. coli in the presence of the anaerobic bacterium Peptostreptococcus. Scand J Immunol. 1998;48:277–82. doi: 10.1046/j.1365-3083.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 20.Herías MV, Midtvedt T, Hanson LÅ, et al. E. coli K5 capsule expression enhances colonization of the large intestine in gnotobiotic rats. Infect Immun. 1997;65:531–6.. doi: 10.1128/iai.65.2.531-536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaila M, Isolauri E, Saxelin M, et al. Viable versus inactivated lactobacillus strain GG in acute in acute rotavirus diarrhoea. Arch Dis Child. 1995;72:51–53. doi: 10.1136/adc.72.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabbé PA, Nash DR, Bazin H, et al. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970;22:448–57. [PubMed] [Google Scholar]
- 23.Wolf HM, Fischer MB, Pühringer H, et al. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood. 1994;83:1278–88. [PubMed] [Google Scholar]
- 24.Wolf HM, Vogel E, Fischer MB, et al. Inhibition of receptor-dependent and receptor-independent generation of the respiratory burst in human neutrophils and monocytes by human serum IgA. Pediatr Res. 1994;36:235–43. doi: 10.1203/00006450-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Moreau M-C, Corthier G. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect Immun. 1988;56:2766–8.. doi: 10.1128/iai.56.10.2766-2768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreau M-C, Coste M, Gaboriau V, et al. Oral tolerance to ovalbumin in mice: effect of some parameters on the induction and persistence of the suppression of systemic IgE and IgG antibody responses. Adv Exp Med Biol. 1995;371B:1229–34. [PubMed] [Google Scholar]
- 27.Telemo E, Karlsson M, Dahlman-Höglund A, et al. Oral tolerance in experimental animals. Int Arch Allergy Immunol. 1997;113:219–23. doi: 10.1159/000237552. [DOI] [PubMed] [Google Scholar]
- 28.Dahlman-Höglund A, Dahlgren UI, Hanson LÅ, et al. Different expression of IL-2 receptor α-chain on a lamina propria T-cell population and goblet cells in rats orally tolerized or sensitized to ovalbumin (OA) after colonization with an OA-producing Escherichia coli. Clin Exp Immunol. 1996;106:534–40. doi: 10.1046/j.1365-2249.1996.d01-875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 30.Willerford DM, Chen J, Ferry JA, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 31.Lundin S, Dahlgren UIH, Hanson LÅ, et al. Oral tolerization leads to active suppression and bystander tolerance in adult rats while anergy dominates in young rats. Scand J Immunol. 1996;43:56–63. doi: 10.1046/j.1365-3083.1996.d01-15.x. [DOI] [PubMed] [Google Scholar]
- 32.Mayrhofer G, Pugh CW, Barclay AN. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983;13:112–22. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- 33.Miller A, Lider O, Abramsky O, et al. Orally administered myelin basic protein in neonates primes for immune responses and enhances experimental autoimmune encephalomyelitis in adult animals. Eur J Immunol. 1994;24:1026–32. doi: 10.1002/eji.1830240503. [DOI] [PubMed] [Google Scholar]
- 34.Strobel S, Ferguson A. Immune responses to fed protein antigens in mice. III. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984;18:588–94. doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]


