Abstract
Leukotrienes (LT) of the 5-lipoxygenase pathway constitute a class of potent biological lipid mediators of inflammation implicated in the pathogenesis of different models of experimental glomerulonephritis. The key enzyme, 5-lipoxygenase (5-LO), catalyses oxygenation of arachidonic acid to generate the primary leukotriene LTA4. This LT, in turn, serves as a substrate for either LTA4 hydrolase, to form the potent chemoattractant LTB4, or LTC4 synthase, to produce the powerful vasoconstrictor LTC4. To investigate the potential role of LT in the pathogenesis of human glomerulonephritis with nephrotic syndrome, we examined the gene expression of 5-LO and LTA4 hydrolase in renal tissue of 21 adult patients with nephrotic syndrome and 11 controls. The patients consisted of 11 cases of membranous nephropathy (MN), seven focal and segmental glomerulosclerosis (FSGS), two non-IgA mesangial glomerulonephritis and one minimal change disease. Total RNA purified from renal tissue was reverse transcribed into cDNA and amplified with specific primers in a polymerase chain reaction (RT-PCR). Eight patients' renal tissue, four MN and four FSGS, co-expressed 5-LO and LTA4 hydrolase. In situ hybridization analysis revealed 5-LO expression and distribution limited to the interstitial cells surrounding the peritubular capillaries. Comparative clinical and immunohistological data showed that these eight patients had impaired renal function and interstitial changes that significantly correlated with 5-LO expression. These findings suggest that leukotrienes may play an important role in the pathogenesis of MN and FSGS. These results are also relevant to elucidating the pathophysiologic mechanisms which underlie progression to renal failure in these diseases.
Keywords: 5-lipoxygenase, LTA4 hydrolase, leukotrienes, nephrotic syndrome
INTRODUCTION
Even though clinical presentation of a nephrotic syndrome is a common feature of membranous nephropathy (MN) and focal and segmental glomerulosclerosis (FSGS), the clinical course in these renal disorders is variable. Approximately 30–40% of the patients in these two renal diseases progress to develop renal insufficiency or end-stage renal disease. Several studies unsuccessfully attempted to correlate glomerular changes with clinical severity or prognosis. Although some investigators reported a close association between tubulointerstitial changes and prognosis in patients with primary MN [1,2], such an association has not be confirmed [3]. These conflicting findings may stem from a lack of knowledge about the pathophysiologic mechanisms responsible for the decline in renal function in these human diseases.
Insights into the immunopathologic mechanisms involved in membranous glomerulonephritis have been largely gained from the experimental model of Heymann nephritis (PHN) [4]. Proteinuria in PHN develops within a few days of glomerular immune complex deposition concurrently with overt haemodynamic changes that include decreased renal plasma flow and glomerular filtration rate [5,6]. Vasoactive arachidonate metabolites have been implicated as major mediators of these renal haemodynamic changes, particularly leukotriene B4 (LTB4) and the potent vasoconstrictive leukotriene D4 (LTD4) [7–9]. Both LTB4 and LTD4 are products of the 5-lipoxygenase (5-LO) pathway which has been identified in leucocytes. The key enzyme 5-LO generates the primary leukotriene LTA4 which, in turn, serves as a substrate for formation of either LTB4, through hydrolysis by LTA4 hydrolase, or LTC4, through conjugation of glutathione by LTC4 synthase [10]. LTB4 is chemotactically active, whereas cysteinyl leukotrienes LTC4, LTD4 and LTE4 increase microvascular permeability and induce smooth muscle contraction [10].
Experimental nephritis with the characteristic renal lesions of FSGS can be induced by administration of purine aminonucleoside (PAN) as described by Glasser et al. [11]. In rats, such treatment causes a nephrotic syndrome which is associated with an acute decline in renal function and interstitial infiltration. Although there is no experimental evidence to directly link leukotrienes to tissue injury in this model, dietary manipulation which produces an essential fatty acid deficiency will ameliorate PAN renal injury [12,13]. Thus, deprivation of essential fatty acid causes arachidonic acid deficiency with the end result being an inability to form eicosanoids [14].
Collectively, these experimental findings and recent development of a molecular approach to examine gene expression of eicosanoid-forming enzymes in alveolar macrophages [15] and renal biopsies [16] have prompted the investigation of the potential role of the lipoxygenase pathway in MN and FSGS. Our findings demonstrate that, in these patients, the expression of the key enzyme 5-LO is associated with an impaired renal function. The reported results also emphasize the potential pathophysiologic role of 5-LO in progression to renal failure.
PATIENTS AND METHODS
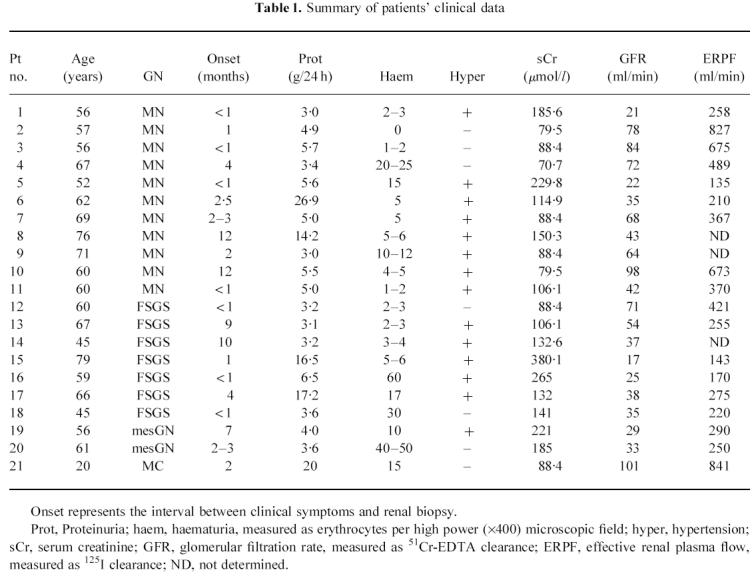
Remnant tissue of diagnostic renal biopsy specimens from 21 patients with nephrotic syndrome was analysed. The specimens included 11 patients with MN, seven patients with FSGS, two non-IgA mesangial glomerulonephritis and one patient with minimal change disease (MC). A summary of the clinical data in these patients is presented in Table 1. The specimen from one patient with normal kidney (both with light and electron microscopy examination and immunofluorescence analysis) biopsied because of persistent microscopic haematuria with normal urography, and 10 more specimens from normal kidneys obtained from nephrectomy for renal carcinoma, were used as controls.
Table 1.
Summary of patients' clinical data
Biopsy renal specimens were processed for light and immunofluorescence microscopy, while taking into account the number of glomeruli showing segmental or global hyalinosis and semiquantitatively evaluating immunofluorescence pattern and extent of mesangial sclerosis. Interstitial fibrosis and interstitial cellular infiltration were scored as follows: 1, focal fibrosis; 2, diffuse fibrosis with focal interstitial infiltration; 3, diffuse fibrosis with extensive interstitial leucocyte infiltration.
RNA extraction and cDNA synthesis
Total RNA of each kidney biopsy was isolated by acid guanidine isothiocyanate and phenol-chloroform extraction [16]. The total RNA concentration and purity was determined spectrophotometrically at 260 nm and 280 nm, respectively.
First strand cDNA was synthesized from 1 μg of total RNA using oligo (dT)12–18 (Pharmacia, Uppsala, Sweden) as a primer and the Superscript Reverse Transcriptase (Gibco, BRL, Grand Island NY) as described previously [16].
Polymerase chain reaction
The cDNA obtained from each biopsy was amplified by polymerase chain reaction (PCR) using specific primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′-ACC ACA GTC CAT GCC ATC ACT GC-3′; 5′-CTC CTT GGA GGC CAT GTG G-3′; 479 bp); β-actin (5′-GAG AAG ATG ACC CAG ATC ATG T-3′; 5′-ACT CCA TGC CCA GGA AGG AAG G-3′; 463 bp); 5-LO (5′-ATC AGG ACG TTC ACG GCC GAG G-3′; 5′-CCA GGA ACA GCT CGT TTT CCT G-3′; 415 bp); LTA4 hydrolase (5′-GAT GAC TGG AAG GAT TTC C-3′; 5′-CCA CTT GGA TTG AAT GCA GAG C-3′; 378 bp); T cell surface marker, CD3 (5′-CTG GAC CTG GGA AAA CCC ATC-3′; 5′-GTA CTG AGC ATC ATC TCG ATC-3′; 267 bp); macrophage surface marker, CD14 (5′-ACA ATC CTG GAC TGG GCG AAC-3′; 5′-TTG GAG CAG CAC CAG GGT TCC-3′; 499 bp); and B cell surface marker, CD19 (5′-ATG AGA ACG AGG ATG AAG AGC-3′; 5′-TAA GGA ATA CAA AGG GGA CTG-3′; 492 bp). The PCR reaction was performed in a 25 μl volume containing 0.2 mm dNTP mix (Perkin-Elmer, Foster City, CA), 1.5 mm MgCl2, 0.625 U AmpliTaq DNA Polymerase (Perkin-Elmer), and 1 mm of each sequence-specific primer pair. cDNA (2.5 μl) was added at the end to the ice-cooled mixture. PCR amplification in a Perkin Elmer thermal cycler consisted of three steps: 94°C 45 s, 62°C 45 s, and 72°C 1 min for 30 cycles (β-actin, 5-LO, LTA4 hydrolase, CD3, CD14 and CD19) and 94°C 1 min, 61°C 2 min, and 72°C 3 min for 30 cycles for GAPDH, assuring that reaction was in the exponential phase by kinetic and titration analysis for each pair of primers. The expression of the two housekeeping genes, GAPDH and cytoplasmic β-actin, was used to normalize for transcription and amplification among test and control specimens.
The PCR products were analysed by PAGE followed by silver staining using Phast System (Pharmacia).
Evaluation of amplified gene products
Evaluation of amplified gene products was performed by laser densitometric analysis of the Phast System gels [16]. The concentration of amplified DNA in each target band was determined relative to equivalent-sized fragments of the standard φX174 RF DNA/Hae III. Based on molecular weight of each target product, the number of amplified DNA molecules (pmol-Equivalent (pmol-Eq)) in β-actin and 5-LO was calculated per microgram of isolated total RNA (603 bp φ174 RF DNA/Hae III fragment: 5.11 pmol = 4.93 OD).
Riboprobe generation
The human 5-LO riboprobe template was a 415-bp blunt-end PCR product generated by amplifying a known positive human kidney cDNA with the same pair of primers used for RT-PCR analysis. This PCR fragment was cloned into a pCR-Blunt vector (Zero Blunt PCR Cloning Kit; Invitrogen, NV Leek, The Netherlands), and to determine the correct orientation of the insert the region included between M13 forward and reverse primer was sequenced. The clone with the PCR product in the right orientation was expanded and used as template for the in vitro transcription of a digoxigenin (DIG)-labelled 5-LO antisense riboprobe, employing the DIG RNA labelling kit (Boehringer Mannheim GmbH, Mannheim, Germany). Riboprobe was purified by chromatography with sterile G-25 spin column (BioRad Labs, Hercules, CA). The labelling efficiency was checked by dot blot.
In situ hybridization
Paraffin sections (5 μm thick) were mounted on silane-coated slides. Tissue sections were deparaffinized and rehydrated in graded alcohol series, then pretreated sequentially in Proteinase K (Gibco) 15 mg/ml in PBS for 10 min; glycine 0.1 m in PBS 30 s; 10% buffered formalin for 20 min, 2× SSC (standard saline citrate buffer 0.3 mm NaCl, 30 mm Na2 citrate pH 7) for 5 min, 0.1 m triethanolamine pH 8 and 0.25% acetic anhydride in diethylpyrocarbonate-treated water for 15 min and finally rinsed in 2× SSC for 5 min.
The slides were hybridized overnight at 42°C in 50 μl of hybridization buffer (50% w/v deionized formamide, 4× SSC, 10% w/v dextran sulfate, 2.5 mg/ml tRNA, 0.02% polyvinylpirrolidone, 0.02% Ficoll, 0.02% bovine serum albumin (BSA)) containing 24 ng of DIG-labelled riboprobe. After hybridization, slides were treated with 0.2× SSC (three times 5 min each), RNase A (20 mg/ml in 0.5 m NaCl, 10 mm Tris–HCl pH 8, 0.1 m EDTA) for 30 min at 37°C and 0.2× SSC (three times 5 min each). The slides were incubated with anti-DIG antibody conjugated with alkaline phosphatase (Boehringer Mannheim) diluted 1:100 and the signal was detected using NBT/BCIP solution (Boeringher Mannheim) as chromogen. Finally, the slides were counterstained with nuclear fast red and mounted in aqueous media.
Immunohistochemistry
Cryostat sections (5 μm thick) were air-dried and fixed in acetone for 10 min. The sections were incubated for 1 h with: anti-5-LO antisera (courtesy of Merk Frosst of Canada) diluted 1:200, anti-CD14 and CD3 MoAbs (Dako Corp., Carpinteria, CA). An avidin-biotin horseradish peroxidase technique (UltraAvidin Peroxidase staining Kit; Leinco Technologies Inc., Manchester, MO) with 3,3′-diaminobenzidine (DAB) as chromogen was used to visualized the antibody–antigen reaction. Slides were counterstained with Harris haematoxylin solution.
Radioisotopic clearances
A dose of 37 kBq/kg of commercially available 51Cr-EDTA (Amersham, Aylesbury, UK) was administered in an antecubital vein. The precise measurement of the injected dose was obtained by weighing the syringe before and after injection. The estimated error introduced by weighing was 0.1%. Venous samples from the arm controlateral to the injection site were drawn at 180, 200, 220 and 240 min. Plasma samples were centrifuged at 1500 g using a fixed-angle centrifuge. A carefully weighed amount of 51Cr-EDTA from the batch used for injection was diluted by a factor of 1000 to obtain a standard. Two millilitres of plasma sample and 2 ml of standard solution were counted in a crystal scintillator detector. Linearity, efficiency and resolution of the counting chain were tested monthly. Clearance values were normalized to 1.73 m2 of body surface area and calculated using the standard formula. The total 51Cr-EDTA plasma clearance was calculated from the correction equation Cl = 0.990 778 × E1 −0.001 218 × E12, where E1 (ml/min) is the ratio of the injected dose and the total area under the monoexponential curve determined from the radioactivity in the plasma samples.
Estimation of the renal plasma flow was done 44 min after injection of 125I-hippurate by means of the classic single plasma concentration determination [17]. Clearance values were corrected to 1.73 m2 body surface area. ERPF was calculated as PAH clearance/E-PAH, where E-PAH was the extraction ratio of hippuran (0.91).
Filtration fraction (FF) was calculated using the following formula: FF = glomerular filtration rate (GFR)/effective renal plasma flow (ERPF).
Statistical analysis
Where applicable, Mann–Whitney rank sum test, Fisher's exact test, and Student's t-test were used for determining the relationship between gene expression and clinical data.
RESULTS
All the patient and control renal specimens were examined for expression of housekeeping genes, β-actin and GAPDH. Evaluation of amplified gene products for β-actin provided similar results in 5-LO+ (1.79 ± 0.329 pmol-Eq/μg RNA) and 5-LO− (1.84 ± 0.223 pmol-Eq/μg RNA) patients. A representative profile for the expression of 5-LO and LTA4 hydrolase and other cellular markers is illustrated in Fig. 1. None of the 11 control specimens showed any PCR product of 5-LO.
Fig. 1.
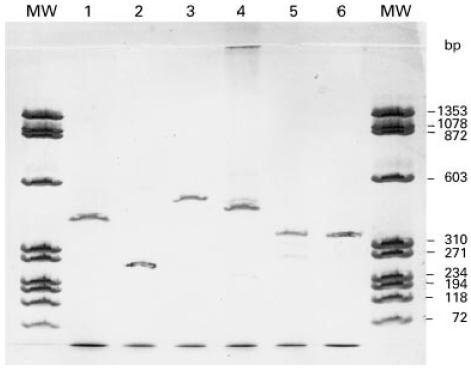
Representative expression profile in renal tissue detected by polymerase chain reaction (PCR). Cytoplasmic β-actin gene (lane 1); CD3, T lymphocyte marker (lane 2); CD14, macrophage marker (lane 3); CD19, B lymphocyte marker (lane 4); LTA4 hydrolase (lane 5); and 5-lipoxygenase (5-LO) (lane 6). Scanned computer image of silver-stained 12.5% Phastgel containing 30 cycles amplified PCR products of target genes. MW, molecular marker of φX174 RF DNA/Hae III digest.
The expression of 5-LO was detected in eight patients (Table 2), whereas all patients' and controls' renal tissues expressed LTA4 hydrolase. Due to the usual association of 5-LO with leucocytes, the potential of leucocytic infiltrate in the renal tissue was determined by expression of gene markers for macrophages (CD14), T lymphocytes (CD3), and B lymphocytes (CD19). As summarized in Table 2, 15 specimens were examined for leucocyte markers. In detail, mononuclear CD14+ cells were detected in 14 out of 15 specimens; CD3+ cells were detected in nine of these specimens (Fig. 2a,b). The B lymphocyte marker CD19 was detectable in only four specimens. In normal tissues, CD14 was weakly detectable in all, and CD3 in four out of five specimens, whereas CD19 could not be detected in any control specimen. Immunohistological data (Table 2) showed that the eight specimens (four MN and four FSGS) which expressed 5-LO had greater interstitial fibrosis and infiltration with mononuclear cells (P = 0.003 for ≥ 1+ score and P = 0.001 for ≥ 2+ score).
Table 2.
Summary of gene expression of 5-lipoxygenase (5-LO), LTA4 hydrolase, and cell surface markers of T cell (CD3), B cell (CD19), and macrophage (CD14) and histological findings in patients' renal biopsies
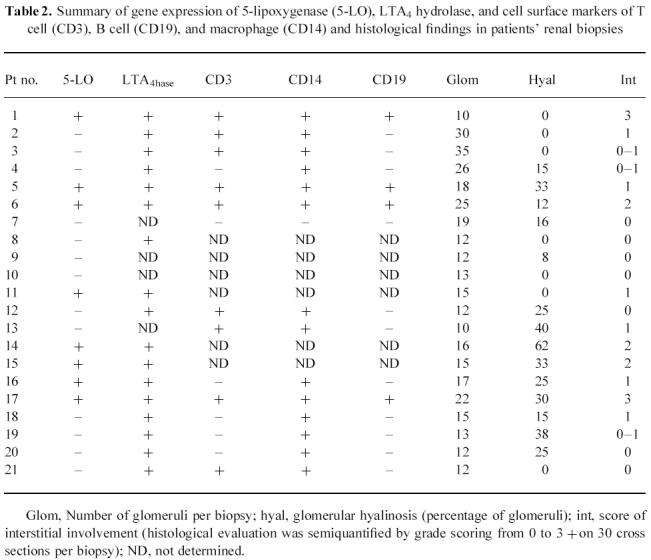
Fig. 2.
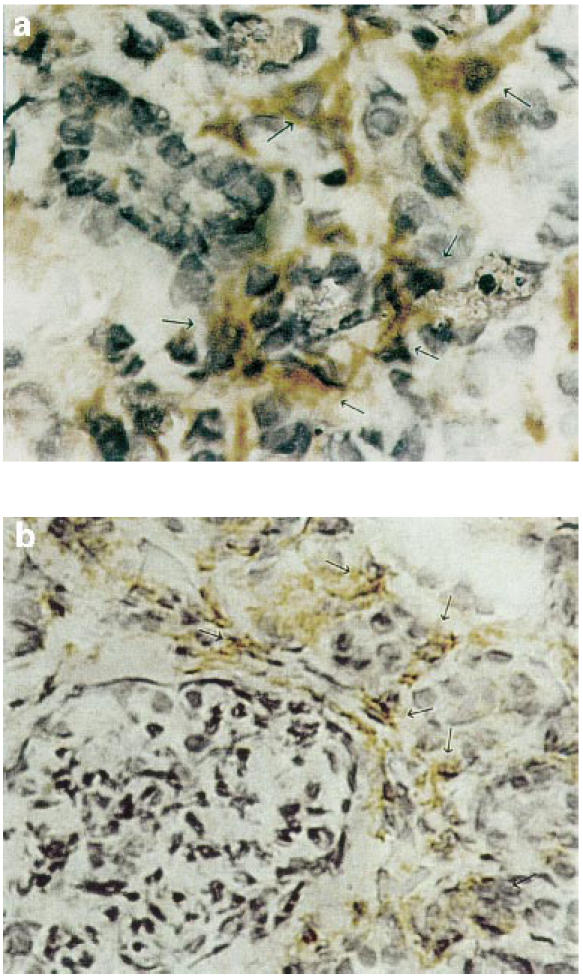
Examples of distributions of CD3+ and CD14+ immunohistochemical staining in frozen sections of a specimen from a nephrotic syndrome patient with membranous nephropathy (no. 6 of Tables 1 and 2). (a) CD3+ interstitial lymphocytes (arrows). (b) CD14+ interstitial mononuclear cells (arrows).
To determine definitely the cellular origin and distribution of 5-LO gene expression, specimens which were positive for 5-LO by RT-PCR were analysed both by immunohistochemistry and in situ hybridization. Figure 3 shows a positive staining for 5-LO in interstitial peritubular (b) and mononuclear cells (a). Furthermore, as illustrated in Fig. 4, intense perivascular staining of the peritubular capillary bed was prominent by in situ hybridization technique. The glomerular capillary tuft was invariably negative, suggesting the site of 5-LO to be confined interstitial cells surrounding vasculature.
Fig. 3.
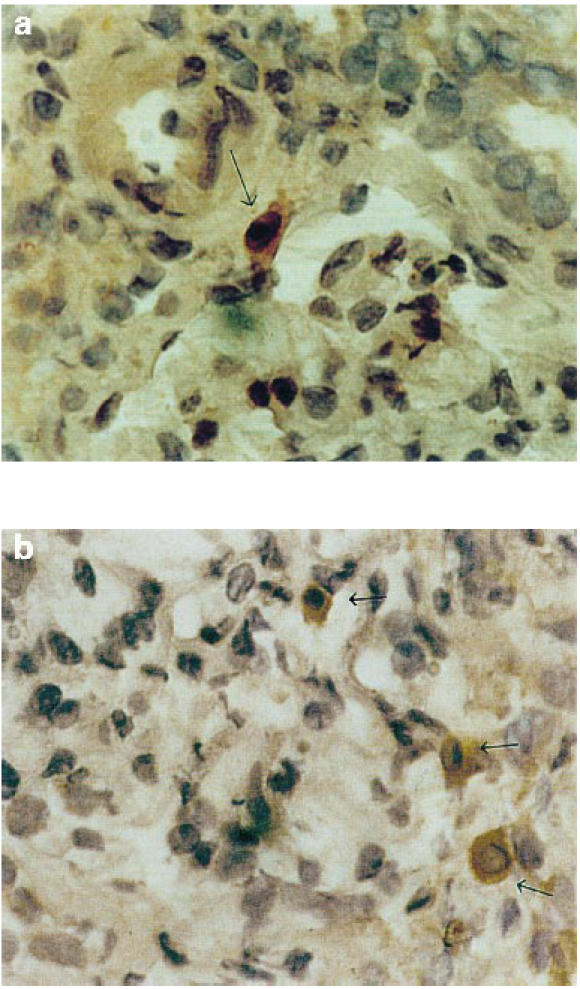
Representative example of immunohistochemistry analysis of 5-lipoxygenase (5-LO) distribution in a frozen section of a specimen from a nephrotic syndrome patient with membranous nephropathy (no. 6 of Tables 1 and 2) showing positive interstitial peritubular spindle-shaped cells (b), and (a) positive interstitial mononuclear cells (arrows).
Fig. 4.
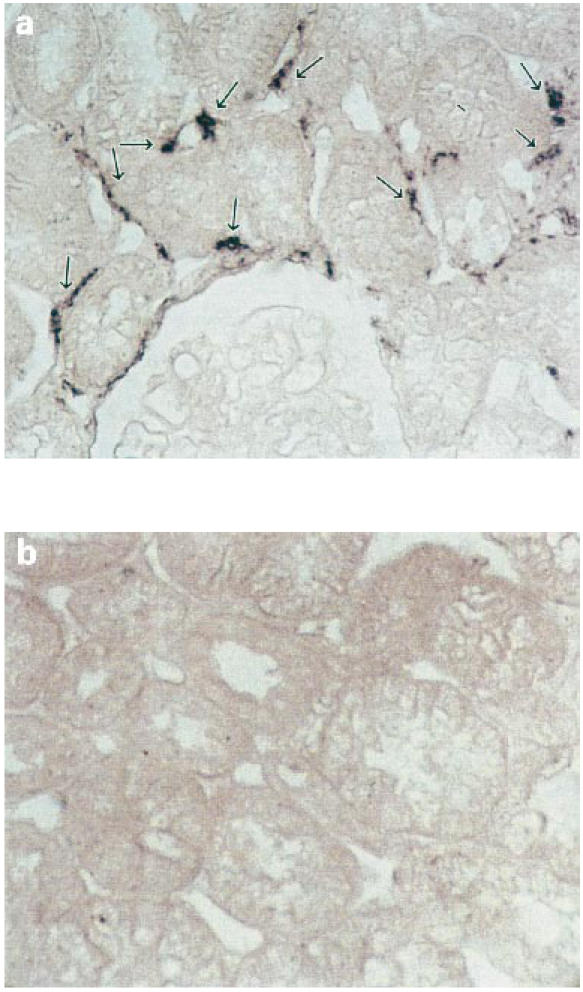
Representative example of in situ hybridization analysis of 5-lipoxygenase (5-LO) gene distribution in a specimen from a nephrotic syndrome patient with membranous nephropathy (no. 5 of Tables 1 and 2). Focal staining surrounding peritubular capillary vessels related to interstitial cells is evident (a). An anti-digoxigenin antibody conjugated with alkaline phosphatase and NBT/BCIP as chromogen were used as detecting system (see Patients and Methods) (×400). Normal tissue is shown for comparison (b).
The expression of 5-LO in renal biopsies was significantly associated with a decline in glomerular filtration rate, renal plasma flow, and increased serum creatinine (Fig. 5). The level of proteinuria in these patients, however, did not correlate with the expression of 5-LO. Furthermore, gene expression of this enzyme was unrelated to time between the onset of presenting symptoms and renal biopsy, age of patient, extent of mesangial sclerosis, glomerular hyalinosis, interstitial fibrosis, or immunofluorescence pattern (data not shown). All eight patients 5-LO+ were hypertensive versus six hypertensive out of the remaining 13 patients who did not express 5-LO (P < 0.05).
Fig. 5.
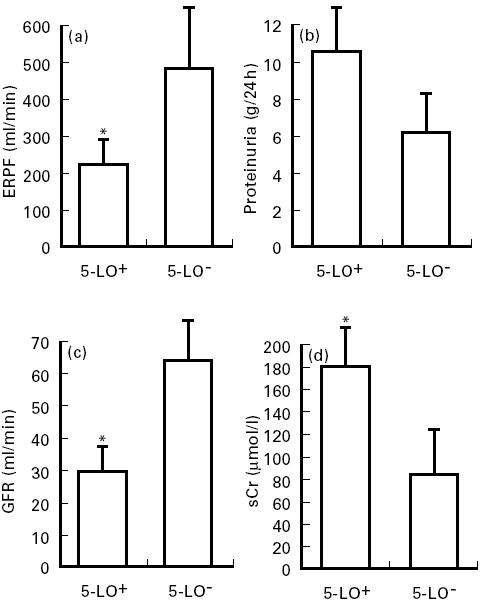
Comparison of clinical data in patients according to 5-lipoxygenase (5-LO) expression. Statistical significance determined by t-test for effective renal plasma flow in (a) (P = 0.01) and glomerular filtration rate in (c) (P = 0.004). Association of proteinuria in (b) (P = NS, Z = 1.23) and level of serum creatinine in (d) (P = 0.01) with 5-LO expression analysed by Mann–Whitney rank sum test. ERPF, Effective renal plasma flow; GFR, glomerular filtration rate; sCr, serum creatinine; 5-LO+ and 5-LO−, five-lipoxygenase-positive and negative cases. *Significant (P ≤ 0.01) compared with 5-LO− cases.
DISCUSSION
The pathophysiologic mechanisms which underlie the manifestation of nephrotic syndrome and progression to renal failure in MN and FSGS are largely unknown. The well characterized role of leukotrienes in the pathogenesis of the PHN model prompted us to investigate whether the lipoxygenase pathway is similarly involved in humans. In contemplating this study, the critical nature of leukotriene generation in the tissue microenvironment for purposes of local action rather than for an effect at a distal site was considered. Furthermore, the extravascular nature of the inflammatory process and ability of renal cells to generate and inactivate lipid mediators suggest that involvement of leukotrienes in renal pathophysiologic processes is unlikely to be apparent by assaying blood or urine samples. To overcome these technical limitations and identify the inflammatory processes in situ, immunohistochemistry techniques and a molecular approach of RNA reverse transcription coupled with PCR to identify key enzymes in the lipoxygenase pathway were used. The presented data demonstrate the gene expression of the 5-LO of the leukotriene pathway in renal tissue of patients with MN and FSGS. Most significantly, there was a strong association between this enzyme expression and decreased renal plasma flow, reduced GFR, and increased serum creatinine.
In the lipoxygenase pathway, 5-LO is the primary enzyme which oxidises arachidonic acid and its conversion to LTA4. This LT plays a pivotal role as a substrate for either LTA4 hydrolase in the formation of LTB4 or LTC4 synthase for generation of sulfopeptidoleukotriene LTC4. Increased production of LTB4 has been demonstrated in isolated glomeruli of PHN [8,9]. However, in this model, there is an apparent lack of correlation between the extent of proteinuria and LTB4 synthesis [8]. In the present study the co-expression of 5-LO and LTA4 hydrolase strongly suggests LTB4 is produced in renal tissues of some nephrotic patients. However, though 5-LO+ patients tended to be more severely proteinuric than 5-LO− ones (Fig. 5), similar to the experimental observations, proteinuria values failed to reach a statistical level of association with 5-LO gene expression, indicating 5-LO expression could be only one of the several variables involved in the pathogenesis of protein excretion in nephrotic states.
It has been postulated that the extracellular metabolism of LTA4 by the ubiquitous tissue distribution of LTA4 hydrolase would result in the production of LTB4 [10]. LTA4 hydrolase is a Zn2+ metallohydrolase and bifunctional enzyme which can be inhibited with Captopril, an inhibitor of dipeptidyl dipeptidase [18]. As expected, LTA4 hydrolase was expressed both in the 11 controls and the patients' renal tissues. The critical parameters of renal dysfunction and progression to renal failure, decline in GFR and renal plasma flow, in MN and FSGS patients, were significantly associated with 5-LO expression (Fig. 5). These findings are consistent with the general decline in renal haemodynamics in response to LTC4 or LTD4 described by Badr et al. [19,20]. More importantly, the pathophysiologic importance of LTD4 has been recently confirmed by Katoh et al. [9] in the experimental model of PHN. The technical difficulty of quantifying LTD4 in a kidney biopsy and lack of molecular information about human LTC4 synthase impede a definitive identification of the 5-LO to LTC4 synthase axis of the lipoxygenase pathway in human MN and FSGS.
Regarding the cellular origin of 5-LO expression, the results of the leucocytic cellular markers' expression analysis (Table 2) suggest a macrophage involvement. T lymphocytes (CD3) do not express 5-LO but B lymphocytes (CD19), which recently have been shown to possess the capacity to convert arachidonic acid to LTB4 [21] due to expression of 5-LO [22], could be involved, too. Interstitial cells surrounding peritubular capillaries appear to be major sites for 5-LO expression (Fig. 4). Interstitial perivascular cell 5-LO gene expression, in nephrotic states, even induced by pathogenically distinct conditions, might be triggered by the reabsorption of abnormally filtered macromolecules. This might represent one of the possible mechanisms of renal injury and dysfunction due to the altered protein traffic through the glomeruli. The locally produced LTA4 might also be translocated to endothelial cells where it could be further metabolized to LTC4/LTD4 or LTB4. Elaboration of the potent vasoactive LTC4 and LTD4 might compromise blood supply along with vasoconstriction, leading to increased efferent arteriolar resistance and diminished renal plasma flow and thereby decline in GFR, while generation of the chemotactic LTB4 could promote leucocyte recruitment and interstitial inflammation which is particularly detrimental in renal glomerular disease progression [23,24]. Accordingly, the role of leukotrienes in this subset of nephrotic patients with a poorer prognosis could be primarily related to the intrinsic glomerular injury.
Treatment with a specific peptidoleukotriene receptor antagonist significantly improves renal haemodynamics in animals with nephritis [25]. Moreover, as recently reviewed [14], a 6-week course of n-3 fatty acid treatment, known to decrease the production of bioactive leukotrienes [26], significantly decreases proteinuria in either MN or FSGS in humans [27]. Finally, initial evidence supports a role for a direct inhibition of leukotriene biosynthesis by a 5-LO activating protein antagonist (which inhibits leukotriene biosynthesis) in decreasing proteinuria in human nephritis [28]. Thus, it is conceivable that interventions which antagonize the action of 5-LO products or reduce their formation result, especially in the early phases of disease, in clinical improvement and amelioration of renal injury.
In conclusion, evidence for the gene expression of 5-LO and LTA4 hydrolase in renal tissues of patients with MN and FSGS was shown. In contrast, 5-LO expression was significantly associated with increased serum creatinine, decline in renal plasma flow and GFR. Our findings suggest that the activity of the 5-lipoxygenase pathway may serve as a suitable therapeutic target for patients with MN and FSGS.
Acknowledgments
The present study has been partially supported by a grant from Sclavo S.p.A, Siena, Italy. The authors are indebted to Andrea De Marchi for his technical assistance.
REFERENCES
- 1.Wehrmann M, Bohle A, Bogensch TZO, et al. Long-term prognosis of chronic idiopathic membranous glomerulonephritis. Clin Nephrol. 1989;31:67–76. [PubMed] [Google Scholar]
- 2.Abe S, Amagasaki Y, Iyori S, Konishi K, Kato E, Sagakuchi H. Significance of tubulointerstitial lesions in biopsy specimens of glomerulonephritic patients. Am J Nephrol. 1989;9:30–37. doi: 10.1159/000167931. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Tomino Y, Fukui M, et al. Correlation between tubulointerstitial changes and prognosis in patients with primary membranous nephropathy. Nephron. 1994;67:362. doi: 10.1159/000187995. [DOI] [PubMed] [Google Scholar]
- 4.Salant DJ, Quigg RJ, Cybulsky AV. Heymann nephritis: mechanism of renal injury. Kidney Int. 1989;35:976–84. doi: 10.1038/ki.1989.81. [DOI] [PubMed] [Google Scholar]
- 5.Gabbai FB, Gushwa LC, Wilson CB, Blantz RC. An evaluation of the development of experimental membranous nephropathy. Kidney Int. 1987;31:1267–78. doi: 10.1038/ki.1987.140. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka T, Rennke HG, Salant DJ, Deen MW, Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect on glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res. 1987;61:531–8. doi: 10.1161/01.res.61.4.531. [DOI] [PubMed] [Google Scholar]
- 7.Badr KF. Glomerulonephritis: roles for lipoxygenase pathways in pathophysiology and therapy. Curr Opin Nephrol Hypertens. 1997;6:111–8. [PubMed] [Google Scholar]
- 8.Lianos EA, Noble B, Hucke B. Glomerular leukotriene synthesis in Heymann nephritis. Kidney Int. 1989;36:998–1002. doi: 10.1038/ki.1989.293. [DOI] [PubMed] [Google Scholar]
- 9.Katoh T, Lianos EA, Fukunaga M, Takahashi K, Badr KF. Leukotriene D4 is a mediator of proteinuria and glomerular hemodynamic abnormalities in passive Heymann nephritis. J Clin Invest. 1993;91:1507–15. doi: 10.1172/JCI116356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. N Engl J Med. 1990;323:645–53. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 11.Glasser RJ, Velosa JA, Michael AF. Experimental model of focal sclerosis. Lab Invest. 1977;36:519. [PubMed] [Google Scholar]
- 12.Diamond JR, Pesek I, Ruggieri S, Karnowsky MJ. Essential fatty acid deficiency during acute puromycin nephrosis ameliorates late renal injury. Am J Physiol. 1989;257:F798–807. doi: 10.1152/ajprenal.1989.257.5.F798. [DOI] [PubMed] [Google Scholar]
- 13.Harris KP, Lefkowith JB, Klahr S, Schreiner GF. Essential fatty acid deficiency ameliorates acute renal dysfunction in the rat after the administration of the aminonucleoside of puromycin. J Clin Invest. 1990;86:1115–23. doi: 10.1172/JCI114816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefkowith JB, Klahr S. Polyunsaturated fatty acids and renal disease. Proc Soc Exp Biol Med. 1996;213:13–23. doi: 10.3181/00379727-213-44031. [DOI] [PubMed] [Google Scholar]
- 15.Funk CD, Fitzgerald GA. Eicosanoid forming enzyme mRNA in human tissues. J Biol Chem. 1991;266:12508–13. [PubMed] [Google Scholar]
- 16.Rifai A, Sakai H, Yagame M. Expression of 5-lipoxygenase and 5-lipoxygenase activation protein in glomerulonephritis. Kidney Int. 1993;43(S39):S-95–S-99. [PubMed] [Google Scholar]
- 17.Tauxe WN, Dubovsky EV, Kidd T. New formulae for the calculation of effective renal plasma flow by the single plasma sample method. Eur J Nucl Med. 1982;7:51–54. doi: 10.1007/BF00251641. [DOI] [PubMed] [Google Scholar]
- 18.Orning L, Krivi G, Bild G, Gierse J, Aykent S, Fitzpatrick FA. Inhibition of leukotriene A4 hydrolase/aminopeptidase by captopril. J Biol Chem. 1991;266:16507–11. [PubMed] [Google Scholar]
- 19.Badr KF, Baylis C, Pfeffer JM, et al. Renal and systemic hemodynamic responses to intravenous infusion of leukotriene C4 in the rat. Circ Res. 1984;54:492–9. doi: 10.1161/01.res.54.5.492. [DOI] [PubMed] [Google Scholar]
- 20.Badr KF, Brenner BM, Ichikawa I. Effects of leukotriene D4 on glomerular dynamics in the rat. Am J Physiol. 1987;253:F239–43. doi: 10.1152/ajprenal.1987.253.2.F239. [DOI] [PubMed] [Google Scholar]
- 21.Jakobsson P-J, Odlander B, Steinhilber D, Rosen A, Claesson HE. Human B lymphocytes possess 5-lipoxygenase activity and convert arachidonic acid to leukotriene B4. Biochem Biophys Res Comm. 1991;178:302–8. doi: 10.1016/0006-291x(91)91814-s. [DOI] [PubMed] [Google Scholar]
- 22.Jakobsson P-J, Steinhilber D, Odlander B, Radmark O, Claesson H-E, Samuelsson B. On the expression and regulation of 5-lipoxygenase in human lymphocytes. Proc Natl Acad Sci USA. 1992;89:3521–5. doi: 10.1073/pnas.89.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee J, Kuncio GS, Neilson EG. Tubulointerstitial injury following glomerulonephritis. Semin Nephrol. 1991;11:361–6. [PubMed] [Google Scholar]
- 24.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 25.Spurney RF, Ruiz P, Pisetsky DS, Coffman TM. Enhanced renal leukotriene production in murine lupus: role of lipoxygenase metabolites. Kidney Int. 1991;39:95–102. doi: 10.1038/ki.1991.12. [DOI] [PubMed] [Google Scholar]
- 26.de Caterina R, Caprioli R, Giannessi D, et al. n-3 fatty acids reduce protein-uria in patients with chronic glomerular disease. Kidney Int. 1993;44:843–50. doi: 10.1038/ki.1993.320. [DOI] [PubMed] [Google Scholar]
- 27.de Caterina R, Endres S, Kristensen SD, Schmidt EB. n-3 fatty acids in renal diseases. Am J Kidney Dis. 1994;24:397–415. doi: 10.1016/s0272-6386(12)80896-1. [DOI] [PubMed] [Google Scholar]
- 28.Guasch A, Zayas CF, Badr KF. Therapeutic effect of leukotriene biosynthesis inhibition in proliferative glomerulonephritis: results of the first human trial. J Am Soc Nephrol. 1996:1334. [Google Scholar]


