Abstract
Proviral load as well as lymphocyte phenotype and function were compared in peripheral blood and lymph node compartments of 17 HIV-1, 12 HIV-2 and three dually infected patients with lymphadenopathy. The mean percentage (95% confidence interval (CI)) of CD4+ cells was higher in lymph node mononuclear cells (LNMC) than in peripheral blood mononuclear cells (PBMC) in both infections, being 26.7% (21.1%, 32.3%) and 15.3% (10.4%, 20.2%), respectively, for HIV-1-infected patients (P = 0.0001) and 32.3% (22.7%, 41.9%) and 22.1% (13.6%, 30.6%), respectively, for HIV-2-infected patients (P = 0.02). In both types of infection, proviral load adjusted for number of CD4+ cells was higher in LNMC than in PBMC: the geometric mean (95% CI) was 8937 (4991; 16 003) and 4384 (2260; 8503), respectively, for HIV-1 patients (P = 0.02) and 1624 (382; 6898) and 551 (147; 2058) DNA copies, respectively, for HIV-2 patients (P = 0.05). Proviral load in both compartments was closely correlated (HIV-1, r = 0.60, P = 0.01; and HIV-2, r = 0.83, P = 0.0003). In both infections, proliferation and interferon-gamma (IFN-γ) production in response to purified protein derivative (PPD) was lower in LNMC than in PBMC, both of which, in turn, were lower than in healthy controls. These results indicate that in HIV-2 as in HIV-1 infection, infected cells have a tropism for the lymph nodes resulting in higher viral load in this compartment and lower lymphocyte responses to the recall antigen PPD which may increase susceptibility to tuberculosis.
Keywords: proviral load, cytokines, HIV-2, lymph node, HIV-1
INTRODUCTION
The central role of lymphoid organs as an important site for viral replication during HIV-1 infection is now clearly understood. Various studies have shown that infected CD4+ T lymphocytes are sequestered in the lymph nodes during HIV-1 infection [1,2] and that viral replication is active in these tissues. HIV-1 viral load is higher in lymph nodes than in peripheral blood during the asymptomatic phase of HIV-1 infection and this dichotomy is reduced during progression to the late stages of HIV disease [2–6]. Limited studies on HIV-2 proviral load in peripheral blood of asymptomatic subjects have shown that it is lower than HIV-1 and is inversely correlated with the percentage of CD4+ cells [7–9], but there is no information on lymph node tropism of HIV-2.
HIV-induced immunosuppression is related to depletion of CD4+ T lymphocytes within peripheral blood and lymph node compartments and also to functional alterations of viable CD4+ lymphocytes. In both HIV-1 and HIV-2 infections, the function of peripheral blood CD4+ T lymphocytes is altered early during the course of the disease when the CD4+ cell count is still high and is further impaired with disease progression [10–14]. The consequences of the higher viral burden in the lymph node compartment on CD4+ lymphocyte function have not been studied.
Therefore, in order to determine if the lymph node serves as a reservoir during HIV-2 infection and to study the outcome of increased viral burden in the lymph node compartment on CD4+ lymphocyte function during HIV infection, we measured proviral load in peripheral blood mononuclear cells (PBMC) and lymph node mononuclear cells (LNMC) of HIV-1- and HIV-2-infected patients and related these measurements to proliferative response and interferon-gamma (IFN-γ) production induced by the recall antigen purified protein derivative (PPD).
SUBJECTS AND METHODS
Subjects
Peripheral blood and lymph nodes were obtained from 17 HIV-1, 12 HIV-2 and three dually seropositive individuals with persistent lymphadenopathy who attended the Medical Research Council (MRC) Laboratories clinic in The Gambia from August 1993 to December 1996. Recruitment was consecutive with the exception of two patients who did not consent. Peripheral blood was also obtained from 27 healthy HIV− blood donors and abdominal lymph nodes from five HIV− donors (two adults with peptic ulcer and three children with intestinal obstruction who had been vaccinated with bacille Calmette–Guérin (BCG)). As blood samples were not obtained from the lymph node donors, their HIV status was determined by polymerase chain reaction (PCR) on the lymph nodes as described below.
HIV serology was determined using a combined HIV-1 and HIV-2 enzyme labelled immunosorbent assay (ELISA) test (Wellcozyme 1 + 2; Murex Diagnostics, Dartford, UK) and if positive, type-specific competitive ELISA (Murex Diagnostics) as well as synthetic peptide assays (PeptiLAV; Diagnostics–Pasteur, Marnes la Coquette, France). The patients and controls or their parents were recruited following informed consent and were counselled prior to HIV testing, and the study was approved by the Gambia Government/MRC Ethical Committee. Because of insufficient cell numbers all analyses could not be performed on every patient studied. Thus the number of patients included in each analysis is detailed in Results.
Isolation of mononuclear cells from blood and lymph node
Venous blood was collected in preservative-free heparin (10 U/ml). Cervical lymph nodes were surgically removed and immediately placed in serum-free RPMI 1640 medium (Whittaker, Verviers, Belgium) and portions taken for microbiological and histological examinations. Cells were teased out with forceps and the cell suspension separated from the lymph node tissue material with a tea strainer. PBMC and LNMC were purified by density centrifugation on Lymphoprep (Nycomed, Oslo, Norway). The resulting preparations yielded > 95% viable cells as determined by trypan blue exclusion.
Flow cytometric analysis
Cell suspensions of LNMC and PBMC were dually stained with FITC-labelled anti-CD4 MoAbs and PE-labelled anti-CD8 MoAbs (Becton Dickinson, San Jose, CA). The lymphocyte population was gated according to side and forward scatter properties and CD4+ and CD8+ cell percentages were counted with a FACScan apparatus (Becton Dickinson, Erembodegem, Belgium).
Analysis of T lymphocyte function
PBMC and LNMC were cultured in RPMI 1640 medium (Whittaker) supplemented with 40 mml-glutamine (Flow, Costa Mesa, CA), 10% heat-inactivated AB serum (Baxter) and 2% penicillin (5000 U/ml)/streptomycin (5000 μg/ml) (Flow) in two sets of triplicate wells (7.5 × 104 cells/200 μl per well) in 96-well round-bottomed microtitre plates (Becton Dickinson, Nutley, NJ). Cells were activated with PPD (Evans Medical Limited, Leatherhead, UK) at 50 U/ml or phytohaemagglutinin (PHA; Murex Diagnostics, Temple Hill, USA) at 2 μg/ml. Proliferative responses were measured on day 4 for PHA cultures and on day 7 for PPD, after pulsing with methyl-3H-thymidine (Amersham Life Sciences, Aylesbury, UK) over 4 h in the case of PHA and over 18 h in the case of PPD-stimulated cultures. Thymidine incorporation was measured by scintillation with an automated Betaplate reader (LKB 1205; Turku, Finland). Data were expressed as stimulation index (SI) = log mean stimulated wells − log mean unstimulated wells. A SI > 2.5 was considered as a positive response. IFN-γ levels were measured in supernatants collected after 6 days of PPD stimulation using a standard ELISA according to the recommendations of the manufacturer (Mabtech AB, Stockholm, Sweden) [15].
Quantification of proviral load
DNA was extracted from PBMC and LNMC of HIV-1, HIV-2 and dually infected patients and proviral load determined by a quantitative DNA PCR method as previously described [9]. The lower limit of detection for both HIV-1 and HIV-2 in this system was three copies per 105 CD4+ cells.
Statistical analysis
Differences in means or log10 means of normally distributed data between PBMC and LNMC were compared by paired t-test or in the case of patients and controls by t-test. Proviral load was compared between HIV-1 and HIV-2 after adjusting for CD4 % by regression analysis. Dual infections were handled in different ways. Thus, in the analyses of type-specific proviral load these patients were counted twice, whereas in the overall analyses and in the correlations between proviral load and proliferation or IFN-γ responses, the dually infected patients were regarded as a single entity and the sum of HIV-1 and HIV-2 proviral load values used. Correlations were assessed by Pearson's correlation coefficient. Discrete data were compared between groups by the χ2 test with Yates’ correction or by Fisher's exact test as appropriate. The SAS System for Windows was used for the analysis. Due to technical errors or lack of cells, all tests could not be done on all patients. Hence numbers vary in different tables.
RESULTS
As is our general experience, HIV-2-infected patients were in a less advanced clinical stage of disease and had higher mean CD4 % in peripheral blood than HIV-1-infected patients (Table 1), although this difference was not statistically significant (difference in means = 6.8%; P = 0.12).
Table 1.
Mean percentage (95% CI) of CD4+ and CD8+ lymphocytes in peripheral blood mononuclear cells (PBMC) and lymph node mononuclear cells (LNMC) obtained from HIV-infected patients and from controls
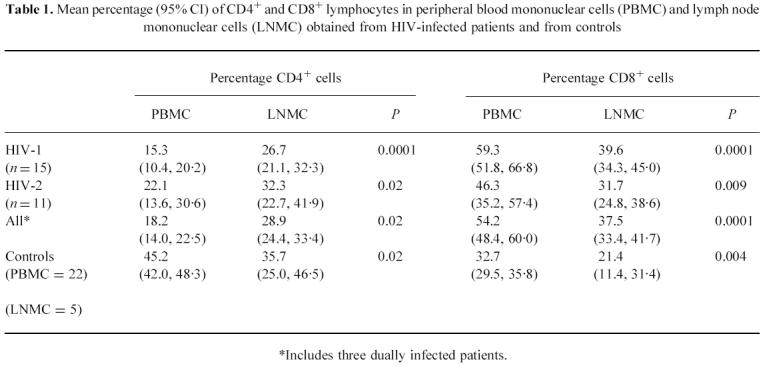
*Includes three dually infected patients
CD4 % is lower in PBMC than in LNMC in HIV infection
The mean percentage of CD4+ and CD8+ lymphocytes in PBMC and LNMC of HIV-infected patients and controls is shown in Table 1. In both HIV-1- and HIV-2-infected individuals, the percentage of CD4+ cells was significantly lower in PBMC than in LNMC, while the opposite was observed in cells obtained from controls. In contrast, the percentage of CD8+ lymphocytes was found to be significantly higher in PBMC than in LNMC both in HIV-infected patients and in healthy controls (Table 2). Percentages of CD4+ lymphocytes in PBMC and LNMC were correlated in HIV-1 (r = 0.75, P = 0.001) and in HIV-2 patients (r = 0.59, P = 0.06) as well as overall (r = 0.65, P = 0.001). Overall, mean CD4 % in PBMC from HIV-infected individuals was significantly lower than in controls (P < 0.0001), while CD4 % in LNMC was similar in both groups (P = 0.2).
Table 2.
Geometric mean (95% CI) proviral load in peripheral blood mononuclear cells (PBMC) and lymph node mononuclear cells (LNMC) of patients
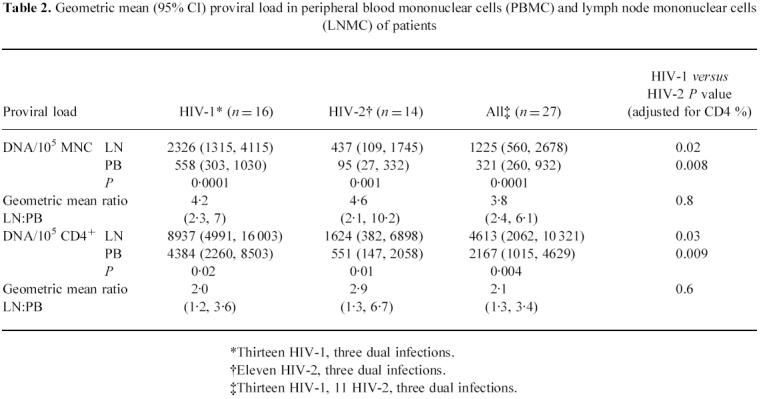
*Thirteen HIV-1, three dual infections.
†Eleven HIV-2, three dual infections.
‡Thirteen HIV-1, 11 HIV-2, three dual infections.
Proviral load is higher in lymph node than in peripheral blood
Proviral load was measured in 13 HIV-1, 11 HIV-2 and three dually infected subjects. Table 2 shows that both HIV-1 and HIV-2 proviral loads were two-to-five-fold higher in LNMC than in PBMC, although the relative amounts expressed as geometric mean ratios did not differ between the two infections, nor did they differ significantly according to stage of disease as determined by CD4 % (data not shown). In either infection and overall, proviral load per 105 CD4+ cells was positively correlated between PBMC and LNMC (r = 0.60, P = 0.01 for HIV-1; r = 0.83, P = 0.0003 for HIV-2; and r = 0.82, P = 0.0001 overall). Similar values were obtained when data expressed as copies per 105MNC cells were analysed. In both compartments, after adjustment for CD4 %, HIV-1-infected patients had significantly higher geometric mean values than HIV-2 patients.
Comparison of peripheral blood and lymph node T cell proliferative responses to PPD
In order to evaluate the possible functional consequences of lymph node tropism of HIV-1 and HIV-2, we compared the responses of peripheral blood and lymph node T lymphocytes to the recall antigen PPD and the mitogen PHA. In The Gambia, virtually all adults have been infected with mycobacteria and healthy subjects respond in vitro to PPD. T cell function was studied in 14 patients with HIV-1, eight with HIV-2 and three with dual infection. PBMC (n = 27) and LNMC (n = 5) obtained from controls showed strong proliferative responses to PPD and PHA (Fig. 1). These responses were significantly lower in HIV-infected subjects (P < 0.001 for both HIV-1 and HIV-2 for PPD and PHA). Among the 13 HIV-infected patients responding to PPD (SI ≥ 2.5) in either PBMC or LNMC populations, the proliferative response induced by PPD in the LNMC was significantly lower than in the PBMC (P = 0.023). This difference was specific for PPD, as the PBMC and the LNMC from these HIV-infected patients showed similar responses to PHA (P = 0.6). The difference was also specific for HIV infection, as proliferation induced by PPD or PHA was similar in PBMC and LNMC obtained from controls (PPD, P = 0.4; PHA, P = 1.0).
Fig. 1.
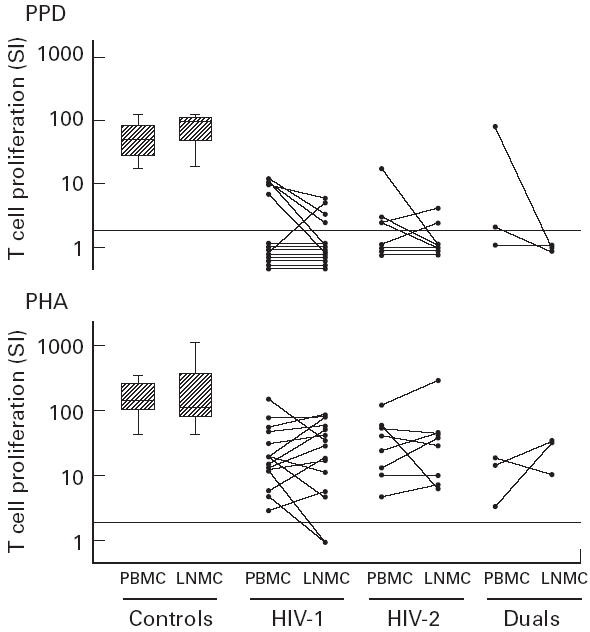
T cell proliferative response to phytohaemagglutinin (PHA) and purified protein derivative (PPD) of peripheral blood mononuclear cells (PBMC) and lymph node mononuclear cells (LNMC) of HIV-infected patients and controls. Box plot shows the median, 25th and 75th percentiles of the controls (27 PBMC and five LNMC). Whiskers represent 10th and 90th percentiles. Dotted line represents cut-off for proliferative response. Proliferative response was measured by thymidine incorporation on day 3 for PHA cultures and on day 7 for PPD cultures.
Both proliferative and proviral load data were available from 12 HIV-1, eight HIV-2 and three dually infected patients. In the six patients (four HIV-1 and two HIV-2) who showed a SI > 2.5 to PPD in LNMC, the geometric mean proviral load was significantly lower than in the other 17 patients who showed a SI < 2.5 (difference in means after adjusting for HIV type = 1.1 log10, 95% confidence interval (CI) = 0.39, 1.78; P = 0.004). In the 11 patients (five HIV-1, four HIV-2 and two dually infected) who had a SI > 2.5 to PPD in the PBMC compartment, the proviral load was also lower than in the 12 non-responders, although this was not statistically significant (difference in means after adjusting for HIV type = 0.43 log10, 95% CI = −0.23, 1.1; P = 0.18).
IFN-γ production in response to PPD
In order to characterize further the response of PBMC and LNMC to PPD, we also measured the production of IFN-γ in all subjects who had proliferative data (Fig. 2). A measurable amount of IFN-γ (≥ 1 U/ml) was produced by the PBMC of all 27 controls compared with only 13 of 23 HIV-infected patients (P = 0.0001). However, the IFN-γ response was significantly higher in the 13 HIV-infected patients with measurable response than in the controls (geometric difference in means = 3.6, 95% CI = 1.5, 8.8; P = 0.007). The five patients who produced high levels had a mean CD4 % of 21.40 (95% CI 13.3, 29.6), whereas the 10 patients who failed to produce had a mean CD4 % of 11.0% (95% CI 4.5, 17.4; P = 0.04). With regard to IFN-γ production in the LNMC, this was measurable in all five controls compared with only six of 23 HIV-infected patients (P = 0.005). IFN-γ response among the six HIV-infected patients with a measurable response was not significantly different from that in the controls (geometric difference in means = 1.02, 95% CI = 0.301, 3.64; P = 0.9). Thus the data show that in HIV-infected subjects, IFN-γ production induced by PPD in PBMC showed a bimodal distribution. As was observed for proliferative responses, IFN-γ production among those who responded was significantly higher in the PBMC than in the LNMC (geometric mean ratio 19.6 (95% CI = 4.7, 81.1), n = 14, P = 0.0006). A similar trend, but of lower magnitude, was observed in the control cultures (geometric mean ratio 1.9 (95% CI = 0.94, 4.0), P = 0.08).
Fig. 2.
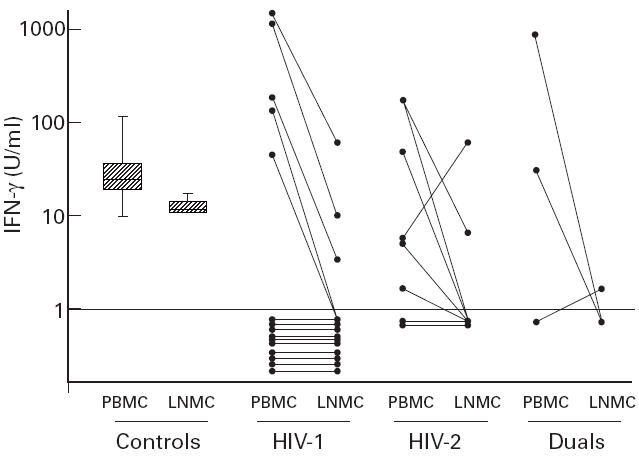
Production of IFN-γ from peripheral blood mononuclear cells (PBMC) and lymph node mononuclear cells (LNMC) of HIV-infected patients and controls. Box plot shows the median, 25th and 75th percentiles of the controls (27 PBMC and five LNMC). Whiskers represent 10th and 90th percentiles. Dotted line represents cut-off for measurable IFN-γ production. IFN-γ was measured on day 6 in purified protein derivative (PPD)-stimulated culture supernatants.
In the six HIV-infected patients who produced a measurable amount of IFN-γ within the lymph node compartment, the geometric mean proviral load was significantly lower than in the other 17 non-responders (difference in means after adjusting for HIV type = 0.80 log10; 95% CI = 0.05, 1.56; P = 0.04). In the 13 patients with a measurable amount of IFN-γ in the peripheral blood compartment, the geometric mean proviral load was lower than in the 10 non-responders, although this was not statistically significant (difference in means after adjusting for HIV type = 0.27 log10; 95% CI = −0.44, 0.98; P = 0.44).
DISCUSSION
Our study shows that lymph nodes are a reservoir for HIV-2, as has been described for HIV-1, and that in both HIV-1- and HIV-2-infected patients the percentage of CD4+ lymphocytes is higher in lymph nodes than in peripheral blood. This finding is in agreement with other studies which show a higher proportion of CD4+ T cells in lymph nodes of HIV-1-infected patients and of baboons infected with HIV-2 [3,16]. The mechanisms underlying the relative increase of CD4+ in the lymph node compartment are not known, but could involve migration of CD4+ into these tissues [17–19]. Alternatively, CD4+ cells within lymph nodes may be less susceptible to HIV-induced depletion.
In our study, the percentage of CD4+ cells in the blood and lymph node was strongly correlated both in HIV-1 and HIV-2 infections, which indicates that measurement of percentage of CD4+ cells in the blood is a reflection of that in the lymph node. This correlation was not observed by Meylan et al. studying lymph node aspirates [3], which is less likely to be more representative of the whole lymph node cell population than the lymph node biopsies we used.
We found HIV proviral load (expressed either as copies per 1 × 105 mononuclear cells or as copies per 1 × 105 CD4+ cells) to be significantly higher in lymph node cells than in peripheral blood cells in both HIV-1 and HIV-2 infections. To our knowledge, this is the first report showing lymph node tropism of HIV-2. As has been described for HIV-1 [1–4], the tropism of HIV-2 for lymph nodes was observed in patients both at an early stage of disease when the CD4 count was normal and at later stages. However, unlike a previous study of HIV-1 [4], the relative amounts of provirus in peripheral blood and lymph node did not vary significantly with stage of disease. Either HIV-2 did not cause as much lymphoid destruction or, more likely, our numbers were too small to demonstrate an effect. The proviral load in peripheral blood and lymph node was strongly correlated both in HIV-1 and HIV-2 infections, indicating that measurements performed in the blood reflected the viral burden in the lymph node which was three to five times higher, and this is relevant for the monitoring of anti-retroviral therapies. The mechanism leading to the higher viral load in the lymphoid organs probably involves trapping of HIV by follicular dendritic cells and increased viral replication but could also be the result of sequestration of infected CD4+ lymphocytes [3,4]. Although CD4+ lymphocytes represent the most important reservoir for HIV, CD8+ lymphocytes can also be infected with HIV-1, especially in patients with advanced disease [20]. The relative contribution of CD4+ and CD8+ lymphocytes as reservoirs for HIV was not assessed in our population.
In order to evaluate the consequences of a higher viral burden on the function of lymph node CD4+ lymphocytes, we studied their response to the recall antigen PPD. CD4+ lymphocytes are the main source of IFN-γ during in vitro stimulation with soluble mycobacterial antigens [21]. We found that, despite a higher proportion of CD4+ lymphocytes, LNMC showed lower proliferation and lower IFN-γ production in response to PPD than PBMC in both HIV-1 and HIV-2 infections, and these responses were related to viral load in the lymph node compartment. In peripheral blood, the percentage CD4+ was an obvious determinant for those with low values to produce IFN-γ, whereas subjects with higher values often produced more than the controls, suggesting a state of hyperactivation. Together, these data suggest that the higher viral load in LNMC could result in a more pronounced defect in the function of CD4+ lymphocytes and could decrease the efficacy of lymph nodes to limit the spread of pathogens such as mycobacteria. Although the function of CD8+ lymphocytes was not assessed in this study, it is likely that the impaired CD4+ cell helper functions also affect the ability of these cells to induce cytotoxic T cell responses of CD8+ lymphocytes which decrease as HIV disease advances [22]. Our findings may also be important if patients treated with anti-retroviral drugs are then offered immunotherapy. Lymphocytes harbouring provirus may be stimulated to release infectious virus, which by infecting bystander cells may diminish the desired immune response.
Acknowledgments
We thank Dr Hazel Dockrell for her advice and guidance and Dr Steve Bickler, Dr Alexander Massen, Dr Lenrie Peters, Ebrima Sarr, Ken Joof, Bakary Sanneh and Elizabeth Harding for their participation in this study. This work was supported by the Medical Research Council and the European Union (INCO-DC Programme).
REFERENCES
- 1.Fox HC, Tenner-Racz K, Racz P, Firpo A, Pizzo PA, Fauci AS. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis. 1991;164:1051–7. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- 2.Graziosi C, Pantaleo G, Demarest JF, et al. HIV-1 infection in the lymphoid organs. AIDS. 1993;7(Suppl. 2):S53–58. doi: 10.1097/00002030-199311002-00012. [DOI] [PubMed] [Google Scholar]
- 3.Meylan ARP, Burgisser P, Werich-Suter C, Spertini F. Viral load and immunophenotype of cells obtained from lymph nodes by fine needle aspiration as compared with peripheral blood cells in HIV-infected patients. J AIDS Hum Retrovir. 1996;13:39–47. doi: 10.1097/00042560-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 5.Lafeuillade A, Tamalet C, Pellegrino P, et al. High viral burden in lymph nodes during early stages of HIV-1 infection. AIDS. 1993;11:1527–8. doi: 10.1097/00002030-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Tamalet C, Lafeuillade A, Yahi N, et al. Comparison of viral burden and phenotype of HIV-1 isolates from lymph nodes and blood. AIDS. 1994;8:1083–8. doi: 10.1097/00002030-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Simon F, Matheron S, Tamalet C, et al. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993;7:1411–7. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ariyoshi K, Berry N, Wilkins D, et al. A community-based study of human immunodeficiency virus type-2 proviral load in a rural village in West Africa. J Infect Dis. 1996;173:245–8. doi: 10.1093/infdis/173.1.245. [DOI] [PubMed] [Google Scholar]
- 9.Berry N, Ariyoshi K, Jobe O, et al. HIV type 2 proviral load measured by quantitative polymerase chain reaction correlates with CD4+ lymphopaenia in HIV type 2-infected individuals. AIDS Res Hum Retrovir. 1994;8:1031–7. doi: 10.1089/aid.1994.10.1031. [DOI] [PubMed] [Google Scholar]
- 10.Clifford Lane H, Depper JM, Greene WC, et al. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 11.Meyaard L, Sshuitemaker H, Miedema F. T-cell dysfunction in HIV infection: anergy due to defective antigen-presenting cell function. Immunol Today. 1993;14:161–4. doi: 10.1016/0167-5699(93)90279-T. [DOI] [PubMed] [Google Scholar]
- 12.Meroni L, Trabattoni D, Balotta C, et al. Evidence for type 2 cytokine production and lymphocyte activation in the early phases of HIV-1 infection. AIDS. 1996;10:23–30. doi: 10.1097/00002030-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Pepin J, Morgan G, Dunn D, et al. HIV-2-induced immunosuppression among asymptomatic West African prostitutes: evidence that HIV-2 is pathogenic but less than HIV-1. AIDS. 1991;5:1165–71. [PubMed] [Google Scholar]
- 14.Whittle H, Egboga A, Todd J, et al. Immunological responses of Gambians in relation to clinical stage of HIV-2 disease. Clin Exp Immunol. 1993;93:45–50. doi: 10.1111/j.1365-2249.1993.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabilan L, Andersson G, Lolli F, et al. Detection of intracellular expression and secretion of interferon-γ at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol. 1990;20:1085–90. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- 16.Barnett WS, Krishna KM, Herndier GB, Levy JA. An AIDS-like condition induced in Baboons by HIV-2. Science. 1994;266:642–6. doi: 10.1126/science.7939718. [DOI] [PubMed] [Google Scholar]
- 17.Meeusen NTE, Premier RR, Brandon RM. Tissue-specific migration of lymphocytes: a key role for TH1 and TH2 cells. Immunol Today. 1996;17:421–4. doi: 10.1016/0167-5699(96)10055-4. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg JY, Anderson OA, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking. Immunol Today. 1998;19:10–17. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 19.Westermann J, Geismar U, Sponholz A, et al. CD4+ T cells of both the naı¨ve and the memory phenotype enter rat lymph nodes and Peyer's patches via high endothelial venules: within the tissue their migratory behaviour differs. Eur J Immunol. 1997;27:3174–81. doi: 10.1002/eji.1830271214. [DOI] [PubMed] [Google Scholar]
- 20.Livingstone WJ, Moore M, Innes D, et al. Frequent infection with peripheral blood CD8-positive T-lymphocytes with HIV-1. Lancet. 1996;348:649–54. doi: 10.1016/s0140-6736(96)02091-0. [DOI] [PubMed] [Google Scholar]
- 21.Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette–Guerin. J Immunol. 1997;158:1949–55. [PubMed] [Google Scholar]
- 22.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]


