Abstract
The aim of this study was to investigate the significance of IgG subclasses and MBL for susceptibility to infection in association with IgA deficiency. The study population consisted of 139 apparently healthy adult blood donors with IgA deficiency and normal serum levels of IgG and IgM, and an increased susceptibility to infection demonstrated at a population level. Additionally, 216 controls matched for age and sex were investigated. IgG4 deficiency was more common and the mean level of IgG4 lower in persons with IgA deficiency than in the controls. No significant associations could be demonstrated between overt IgG subclass deficiencies and increased susceptibility to infection. However, when the mean concentrations of IgG subclasses were analysed with regard to medical history, that of IgG1 was lower in persons who reported recurrent viral respiratory infections, that of IgG3 in persons who had episodes of severe infection in their history, and that of IgG4 in persons who had recurrent mild respiratory infections, compared with those who had no particular history of infections. In contrast, MBL deficiency—alone or combined with that of the IgG subclass—was not associated with increased susceptibility to infection in persons with IgA deficiency. The results indicate that the proneness to infections observed in a population of otherwise healthy persons with IgA deficiency can only for a small part be accounted for by concomitant deficiencies of IgG subclasses. Contrary to expectations, no synergism between the deficiencies of IgA and MBL could be demonstrated.
Keywords: IgA, IgG subclasses, mannan-binding lectin, infection
INTRODUCTION
IgA deficiency is a common immunological defect. It is associated with increased susceptibility to infection [1,2]. However, some persons with IgA deficiency are asymptomatic [1]. It has been suggested that IgA deficiency must be associated with those of the IgG subclasses, especially of IgG2 and IgG4, to be manifested with clinical symptoms [3–6]. However, since not all persons with IgA deficiency and increased susceptibility to infection evince such a combined immunodeficiency, it must be assumed that other concomitant immunodefects can also render IgA deficiency clinically significant.
MBL is an acute-phase protein secreted by the liver. It binds to oligosaccharide structures on the surface of microbes and opsonizes them for the phagocytic cells either directly or by activating the complement lectin pathway [7]. MBL deficiency causes an opsonic defect which predisposes the host to infections [8]. However, in the majority of persons with MBL deficiency, increased susceptibility to infection cannot be demonstrated [9,10]. This suggests that other factors may be needed to render MBL deficiency significant. Recently, in paediatric patients with increased susceptibility to infection, MBL deficiency was found to be associated with IgG subclass deficiencies [11]. Furthermore, a connection between MBL and IgA deficiencies has been proposed [7,12], although no evidence to support such a concept has been published.
In this study, we investigated the significance of IgG subclasses and MBL in relation to susceptibility to infection in 139 apparently healthy adult blood donors with IgA deficiency, and an increased susceptibility to infection demonstrated on a population level [1].
SUBJECTS AND METHODS
Study subjects
The study population consisted of 139 persons with IgA deficiency (95 males and 44 females), aged 44 years (median; range 33–81 years). They had been screened from apparently heathy adult blood donors by double diffusion and haemagglutination inhibition methods in the Finnish Red Cross Blood Transfusion Service in the years 1971–80 [13]. In 1992, the concentration of IgA was re-determinated in fresh serum samples by an enzyme immunoassay (EIA) [14,15]. In all persons, the concentration of IgA was < 0.05 g/l, and the concentrations of IgG and IgM were normal.
Samples
The determinations were made on samples drawn in 1992. For control purposes, serum samples from 216 apparently healthy age- and sex-matched blood donors with normal serum concentrations of IgG, IgM and IgA were analysed. The samples were stored at −70°C until analysed.
Clinical data
In 1992, all persons with IgA deficiency completed a questionnaire on their medical history. The data were checked and completed by means of personal interview and examination of patient records or records of drug reimbursements paid under the National Sickness Insurance Scheme [1].
Definitions
On a population level, the study subjects have been proved to be significantly more susceptible to severe and recurrent respiratory infections than their age- and sex-matched controls [1]. Septicaemia, tuberculosis and pneumonia were classified as severe infections. Milder respiratory infections were defined as recurrent and bacterial if three or more episodes of acute bronchitis, tonsillitis, sinusitis, and/or otitis media requiring antibiotic therapy had occurred during each of 3 or more successive years. Tonsillectomy and adenoidectomy were also considered signs of recurrent mild bacterial respiratory infections. Three or more episodes of common cold, pharyngitis, laryngitis or bronchitis, not necessitating the use of antibiotics, during each of the 3 years before the study were regarded as recurrent viral respiratory infections [1].
Determinations
Serum concentrations of IgG subclasses were determined using a Behring nephelometer (Behringwerke AG, Marburg, Germany) according to manufacturer's instructions. Serum MBL concentration was determined by an EIA as previously described in detail [16]. The reference values for the parameters were: IgG1 4.9–11.4 g/l; IgG2 1.50–6.40 g/l; IgG3 0.20–1.10 g/l; IgG4 0.08–1.40 g/l; MBL ≥ 0.02 mg/l. Values below the reference interval were defined as indicative of deficiency.
Statistical analysis
Differences in the mean concentrations were compared by Student's t-test. Since not all parameters were normally distributed, square root transformations of values were used when appropriate. Differences in deficiency prevalences were compared using χ2 or Fisher's exact tests.
Ethics
The study plan was accepted by the ethical committees of the Finnish Red Cross Blood Transfusion Service and Tampere University Hospital.
RESULTS
The deficiency prevalences and the mean concentrations of IgG subclasses and MBL in persons with IgA deficiency and controls are shown in Table 1. Of 139 persons with IgA deficiency, 37 (27%) had IgG4 deficiency as opposed to only 17 (8%) of the 216 controls. Also the mean concentration of IgG4 in the study subjects was significantly lower than that in the controls. In contrast, the prevalences of IgG1 and IgG3 deficiencies in the study subjects were lower and the mean concentrations of IgG1, IgG2 and IgG3 higher than in the controls.
Table 1.
Deficiency prevalences and mean concentrations of IgG subclasses and MBL in persons with IgA deficiency and their age- and sex-matched controls
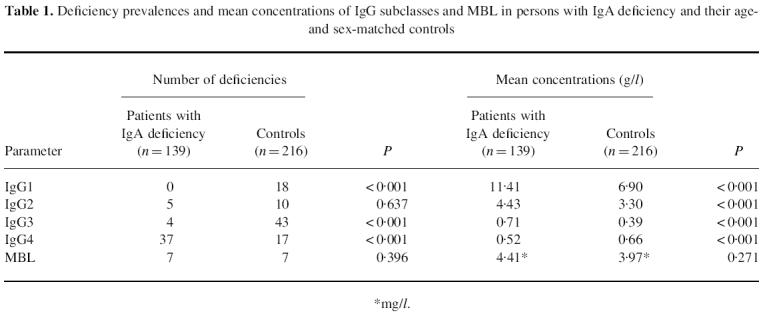
*mg/l.
Susceptibility to infection
The mean concentrations of IgG subclasses in persons with IgA deficiency, grouped according to their medical history in respect of infections, are illustrated in Fig. 1. The mean concentration of IgG1 was significantly lower in persons with a history of recurrent viral respiratory infections than in those without infections (10.59 g/l and 11.69 g/l, respectively; P = 0.034). IgG3 was lower in persons with a history of severe bacterial infections than in those without infections (0.58 g/l and 0.72 g/l, respectively; P = 0.059). Furthermore, in persons who reported recurrent mild bacterial respiratory infections, IgG4 was slightly lower than in those without infections (0.43 g/l and 0.64 g/l, respectively; P = 0.097). No differences between different subgroups were observed with regard to prevalence of IgG subclass deficiencies. With regard to MBL, neither the mean concentration nor the deficiency prevalence differed between subgroups of persons with IgA deficiency. Among the persons with combined deficiency of MBL and IgA, the prevalence of IgG subclass deficiency was not significantly higher in those with increased susceptibility to infection than in those without (3/5 and 0/2, respectively; P = 0.429)
Fig. 1.
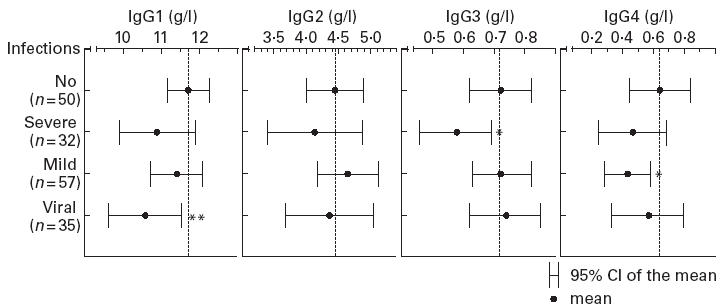
Mean concentrations of IgG subclasses in persons with IgA deficiency, grouped according to medical history with regard to infections. Of the total of 89 persons who had a history indicating increased susceptibility to infection, 27 belonged to two and four to three subgroups. The mean concentrations were compared by Student's t-test with those in persons who had no history of increased susceptibility to infection. No, Persons who had no history indicating increased susceptibility to infection; severe, persons who had had severe infections; mild, persons who had had mild recurrent bacterial respiratory infections; viral, persons who had had recurrent viral respiratory infections. **P < 0.05; *P < 0.10.
DISCUSSION
The prevalence of IgG4 deficiency was significantly higher and the mean concentration of IgG4 lower in persons with IgA deficiency than in controls. Although IgA deficiency has been found to be associated with IgG subclass deficiencies, the genetic backround of this connection is incompletely understood. However, it is likely that persons with IgA deficiency and those with common variable immunodeficiency (CVID) share an allelic condition with a variable expression of a common gene defect which may be involved in the regulation of immunoglobulin class switching [17]. Furthermore, the distribution of Gm allotypes in persons with IgA deficiency differs from that in those without [18].
The prevalences of IgG1 and IgG3 deficiencies were lower and the mean concentrations of IgG1, IgG2 and IgG3 higher in persons with IgA deficiency than in controls. These findings are in concordance with the results of earlier studies [3,5,18,19]. IgA is the first-line defence mechanism on mucous membranes and its deficiency causes an immune defect for which IgG tends to compensate.
Among the persons with IgA deficiency, the mean concentration of IgG1 was significantly lower in those who had a history of recurrent viral respiratory infections than in those without infections. It is known that IgG antibodies against viral antigens are mainly of IgG1 subclass [20]. It appears that even a minor defect in IgG1-mediated immune response may be sufficient to predispose an IgA-deficient host to viral respiratory infections.
IgG3 was lower in persons with IgA deficiency who had a history of severe infections compared with those without infections. Among cases with such a history, pneumonia was the principal diagnosis, accompanied by two cases with lung tuberculosis and one with septicaemia. This finding is in concordance with that of Björkander et al., who observed that low IgG3 in persons with IgA deficiency and repeated respiratory infections is associated with impaired lung function suggestive of severe pulmonary damage [4].
IgG4 deficiency was not straightforwardly associated with increased susceptibility to infection in persons with IgA deficiency. Also, the mean concentration of IgG4 was only slightly lower in those who had recurrent mild bacterial respiratory infections than in those without infections. French et al. [6] have suggested that IgG4 deficiency in conjunction with specific defects in antibody response to polysaccharide antigens might be an essential risk factor for severe respiratory infections in persons with IgA deficiency. However, in that study the subjects were selected based on their infectious morbidity. Hence, the clinical significance of a combined IgA and IgG4 deficiency may have been overestimated. Also, the current study population has been proved to be significantly more susceptible to severe and recurrent respiratory infections than the age- and sex-matched controls [1]. However, owing to the selection of the cases among apparently healthy blood donors, extreme cases with a continuing susceptibility to severe infections were likely to be excluded. Probably for the same reason, the previously observed connection between the deficiencies of IgA and IgG2 was not seen in our study [3–6].
The mildly decreased levels of IgG4 in IgA-deficient persons with recurrent mild bacterial respiratory infections may be accompanied by specific, uncompensated defects in IgG-mediated antibody response as the actual cofactors for IgA. Such a concept is supported by results published by Lane et al. [21], who observed that specific defects in IgG-mediated antibody response may cause a clinically significant immune defect in persons with IgA deficiency, although the IgG subclass concentrations remain at the normal level.
The deficiency of MBL—alone or combined with that of the IgG subclass—did not seem to have an effect on susceptibility to infection in persons with IgA deficiency. In an evolutionary perspective, MBL is an early defence mechanism subsequently secured by more specific mechanisms such as antibody response [7]. Furthermore, MBL acts mainly in the circulation, while IgA is secreted on mucous membranes. There may not exist such a synergism between these two factors as has been proposed [7,12] and recently observed between MBL and IgG [11].
In conclusion, although IgG4 deficiency was associated with IgA deficiency, it did not affect susceptibility to infection among otherwise healthy blood donors. On the other hand, decreased concentrations of IgG1 and IgG3 in conjunction with IgA deficiency were associated with proneness to viral respiratory infections and severe bacterial infections, respectively, on a population level. The results indicate that the proneness to infections in the current population consisting of otherwise healthy persons with IgA deficiency can only for a small part be accounted for by concomitant deficiencies of IgG subclasses. Furthermore, no synergism could be demostrated between the deficiencies of IgA and MBL. In future studies on susceptibility to infection in persons with IgA deficiency, specific defects in antibody response as well as immune mechanisms affecting mucosal defence should be addressed.
Acknowledgments
The valuable discussions with Professor Pekka Jousilahti (Department of Epidemiology, Tampere School of Public Health, University of Tampere, Tampere, Finland) are gratefully acknowledged. This study was supported by a grant from the Medical Research Fund of Tampere University Hospital, Tampere, Finland.
REFERENCES
- 1.Koskinen S. Long-term follow-up of health in blood donors with primary selective IgA deficiency. J Clin Immunol. 1996;16:165–70. doi: 10.1007/BF01540915. [DOI] [PubMed] [Google Scholar]
- 2.Burrows PD, Cooper MD. IgA deficiency. Adv Immunol. 1997;65:245–76. [PubMed] [Google Scholar]
- 3.Oxelius V-A, Laurell A-B, Lindquist B, Colebiowska H, Axelsson U, Björkander J, Hanson LÅ. IgG subclasses in selective IgA deficiency; importance of IgG2-IgA deficiency. N Engl J Med. 1981;304:1476–7. doi: 10.1056/NEJM198106113042408. [DOI] [PubMed] [Google Scholar]
- 4.Björkander J, Bake B, Oxelius VA, Hanson LA. Impaired lung function in patients with IgA deficiency and low levels of IgG2 or IgG3. N Engl J Med. 1985;313:720–4. doi: 10.1056/NEJM198509193131203. [DOI] [PubMed] [Google Scholar]
- 5.French MA, Harrison G. An investigation into the effect of the IgG antibody system on the susceptibility of IgA deficient patients to respiratory tract infections. Clin Exp Immunol. 1986;66:640–7. [PMC free article] [PubMed] [Google Scholar]
- 6.French MAH, Denis KA, Dawkins R, Peter JB. Severity of infections in IgA deficiency: correlation with decreased serum antibodies to pneumococcal polysaccharides and decreased serum IgG2 and/or IgG4. Clin Exp Immunol. 1995;100:47–53. doi: 10.1111/j.1365-2249.1995.tb03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 8.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;ii:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 9.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–3. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 10.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Brit Med J. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aittoniemi J, Baer M, Soppi E, Vesikari T, Miettinen A. Mannan binding lectin deficiency and concomitant immunodefects. Arch Dis Child. 1998;78:245–8. doi: 10.1136/adc.78.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner MW. Deficiency of mannan binding protein—a new complement deficiency syndrome. Clin Exp Immunol. 1991;86(Suppl. 1):53–56. doi: 10.1111/j.1365-2249.1991.tb06208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koistinen J. Selective IgA deficiency in blood donors. Vox Sang. 1975;29:192–202. doi: 10.1111/j.1423-0410.1975.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirvonen M, Koskinen S, Tölö H. A sensitive enzyme immunoassay for the measurement of low concentrations of IgA. J Immunol Methods. 1993;163:59–65. doi: 10.1016/0022-1759(93)90239-4. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen S, Tölö H, Hirvonen M, Koistinen J. Long-term persistence of selective IgA deficiency in healthy adults. J Clin Immunol. 1994;14:116–9. doi: 10.1007/BF01541344. [DOI] [PubMed] [Google Scholar]
- 16.Aittoniemi J, Miettinen A, Laippala P, Isolauri E, Viikari J, Ruuska T, Soppi E. Age-dependent variation in the serum concentration of mannan-binding protein. Acta Pædiatr. 1996;85:906–9. doi: 10.1111/j.1651-2227.1996.tb14182.x. [DOI] [PubMed] [Google Scholar]
- 17.Truedsson L, Baskin B, Pan Q, Rabbani H, Vorechovský I, Smith CIE, Hammarström L. Genetics of IgA deficiency. APMIS. 1995;103:833–42. doi: 10.1111/j.1699-0463.1995.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 18.Oxelius VA, Carlsson AM, Hammarström L, Björkander J, Hanson LÅ. Linkage of IgA deficiency to Gm allotypes; the influence of Gm allotypes on IgA-IgG subclass deficiency. Clin Exp Immunol. 1995;99:211–5. doi: 10.1111/j.1365-2249.1995.tb05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberton DM, Björkander J, Henrichsen J, Söderström T, Hanson LA. Enhanced IgG1 and IgG3 responses to pneumococcal polysaccharides in isolated IgA deficiency. Clin Exp Immunol. 1989;75:201–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Schur PH. IgG subclasses—a review. Ann Allergy. 1987;58:89–96. [PubMed] [Google Scholar]
- 21.Lane PJ, MacLennan IC. Impaired IgG2 anti-pneumococcal antibody responses in patients with recurrent infection and normal IgG2 levels but no IgA. Clin Exp Immunol. 1986;65:427–33. [PMC free article] [PubMed] [Google Scholar]


