Abstract
In order to study the mechanism of abortion, the proportions of NK cells in the peripheral blood and decidual lymphocytes were evaluated in both chromosomally normal and abnormal missed abortions. In normal pregnancy, CD56+16−3−NK cells are a major element of decidual lymphocytes. The percentages of CD56+16−3−NK cells of peripheral lymphocytes in normal pregnancies were not statistically significantly different from those of chromosomally normal and abnormal abortions. In the decidua, the percentages of CD56+16−3− NK cells of decidual lymphocytes showed no statistically significant differences between normal pregnancies and chromosomally abnormal abortions. However, the percentages of CD56+16−3−NK cells of chromosomally normal abortions were lower than those of chromosomally abnormal (P = 0.0025). Moreover, the percentages of CD56+16− NK cells in abortions with normal chromosomes were lower than those in normal pregnancies or abortions with abnormal chromosomes (P = 0.0037, P = 0.0025). However, when the proportion of CD56+NK cells expressing CD16 was evaluated, there were no statistically significant differences in the percentages of CD56+16+ NK cells in normal pregnancies and missed abortions with normal chromosomes and abnormal chromosomes. We conclude that the expression of decidual CD56+16−3− NK cells in missed abortions with normal chromosomes is different from abortions with abnormal chromosomes and that this phenomenon may depend on an abnormal immune response of the maternal side.
Keywords: natural killer cell, pregnancy, spontaneous abortion, chromosomal abnormality, reproduction
INTRODUCTION
The causes of spontaneous abortion may be abnormal development of the zygote and/or maternal factors, one being due to chromosomal anomalies. In humans, 50–60% of early spontaneous abortions are associated with a chromosomal anomaly of the conceptus as reported in several studies [1]. As reported, maternal factors for spontaneous abortion include uterine abnormalities, infection, abnormal antibodies for blood types, endocrine defects, chemicals and immunological factors [2].
The reasons for abortion with normal chromosomes are difficult to determine, although the following are possibilities; a genetic abnormality such as an isolated mutation or polygenic factors, maternal factors and paternal factors. The maternal immune response in abortion with normal chromosomes is not yet well known. It was reported that NK cells may have a homeostatic role in reproduction and the control of placentation [3,4]. A large proportion of decidual lymphocytes are NK cells (CD56+16−3−) which express the cell surface marker CD56 but do not express CD16 and CD3 [5]. NK cells appear to display deleterious effects on fetal development resulting in spontaneous abortions [6–9]. However, CD56+16− NK cells secrete various cytokines, including macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), which are thought to promote placental growth. It appears that NK cells may play an important role in maintaining pregnancy [10,11]. To investigate the mechanism of abortion when the fetal chromosomes are normal, the proportions of NK cells in the peripheral blood and decidual lymphocytes were evaluated by flow cytometry.
MATERIALS AND METHODS
Materials
Maternal peripheral blood samples, villi and decidua were taken from 28 cases of missed abortion and 15 cases of normal pregnancy from weeks 5 to 11 of gestation. All women gave informed consent. Missed abortions were diagnosed by using vaginal ultrasound. The term missed abortion referred to the prolonged retention of a fetus that died during the first trimester of pregnancy. The samples obtained from women undergoing termination of normal pregnancies and therapeutic extraction of missed abortions were taken by dilatation and curettage (D&C) in our institution within 1 week after the death of the fetus was confirmed. In the control samples (normal women), it was confirmed that the development of the fetuses was within normal limits by measuring gestational sac and crown-rump length and that the chromosomes from their villi were normal.
In the study cases, no uterine abnormalities, infections, abnormal antibodies involving blood types or autoantibodies such as anti-cardiolipin antibody were found.
Methods
Chromosomal analysis
Chorionic villi were sampled for cytogenetic analysis. Primary cultures were established using Chang's medium. From cultured specimens, cells were harvested and trypsin–Giemsa-banded, and 40 metaphases for each tissue were analysed. The tissues were classified as mosaic if 20% of the cells had an identical karyotype differing from the rest of the cells analysed from the same specimen.
Isolation of decidual lymphocytes and peripheral blood lymphocytes
Decidual cell suspensions were prepared from decidual tissue by a modification of Petrovic's method [12]. Fragments of decidua were washed with PBS and the maternal blood removed, then decidual tissues were identified macroscopically and minced with scissors. Tissues were incubated at 37°C in 5% CO2 with continuous agitation in RPMI 1640 medium (Gibco, Grand Island, NY) containing 0.4% trypsin (Sigma, St Louis, MO). After 90 min, the cell suspension was filtered through a 50-μm nylon mesh (Kurabou, Tokyo Japan), washed three times with RPMI 1640 by centrifuging at 400 g. The cell pellet was resuspended in RPMI 1640 containing 10% fetal calf serum (FCS; Gibco). The resuspended cells were layered onto Lymphoprep (Nycomed Pharma AS, Oslo, Norway) solution and centrifuged for 30 min at 400 g. Cells aspirated from the interface were washed three times with PBS, adjusted to a concentration of 1 × 107 cells/ml in RPMI. Cell viability was confirmed to be usually > 95% by trypan blue dying. Peripheral blood lymphocytes were also separated using Lymphoprep.
Antibody labelling
Peripheral blood (100 μl) and decidual lymphocytes (1 × 106 cells/100 μl) were labelled with 20 μl of FITC-conjugated anti-CD16 MoAb (clone 3G8; Pharmingen, San Diego, CA), PE-conjugated anti-CD56 antibody (clone B159; Pharmingen) and PerCP-conjugated anti-CD3 antibody (clone SK7; Becton Dickinson, San Jose, CA).
Flow cytometry
Antibody-labelled cells were analysed by flow cytometry using FACS Caliber (Becton Dickinson). Three-colour flow cytometry utilized an argon laser with 15 mW at 488 nm excitation. The first gates were set around the lymphocytes on forward scatter and side scatter dot plots to exclude other cells from analysis. Then, crossovers of FL1, FL2 and FL3 signals were adjusted by compensation using FITC-, PE- or PerCP-conjugated antibodies. The second gate was set around CD3− lymphocytes. Results with all other antibodies were expressed as the percentage of these gated lymphocytes. For each experiment, 10 000 lymphocytes were evaluated. The percentages of antibody-positive cells were quantified using CELL Quest (Becton Dickinson), counting CD56+dim cells as CD56+.
Statistical analysis
Student's t-test and Mann–Whitney U-test were used to determine the levels of significance of differences. Differences at P < 0.05 were considered statistically significant.
RESULTS
Chromosomal analysis
Seventeen cases of the 28 missed abortions had abnormal chromosomes, while all of the 15 normal pregnancy cases had normal chromosomes. Distribution of abnormal chromosomes in missed abortion cases is shown in Table 1. The median and range of the maternal age and gestation of normal control and missed abortion with normal chromosomes and abnormal chromosomes were 29 years (range 18–41 years) and 7 weeks (6–10 weeks), 33 years (23–41 years) and 9 weeks (6–12 weeks), and 30 years (21–40 years) and 9 weeks (7–11 weeks), respectively. There were no statistically significant differences in the three groups.
Table 1.
Distribution of chromosomal abnormalities in missed abortion cases
Flow cytometry analysis
NK cells consist of CD56+16+, CD56+16−, CD56+16−3− and other NK cell types. First, the changes of total CD56+ NK cells were analysed, followed by analysis of the changes of CD56+16−, which have been previously reported in normal pregnancy and abortion. Finally, using three-colour flow cytometry, CD56+16−3− NK cells, which were a large proportion of the decidual lymphocytes, were analysed.
The percentages of CD56+ NK cells were 54.6% (31.2–73.3%) in the decidua lymphocytes taken from normal pregnant women. The CD56+ NK cells were the largest proportion of the decidual lymphocytes. When the percentages of decidual CD56+ cells were evaluated in missed abortions, the percentages of CD56+ cells of decidual lymphocytes in abortions with normal chromosome and abnormal chromosome were 31.0% (range 6.2–72.6%) and 54.3% (30.7–82.4%), respectively. The percentages of CD56+ cells in abortions with normal chromosomes were lower than those in normal pregnancies or abortions with abnormal chromosomes (P = 0.004, P = 0.003).
Moreover, when the percentages of decidual CD56+16− NK cells were evaluated in missed abortions and normal pregnancies, the percentages of CD56+16− NK cells of decidual lympho-cytes in normal pregnancies and abortions with abnormal chromosomes were 48.9% (median) (range 21.7–68.1%) and 49.5% (22.7–78.9%), respectively, both without any statistically significant difference. However, the percentages of CD56+16− NK cells in abortions with normal chromosomes were 24.0% (3.4–53.8%), lower than those in normal pregnancies or abortions with abnormal chromosomes (P = 0.0064, P = 0.009).
We also determined the proportion of CD56+ NK cells expressing CD16. The percentages of CD56+16+ NK cells in normal pregnancies and missed abortions with normal chromosomes and abnormal chromosomes were 7.9% (1.5–17.8%), 2.8% (1.4–18.8%) and 4.7% (1.0–30.4%), respectively, without any statistically significant difference.
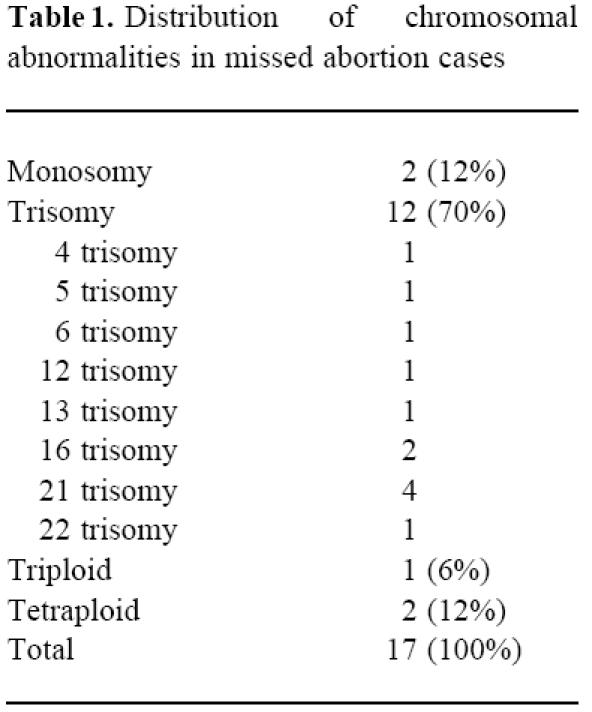
When the percentages of decidual CD56+16−3− NK cells in missed abortions and normal pregnancies were evaluated, the percentages of CD56+16−3− NK cells of decidual lymphocytes in normal pregnancies and abortions with abnormal chromosomes were 41.3% (median) (range 22.0–65.3%) and 46.4% (23.2–84.0%), respectively, both without any statistically significant difference. However, the percentages of CD56+16−3− NK cells in abortions with normal chromosomes were 22.45 (2.8–52%), lower than those in abortions with abnormal chromosomes or normal pregnancies (P = 0.0025, P = 0.0065)(Fig. 1). When we looked for a correlation between cell numbers and gestational age, no correlation was found (r = 0.228, P = 0.14).
Fig. 1.
Percentages of CD56+16−3− NK cells in decidual lymphocytes taken from chromosomally normal, abnormal missed abortions and normal pregnancies. The bars indicate median values. P = 0.0025: missed abortion chromosomally normal versus chromosomally abnormal. P = 0.0065: missed abortion chromosomally normal versus normal pregnancies. P = 0.2: chromosomally abnormal versus normal pregnancies (Mann–Whitney U-test). The lymphocytes were isolated from the decidua and stained with FITC–anti-CD16, PE–anti-CD56 and PerCP–anti-CD3 MoAb. The first gate was set around the lymphocytes. The second gate was set around CD3− lymphocytes. Ten thousand lymphocytes were analysed on a FACS Caliber. Results with all antibodies were expressed as the percentages of these gated lymphocytes. CD56+dim cells were counted as CD56+.
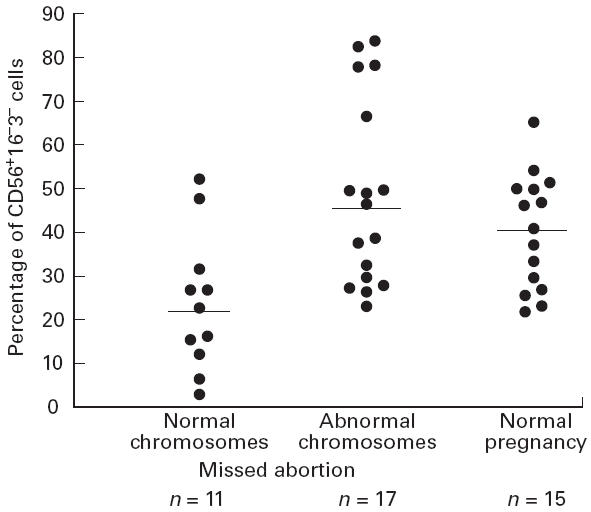
By the dot plot analysis of decidual lymphocytes, a large number of CD56+16−3− NK cells of missed abortions with abnormal chromosomes were found (left panel of Fig. 2). Decreased numbers of CD56−16−3− NK cells of a missed abortion case with normal chromosomes are found (right panel of Fig. 2).
Fig. 2.
Dot plot analysis of decidual lymphocytes. FL1 (CD16) and FL2 (CD56) were gated with CD3− cells. A large number of CD56+16−3− cells of missed abortions with abnormal chromosomes are found in the left panel. Decreased number of CD56+16−3− NK cells in missed abortions with normal chromosomes are found in the right panel. A large proportion of the NK cells in this group were still CD56+ (CD56+dim), but they were less bright than in the group with abnormal chromosomes. The lymphocytes were isolated from the decidua and stained with FITC–anti-CD16, PE–anti-CD56 and PerCP–anti-CD3 MoAb. The first gate was set around the lymphocytes. The second gate was set around CD3− lymphocytes. Ten thousand lymphocytes were analysed on a FACS Caliber. Results with all antibodies were expressed as the percentage of these gated cells. CD56+dim cells were counted as CD56+.
A large proportion of the NK cells in this group were still CD56+ (CD56+dim), but they were less bright than in the group with abnormal chromosomes.
We checked the peripheral lymphocytes, and found the percentages of CD56+16−3− NK cells in normal pregnancies and abortions with normal and abnormal chromosomes were 0.57% (0.14–3.79%), 1.26% (0.35–10.5%) and 1.6% (0.42–4.81%), respectively, with no statistically significant difference. No differences were found in the percentages of CD56+, CD56+16− and CD56+16+ cells in those groups.
DISCUSSION
The most abundant cell type of decidual lymphocytes in early normal pregnancy were CD56+16−3− NK cells. We evaluated the percentages of decidual CD56+16−3− NK cells in missed abortions and normal pregnancy controls. No significant differences in the percentages of these cell populations were found. However, when the cases were divided into abnormal and normal chromosomes in missed abortion, the percentages of decidual CD56+16−3− NK cells of normal chromosomal cases were significantly lower than those of abnormal chromosomal cases and normal pregnancies (Fig. 1). These changes were not found in maternal peripheral blood lymphocytes.
NK cells appear to display deleterious effects on fetal development, resulting in spontaneous abortion in mice and humans. Gendron & Baines reported that the infiltration of NK cells into the decidua is associated with spontaneous abortion in mice [6]. Yokoyama reported the presence of cytotoxic cells directed against placental cells in human habitual abortions by an in vitro terminal labelling assay [9]. However, we could not detect higher percentages of CD56+, CD56+16−, CD56+16+ and CD56+16−3− cells in our missed abortion cases. Maruyama et al. reported no significant differences were found between normal pregnancy and spontaneous abortion in the percentages of CD3−CD16+ and/or CD56+ (NK cells) in the decidua [13]. Chao et al. also reported decidual NK cells (CD16+ or CD56+) were increased to approximately the same extent in normal pregnancy and abortion (anembryonic pregnancy) [14].
As CD16+ cells trigger NK cell antibody-dependent cellular cytotoxicity and CD16+ cells consistently exhibit higher cytolytic activity than CD16− NK cells, this increased proportion of CD56+16+ cells may be involved in the demise of the conceptus, either directly or indirectly when exposed to antibody-coated fetal-derived cells [15]. However, increased percentages of CD56+16+ cells were not found in missed abortions with normal chromosomes.
It has been reported that NK cells may play an important role in maintaining pregnancy. Michael et al. reported that the number of cells with large granules decreased in the decidua from women with missed abortions, although it is not clear whether these cells originated from CD56+16− NK cells [16]. Wegman suggested CD56+16− NK cells secrete various cytokines, including M-CSF and GM-CSF, which are thought to promote placental growth [11]. Saito et al. also demonstrated that CD56+16− NK cells secrete cytokines such as GM-CSF, M-CSF and leukaemia inhibitory factor (LIF) [10]. The result of lower percentages of CD56+16−3− NK cells in chromosomally normal missed abortion cases may indicate an inappropriate development of decidual NK cells. Lachapelle reported that the decrease of CD56bright16− NK cells was observed in the non-pregnant endometrium in recurrent aborters and that this may be associated with an altered cytokine expression profile and a failure to provide the conceptus with the proper growth environment [17].
We conclude that the expression of decidual CD56+16−3− NK cells in missed abortion with normal chromosomes is different from that of abortions with abnormal chromosomes, and that it may depend on an abnormal immune response in the maternal tissues.
REFERENCES
- 1.Cunningam FG, MacDonald PC, Gant NF, et al. Abortion: Reproductive success and failure. In: Cunningam FG, MacDonald PC, editors. Williams obstetrics. 20. Tokyo: Prentice Hall; 1997. pp. 579–605. [Google Scholar]
- 2.Stray-Pederson B, Stray-Pederson S. Etiologic factors and subsequent reproductive performance in 195 couples with a prior history of habitual abortion. Am J Obstet Gynecol. 1984;148:140–6. doi: 10.1016/s0002-9378(84)80164-7. [DOI] [PubMed] [Google Scholar]
- 3.King A, Loke YW, Chaouat G. NK cells and reproduction. Immunol Today. 1997;18:64–66. doi: 10.1016/s0167-5699(97)01001-3. [DOI] [PubMed] [Google Scholar]
- 4.King A, Hiby SE, Verma S, Burrows T, Gardner L, Loke YW. Uterine NK cells and trophoblast HLA Class 1 molecules. Am J Reprod Immunol. 1997;37:459–62. doi: 10.1111/j.1600-0897.1997.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 5.Starkey PM, Sargent IL, Redman CWG. Cell population in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988;65:129–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Gendron RL, Baines MG. Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell Immunol. 1988;113:261–7. doi: 10.1016/0008-8749(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 7.Makita R, Minami M, Takamizawa M, et al. NK cell activity and immunotherapy for recurrent spontaneous abortion. Lancet. 1991;338:579. doi: 10.1016/0140-6736(91)91153-l. [DOI] [PubMed] [Google Scholar]
- 8.Aoki K, Kajiura S, Matsumoto Y, et al. Preconceptional natural killer cell activity as a predictor of miscarriage. Lancet. 1995;345:1340–2. doi: 10.1016/s0140-6736(95)92539-2. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama M. Cytotoxic cells directed against placental cells in human habitual abortions by an in vitro terminal labeling assay. Am J Reprod Immunol. 1994;31:197–204. doi: 10.1111/j.1600-0897.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Nishikawa K, Morii T, et al. Cytokine production by CD16−CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol. 1993;5:559–63. doi: 10.1093/intimm/5.5.559. [DOI] [PubMed] [Google Scholar]
- 11.Wegman TG. Placental immunotrophism: maternal T cells enhance placental growth and function. Am J Reprod Immunol Microbiol. 1987;15:67–69. doi: 10.1111/j.1600-0897.1987.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 12.Petrovic O, Gudelj L, Rubesa G, et al. Decidual–trophoblast interaction: decidual lymphoid cell function in normal, anembryonic, missed abortion and ectopic human pregnancy. J Reprod Immunol. 1994;26:217–31. doi: 10.1016/0165-0378(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama T, Makino T, Sugi T, et al. Flow-cytometric analysis of immune cell populations in human decidua from various types of first trimester pregnancy. Hum Immunol. 1992;34:212. doi: 10.1016/0198-8859(92)90114-3. [DOI] [PubMed] [Google Scholar]
- 14.Chao KH, Yang YS, Ho HN, et al. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am J Reprod Immunol. 1995;34:274–80. doi: 10.1111/j.1600-0897.1995.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 16.Michael M, Underwood J, Clark DA, et al. Histologic and immunologic study of uterine biopsy tissue of women with incipient abortion. Am J Obstet Gynecol. 1989;161:409–14. doi: 10.1016/0002-9378(89)90533-4. [DOI] [PubMed] [Google Scholar]
- 17.Lachapelle MH. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. J Immunol. 1996;156:4027–34. [PubMed] [Google Scholar]


