Abstract
Bacteria or bacterial products may constitute important inducers of surface molecule expression on endothelial cells and leucocytes. This study was undertaken to determine the effects of the Salmonella typhimurium porins, LPS-S and LPS-R on the transendothelial migration of leucocytes through human umbilical vein endothelial cells (HUVEC). Treatment of the HUVEC with either porins or LPS-S or LPS-R increased the transmigration of different leucocyte populations, in particular that of neutrophils. The maximal increase occurred using LPS-S treatment, whereas porin stimulation fell between LPS-S and LPS-R. The transmigration increase was dose-dependent and reached its maximum at about 100–1000 ng/ml of stimulus. Optimal endothelial activation occurred after 2–4 h and 4–6 h using LPS and porin, respectively. Stimulation of leucocytes with either porins or LPS slightly increased their transmigration through non-activated endothelial cells. Transmigration increased remarkably during the simultaneous stimulation of endothelial cells by IL-1ß together with either porins or LPS. To assess participation of E-selectin, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and leucocyte adhesion complex (CD11/18) in porin- or LPS-mediated leucocyte migration, blocking MoAbs were used. Each blocking MoAb partially and selectively decreased leucocyte transmigration. The obtained results contribute to clarify some aspects of the inflammatory process at sites of infection.
Keywords: transmigration, leucocytes, HUVEC, porins, lipopolysaccharide
INTRODUCTION
Leukocyte–endothelial cell interactions both in vivo and in vitro are active multistep processes [1], clearly demonstrated in studies of neutrophil interactions with inflamed venues [2,3]. The initial adhesion of circulating leucocytes to vascular endothelium is induced by interaction of constitutively functional leucocyte homing receptors with regulated endothelial cell ligands or counter receptors. A rapidly expanding body of data has revealed the structure, function and expression of endothelial cell surface molecules that support the adhesion and extravasation of leucocytes. During inflammation a dramatic increase of endothelial cell surface molecule expression occurs that supports the adhesion of blood leucocytes. Bacteria or bacterial products may constitute important inducers of surface molecule expression on endothelial cells. LPS and membrane proteins can frequently reach active concentrations at sites of infection whether directly secreted by bacteria [4] or derived from the lysis of the bacterial cell. In the presence of antibiotics the concentration of these compounds might be even greater. A number of recent studies have provided evidence that different antibiotics significantly vary in their capacity to mediate the release of LPS in in vitro cultures using Gram-negative organisms, but little is known regarding the induction of adhesion molecules by LPS [5,6]. LPS-S induce adhesion of leucocytes to endothelial cells as potent as that induced by IL-1ß; LPS-R and lipid A are less potent than the S form of LPS [7].
It has long been recognized that Salmonella typhimurium provokes an intense intestinal inflammatory response, consisting largely of neutrophil migration across the epithelial lining of the intestine [8]; this inflammatory event manifests itself as an epithelial dysfunction, namely, diarrhoea [8,9]. The mechanisms and cell types responsible for coordinating mucosal inflammatory responses to such pathogens remain obscure. In an in vitro model, McCormick et al. [10] showed that S. typhimurium transepithelial signalling to polymorphonuclear neutrophils (PMN) plays a direct and substantial role in stimulating enteritis in humans.
Among the outer membrane proteins of Gram-negative bacteria, S. typhimurium porins have been found in previous studies to affect several biological functions of cells involved in the immune response [11,12] as well as in inflammation [13,14]. Apart from their transport functions there are several reports of their ability to stimulate cytokine synthesis. Isolated porins from S. typhimurium [11,15], Yersinia enterocolitica [16] or Helicobacter pylori [17] have been shown to stimulate monocytes or lymphocytes to release a range of proinflammatory and immunomodulatory cytokines including IL-1, IL-4, IL-6, IL-8, tumour necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interferon-gamma (IFN-γ). Salmonella typhimurium porins induced a dose-dependent oedema into the paws of rats [14]; oedema was still present in animals which had had their complement levels depleted, demonstrating that inflammation was not associated with complement activation; however, it could be somehow decreased by indomethacin and was significantly reduced by dexametasone. Rat peritoneal cells incubated with porins [14] released histamine but small amounts of prostacyclin, suggesting that porins have little ability to induce the prostanoid-producing enzyme cyclooxygenase II.
The roles of outer membrane proteins (OMP) and porins in inflammatory and immunomodulatory responses have been recently reviewed by Henderson et al. [18]. Considering the porins' role in such responses and their ability to induce the release of adhesion molecules from endothelial cells [19], we have attempted to clarify the mechanisms by which S. typhimurium porins contribute to leucocytes' endothelial transmigration. We investigated the in vitro effects of porins, and compared them with those of LPS-S and LPS-R from S. typhimurium, on leucocyte migration through the endothelium using monolayers of cultured endothelial cells as a barrier to migration.
MATERIALS AND METHODS
Preparation of porins
A strain of S. typhimurium SH5014, kindly provided by Nurminen (National Public Health Institute, Helsinki, Finland) was used to extract and purify porins and LPS-R. A strain of S. typhimurium74 NCTC was used to extract LPS-S. Porins were isolated from the lysozyme-EDTA envelopes as described by Nurminen [20]. Briefly, 1 g of envelope was treated with 2% Triton X-100 in 0.01 m Tris–HCl (pH 7.5, containing 10 mm EDTA); after addition of trypsin (10 mg/g of envelope), the pellet was dissolved in SDS buffer (4% w/v SDS in 0.1 m sodium phosphate pH 7.2) and applied to a Ultragel ACA34 column equilibrated with 0.25% SDS buffer. The fraction containing proteins, identified by A280, was extensively dialysed and analysed SDS–PAGE in slabs by the method of Laemmli [21]. The molecular weights of the bands in the 34–36-kD region were measured by comparison with known molecular weight standards. Proteins were assayed by the method of Lowry et al. [22]. All possible traces of LPS were identified by gel-electrophoresis minislabs and staining with silver nitrate as described by Tsai & Frasch [23] and by the Limulus amoebocyte lysate assay, in endotoxin-free water using S. typhimurium SH5014 LPS-R as a control. In same experiments porins in the presence of polymixin B were used (Sigma, St Louis, MO) to neutralize the biological activity of traces of LPS; the porins were incubated with polymixin B at room temperature for 1 h in the ratio of 1:10 [24].
Preparation of LPS
LPS-R was isolated from S. typhimurium SH5014 with phenol/chloroform/ether as described by Galanos et al. [25]. Briefly, liquid phenol (90 g of dry phenol plus 11 ml of water–chloroform–petroleum ether in a volume ratio of 2:5:8) was added to 1 g of dried bacteria. After 2 min homogenization bacteria were centrifuged and extracted twice. The supernatant was filtered through filter paper and treated as described by Galanos et al. [25].
LPS-S was isolated from S. typhimurium 74 NCTC. Briefly, 1 g of dried bacteria in 12.5 ml of water was extracted by equal volume of 90% phenol. The aqueous layers were pooled and treated as described by Westphal et al. [26].
Isolation and culture of human vascular endothelial cells
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cord vein according to the method of Gimbrone [27]. The umbilical vein was cannulated, washed with PBS, perfused for 20 min with PBS containing collagenase (Sigma; 1 mg/ml) at 37°C in 5% CO2 and rinsed with PBS. HUVEC were collected and established as primary cultures in medium 199 (Seromed, Germany) containing 20% fetal calf serum (FCS; Seromed). Cells were serially passaged and maintained using M199 supplemented with 20% FCS and porcine intestinal heparin (50 μg/ml; Sigma) in tissue culture flasks with 0.1% gelatine. Passages 2–3 of HUVEC were used for experimental assay.
Leucocytes
Venous blood was drawn from 30- and 40-year-old healthy individuals into heparinized tubes. Buffy coat, diluted three-fold with PBS, was applied on Ficoll–Hypaque gradient (Monopoly Resolving Medium; Flow Labs, Irvine, UK) and centrifuged for 30 min at 300 g at 4°C. Mononuclear cells and neutrophils were then isolated by Ficoll–Hypaque gradient centrifugation, density of 1.077 (Biochrom), for 30 min at 300 g at 4°C. Neutrophils (95 ± 2% by May–Grünwald–Giemsa panoptic staining) obtained from the pellet of the Ficoll gradient were washed twice with PBS and resuspended in medium 199 supplemented with 1% of heat-inactivated FCS.
Mononuclear cells were washed twice with PBS, resuspended in medium 199 containing 10% heat-inactivated FCS and were then incubated in plastic trays (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) for 2 h at 37°C in 5% CO2–95% air. At least 97 ± 2% of the adherent cells were monocytes as determined by staining with α-naphthylbutyrate and uptake of colloidal carbon [28]; at least 95 ± 2 of the non-adherent cells were lymphocytes, as checked by flow cytofluorometry analysis (FACS IV; Becton Dickinson). Cells were > 98 ± 2% viable as determined by trypan blue dye exclusion (0.1% w/v in PBS).
Leucocyte migration across endothelial monolayer
HUVEC (2 × 105 endothelial cells per insert) from second subcultures were grown on fibronectin-coated inserts (3 μm pore size; 6.4 mm; Becton Dickinson, Bedford, MA) which were then placed in a 24-well plate until the monolayer was confluent. The integrity of the endothelial cell monolayer was verified before and after each experiment of leucocyte migration by phase-contrast microscopy and crystal violet staining [29]. The cultures were washed with medium and stimuli were added at various concentrations in a final volume of 1 ml for the indicated times. The cultures were washed twice with medium and each leucocyte population (1.5 × 106 cells/well) was added singly to the wells and allowed to migrate for the indicated times. Leucocyte migration was quantified by microscopical count in a haemocytometer [29]. Briefly, after incubation for the desired period of time, non-adherent cells in the upper chamber were removed by washing with three rapid changes of serum-free medium M199, and the lower surface of the filter was rinsed with 0.5 ml of ice-cold medium containing 5 mm EDTA to remove adherent transmigrated cells; leucocytes migrated and fallen into the lower chamber were combined with those detached from the filter, stained with 0.2% trypan blue and counted; the percentage of migration was calculated as follows: cells migrated/total cells added × 100.
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) assay on washing media was carried out according to the manufacturer's instructions (Boehringer Mannheim, Mannheim, Germany).
Assessment of the inhibition of leucocyte–HUVEC interaction
HUVEC were incubated with MoAbs against the various adhesion molecules before the transmigration assay. The MoAbs were prepared as Fab fragments with a commercial kit (Immuno Pure; Pierce, Rockford, IL). Endotoxin contamination was eliminated from the monoclonal reagents [30]. Monolayers of HUVEC were incubated with 15–25 pg/ml of MoAb fragments in M199 plus 5% human AB serum heat-inactivated for 45 min at 37°C. Leucocytes were incubated with 10–40 pg/ml of MoAb fragments in M199 plus 10% FCS heat-inactivated for 30 min at 56°C. Control HUVEC and leucocytes were not treated.
Blocking experiments
To block cytokine induction, MoAbs (0.5 μg/ml) anti-human IL-1α, anti-human IL-1ß and anti-human IL-6 were added to cell culture media immediately following bacterial component stimulation. The HUVEC were incubated for the usual length of time before transmigration was assayed.
MoAbs, cytokines and reagents
The MoAbs used in this study included: anti-E-selectin BMS 110 antibodies (clone CI26CI0B7 IgG2a; Bender MedSystems, Vienna, Austria); anti-intercellular adhesion molecule-1 (ICAM-1)/CD54 antibodies (clone 15.2, IgG1; Boehinger Mannheim Biochemical); anti-vascular cell adhesion molecule-1 (VCAM-1) antibodies (clone 1.G11B1, IgG; Prodotti Gianni, Milano, Italy); anti-CD11a antibodies (clone R7.1, IgG1; Bender MedSystems); anti-CD11b antibodies (clone M1/70 IgG2b; Pharmingen, San Diego, CA). As isotype-matched controls we used: anti-collagen (clone 1042, IgG2b; Boehinger Mannheim Biochemical); anti-heat shock protein 72/73 (clone W27, IgG2a; Boehinger Mannheim Biochemical); anti-HLA-DQ (clone BL-1a/DQ, IgG1; Boehinger Mannheim Biochemical). MoAbs anti-human TNF-α, anti-human IL-1α and anti-human IL-6 were obtained from Genzyme (Cambridge, MA). Human IL-1ß was purchased from Becton Dickinson Labware (Bedford, MA). Phenol, chloroform and ether were purchased from Sigma.
Statistical analysis
All data are presented as mean ± s.d. The statistical significance of differences between groups was determined using Student's paired t-test.
RESULTS
Porins and LPS preparations
The purification and contamination of porins by LPS have been addressed in previous studies [12–14]. The purity of the porin preparation, checked by SDS–PAGE, is shown in Fig. 1, lane B. SDS–PAGE revealed two bands with mol. wts of 34 kD and 36 kD.
Fig. 1.

SDS–PAGE analysis of porins and LPS preparations from Salmonella typhimurium. Lane A, molecular weight standards (phosphorylase b, 94 000; albumin, 67 000; ovalbumin, 43 000; carbonic anydrase, 30 000; trypsin inhibitor, 20 100; α-lactalbumina, 14 000). Lane B, SH5014 porins. Lane C, SH5014 LPS-R. Lane D, 74 NCTC LPS.
The Limulus test showed the presence of LPS at a concentration of 50 pg/μg of porins; these traces of LPS did not show any biological activity under our experimental conditions (data not shown).
The patterns of LPS-R and LPS-S preparations is revealed on SDS–PAGE by silver nitrate staining (Fig. 1, lanes C and D).
Neutrophils, lymphocytes and monocytes transmigration through HUVEC stimulated by either porins or LPS
To examine the role of porins in transendothelial migration of leucocyte purified subpopulations, we used an in vitro model consisting of HUVEC grown to confluence on human fibronectin-coated culture insert (6.4 mm diameter leucocyte permeable membrane with 3-mm pores). Previous studies have shown that in the absence of stimulus, only 3 ± 2% leucocytes added to these cultures penetrated the HUVEC monolayer. After treating the monolayer for 4 h with 5 U/ml IL-1ß, 30–50% of added leucocytes migrated across the endothelium, a finding in agreement with others [31]. Treatment of the monolayer with either LPS-S, LPS-R or porins induced a considerable increase of neutrophil, lymphocyte and monocyte migration across the endothelium. The dose–response curve is shown in Fig. 2. In the experiments presented HUVEC were incubated with the stimuli, which were removed before adding leucocytes. The percentage of neutrophil and monocyte migration across the endothelium treated with 100 ng/ml of porins was not significantly different from the percentage of neutrophil and monocyte migration through HUVEC stimulated with LPS-S (100 ng/ml), but was greater than the percentage migration across the LPS-R (100 ng/ml)-treated endothelium. The porin activation of lymphocytes overlapped that of LPS-R; furthermore the activation by porins or LPS-R was always weaker than the activation by LPS-S. Amongst the leucocytes, neutrophils showed a particular increase in transmigration across HUVEC stimulated by either porins or LPS. As a control of stimulation specificity, parallel experiments identical to test samples were run using 0.2% bovine serum albumin (BSA) instead of a stimulus. No effect was detectable (data not shown). Data in Fig. 3 present the kinetics of each leucocyte subpopulation transendothelial migration across HUVEC. LPS-S or LPS-R treatment reached maximum stimulatory effect after 2–4 h, whereas porin treatment induced maximum effect after about 4–6 h. LDH assays on washing media during stimulation of the HUVEC with the various agents did not reveal significant differences between controls and tests, suggesting that the treatment does not cause toxic effects in the HUVEC (data not shown). Treatment of the HUVEC monolayers with combinations of IL-1ß, LPS and porins did not affect the integrity of the monolayers. The transmigration of leucocytes, at the end of incubation, did not affect the integrity of the monolayers. The biological effect of the porin–polymixin B was shown to be practically the same as porins used on their own, therefore the following experiments were carried out using only the porins.
Fig. 2.
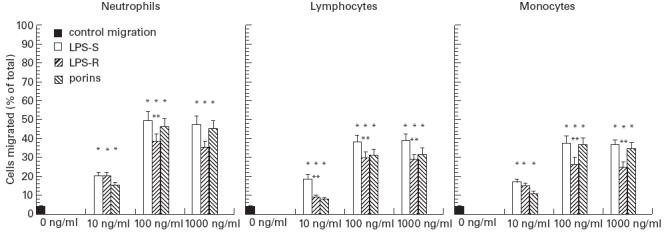
Porin-, LPS-S-, LPS-R-stimulated migration of leucocytes across human umbilical vein endothelial cells (HUVEC) cultured on human fibronectin-coated culture insert (6.4 mm diameter) in a dose-dependent manner. Monolayers of HUVEC were incubated with either medium (control) or LPS for 4 h or porins for 6 h and washed; leucocytes were then added and allowed to migrate for 2 h. Data represent the mean ± s.e.m. of three independent experiments. *P < 0.001 versus control migration; **P < 0.05 versus LPS-S-stimulated leucocytes.
Fig. 3.

Time course of porin-, LPS-S- and LPS-R-induced leucocyte transendothelial migration. Human umbilical vein endothelial cells (HUVEC) were incubated with either medium (control, ▴) or 100 ng/ml of stimulus for 2 h and at the indicated time periods leucocytes were added and allowed to migrate for 2 h. □, LPS-S; •, porins; ○, LPS-R. Data shown are mean ± s.e.m. of three independent experiments. (P < 0.001 comparison with control migration).
Blocking experiments of transmigration by anti-cytokine MoAbs to IL-1α, IL-1ß and IL-6 did not significantly modify leucocyte subpopulation transmigration through HUVEC. Cells were incubated with stimuli and MoAbs. The MoAb concentrations used were considerably higher than necessary concentrations to neutralize the possible cytokine release (data not shown).
Transmigration of porin- and LPS-stimulated neutrophil, lymphocyte and monocyte subpopulations across non-IL-1ß-activated HUVEC
Leucocyte-purified subpopulations (1.5 × 106 cells/ml) were treated for 2 h with porins (100 ng/ml) or LPS-S (100 ng/ml) or LPS-R (100 ng/ml), then washed twice with medium and finally layered on non-activated HUVEC.
The results are shown in Fig. 4. The percentage of treated leucocyte migration was increased compared with non-treated controls. The increase induced by LPS-R or porin treatment was lower than that induced by LPS-S treatment.
Fig. 4.

Transendothelial migration of stimulated leucocytes across non- stimulated human umbilical vein endothelial cells (HUVEC). Leucocytes were treated separately with stimuli (100 ng/1.5 × 106 cells/ml) for 2 h, were then washed and added to non-stimulated monolayer. The data represent mean ± s.e.m. of three independent experiments. *P < 0.001 versus control migration; **P < 0.05 versus LPS-S-stimulated leucocytes.
Neutrophil, lymphocyte and monocyte subpopulation transmigration through HUVEC activated by IL-1ß and stimulated by porins and LPS
The results of the experiments with IL-1β-activated endothelial monolayers treated with porins or LPS are reported in Fig. 5. The endothelium activated by IL-1ß and subsequently stimulated with LPS or porins allowed a rapid increase of migration of all the leucocyte populations compared with controls.
Fig. 5.

Migration of leucocytes across endothelial monolayer activated by IL-1ß for 4 h and then stimulated by porins, LPS-S and LPS-R for 2 h. The data represent mean ± s.e.m. of three independent experiments.
Porin and LPS-R involvement of endothelial adhesion molecules
In order to establish specificity of the transmigration induced by porins or LPS, HUVEC, treated as indicated in each experiment, were incubated with specific MoAbs. Only results obtained with LPS-R are presented because previous experiments with LPS-S have shown a similar pattern (data not shown). Equal concentrations of MoAbs directed against irrelevant antigens had no significant effect on porin- or LPS-R-stimulated migration.
It is known that expression of E-selectin, ICAM-1 and VCAM-1 in stimulated HUVEC is dependent on the stimulus as well as the length of cytokine stimulation [32,33]. Involvement of E-selectin in this migration was assessed by 45 min incubation before the assay of either porins or LPS-treated HUVEC with the anti-E-selectin BMS110 which are specific blocking MoAbs (clone CI26CI0B7; IgG2a) (Figs 6,7 and 8). The same MoAbs was also present during the assay. MoAbs produced a significant reduction of neutrophil migration compared with controls untreated with MoAbs. When IL-1β-stimulated endothelium cells were treated with porins or LPS-S, the anti-E-selectin MoAbs failed to inhibit migration significantly. We assessed the participation of ICAM-1 by incubating porins or LPS-treated HUVEC with the specific blocking MoAbs anti-ICAM-1/CD54 (clone 15.2; IgG1). This MoAb inhibited migration of neutrophils, monocytes and lymphocytes across the HUVEC monolayer. Neutrophils were the most inhibited. Negligible inhibition of HUVEC activated by IL-1ß and stimulated by porins was observed.
Fig. 6.

Migration inhibition of neutrophils across human umbilical vein endothelial cell (HUVEC) monolayer by pretreatment with MoAb for 45 min. Monolayers of HUVEC were incubated with either IL-1ß for 4 h or porins for 6 h or LPS-R for 4 h. In each separate experiment the control MoAbs (W27, BL-1a/DQ) of the same isotype of MoAbs used had no significant effect on porin- or LPS-R-stimulated migration. Results are representative of three independent experiments; bars, mean ± s.e.m.; *P < 0.001.
Fig. 7.
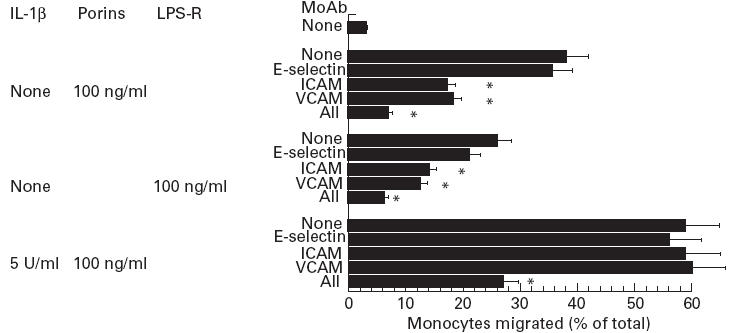
Migration inhibition of monocytes across human umbilical vein endothelial cell (HUVEC) monolayer by pretreatment with MoAbs for 45 min. Monolayers of HUVEC were incubated with either IL-1ß for 4 h or porins for 6 h or LPS-R for 4 h. In each separate experiment the control MoAbs (W27, BL-1a/DQ) of the same isotype of MoAbs used had no significant effect on porin- or LPS-R-stimulated migration. Results are representative of three independent experiments; bars, mean ± s.e.m.; *P < 0.001.
Fig. 8.

Migration inhibition of lymphocytes across human umbilical vein endothelial cell (HUVEC) monolayer by pretreatment with MoAbs for 45 min. Monolayers of HUVEC were incubated with either IL-1ß for 4 h or porins for 6 h or LPS-R for 4 h. In each separate experiment the control MoAbs (W27, BL-1a/DQ) of the same isotype of MoAb used had no significant effect on porin- or LPS-R-stimulated migration. Results are representative of three independent experiments; bars, mean ± s.e.m.; *P < 0.001.
Participation of VCAM-1 was assessed by incubating porin- or LPS-treated cells with the specific blocking MoAbs anti-VCAM-1 (clone 1.G11B1; IgG). As expected, incubation of HUVEC with MoAbs anti-VCAM-1 did not reduce porin- or LPS-induced migration of neutrophils, whereas migration of lymphocytes and monocytes was significantly reduced. Simultaneous exposure of IL-1β-activated endothelium to porin stimulation after pretreatment with the three MoAbs (anti-E-selectin, anti-VCAM-1 and anti-ICAM-1) caused a remarkable transmigration reduction.
Anti-CD18 MoAbs inhibited the enhanced migration of leucocytes across the HUVEC monolayer activated by IL-1ß. Anti-CD11b/CD18 MoAbs selectively did not inhibit the enhanced migration of lymphocytes [34]. Anti-CD11a/CD18 MoAbs also inhibited enhanced migration of leucocytes stimulated by either porins or LPS across HUVEC activated by IL-1ß or porins or LPS (Fig. 9).
Fig. 9.

Migration inhibition across human umbilical vein endothelial cell (HUVEC) monolayer activated by IL-1ß (5 U/ml) of leucocytes pretreated with MoAbs for 45 min. Monolayers of HUVEC were incubated with porins for 6 h or LPS-R for 4 h. In each separate experiment the control MoAbs (BL-1a/DQ, 1042) of the same isotype of MoAbs used had no significant effect on porin- or LPS-R-stimulated migration. Results are representative of three independent experiments; bars, mean ± s.e.m.; *P < 0.001.
DISCUSSION
Bacterial surfaces contain an interesting assortment of molecules that probably interfere with the complex network regulating the leucocyte traffic. When these molecules are released at the site of inflammation or in the blood stream during sepsis, they influence both leucocytes and endothelial cells by changing their adhesion molecule expression. Previous studies have demonstrated that Gram-positive and Gram-negative bacteria contain molecules interacting with endothelial cells [35–40]. LPS plays an important role in infections by Gram-negative bacteria. The roles of other surface molecules of Gram-negative bacteria are less understood [41,42]. Several studies have shown the activity of endotoxin-associated surface proteins [43]. Our previous investigations have indicated that, among the major surface proteins, the 34 k and 36 k porins from S. typhimurium have an inflammatory effect by activating the complement [13], releasing histamine [14], and inducing cytokine release [11]. The porin concentration of Gram-negative bacterial cells is approx. 2 × 105 molecules/cell, covering an area of about 1.8 μm2 or roughly a third of the cell surface area [44]. Therefore, lysis of about 107 cells is sufficient to release porins in the range that in our experiments induces endothelium activation.
The LPS-R concentration in the porin preparation was 50 pg/mg porins. This amount in parallel trials had no effect on endothelial cells or leucocytes. According to our previous results [11,15], the porins combined with polymixin B do not affect their biological activity. Activation in the different assays by porins may therefore be attributed to the protein fraction of preparation. Inhibition of IL-1α, IL-1β and IL-6 induction did not block the effect of porins and LPS, indicating that their effect is not indirect.
Amongst the leucocyte populations the transmigration of neutrophils was particularly increased. It has been previously shown that S. typhimurium provoked an intestinal inflammatory response with accumulation of neutrophils [8]. After 6 h the transmigration increases induced by porins overlapped those induced by LPS. It has been demonstrated that LPS-S, containing an O-specific chain, induces a maximal level of adhesion, comparable to adhesion induced by IL-1ß; LPS-R and free lipid A, leaking the O-specific chain, stimulated adhesion to a lower degree [7]. Therefore it seems that the protein and the polysaccharide fractions markedly increased lipid A activity [45,46].
Moreover, porin treatment caused transmigration that lasted several hours longer than that caused by LPS. Here we show that porins modulate leucocyte migration by acting on endothelial cells and leucocytes, although at a slower rate than LPS. Like LPS, porins activate those cells, and further increase the activation of those cells already activated by IL-1ß. Consequently, the in vivo activity of the two molecules shows an effect prolonged in time [15]. In a previous study we have shown that the synthesis of specific cytokine mRNA begins later and persists longer in cells treated with porins than in cells treated with LPS [15]. Treatment of leucocytes with porins equally causes increase in migration of various classes of leucocytes across non-activated endothelial cells. Activation of lymphocytes in vitro by lectins and other mitogens, by antigen stimulation or phorbol-myristate acetate, leads to profound alterations in the migration properties of these cells [47–49]. Upon activation by periodate, LPS or concanavalin A (Con A), lymphocytes display alterations in l-selectin expression: during the initial 2 days and at suboptimal concentrations of stimulus expression of l-selectin is increased, whereas at higher concentrations this expression is reduced [50]. Expression of the l-selectin antigen is regulated in a complex manner in response to activation signals, with both up- and down-regulation occurring under distinct conditions of stimulus concentration and blast transformation [50].
The simultaneous stimulation of endothelial cells with IL-1ß and either porins or LPS causes overlapping effects leading to a very high migration index. In a natural inflammatory process the combination of several (external and internal) stimuli probably induces high endothelial permeability of vessels to migrating cells.
Our experiments have shown that the activation of both leucocytes and endothelial cells allows all three studied populations of leucocytes to reach high levels of transmigration. In vivo, the conditions for a full adhesion and transmigration to the circulating cells probably occur at the site of inflammation. Some bacterial components, and among these the porins, behave as the chemoattractants [51]. Exposure of neutrophils to chemoattractants induces a dramatic change in morphology features and adhesiveness [52,53].
Neutrophil transmigration was partially inhibited by MoAbs binding to E-selectin; the transmigration of lymphocytes and monocytes was partially inhibited by MoAb anti-VCAM-1; the transmigration of neutrophils, lymphocytes and monocytes was partially inhibited by MoAb anti-ICAM-1.
As expected, monocyte and granulocyte transmigration through endothelial cells was inhibited by MoAbs binding to CD11a/CD18 and CD11b/CD18. Lymphocyte transmigration was inhibited by MoAbs CD11a/CD18 and not by CD11b/CD18. Modulation of the transmigration occurs through a qualitative change in the expression of CD11a/CD18 and a quantitative change in the expression of CD11b/CD18 [54]. During the transmigration events a variety of factors may stimulate CD11/CD18 activity; in addition, it has been suggested that the molecular interaction of E-selectin with leucocyte cell surface ligands stimulates CD11a/CD18 function [55]. While the MoAbs, each separately used, do not inhibit the transmigration of leucocytes across endothelium activated by IL-1ß and stimulated by porins, the three MoAbs used together were able to inhibit this transmigration. Evidently the activation by IL-1ß and porins caused other changes, probably exposing other unknown receptors on the cell membrane. The inhibition observed with the contemporary use of the three MoAbs suggested co-operation among receptors in abnormally stimulated cells. The process of leucocyte adherence and extravasation involves an initial phase of loose adherence to endothelium, followed by fine adherence and subsequent extravasation.
Activated cells (leucocytes and HUVEC) have a higher capacity to promote cell migration; this property may be related to the increased levels of adhesion molecules, but also differences in the co-operation of molecules, in their ability to activate or send out other signals required for extravasation may additionally contribute to the preferential attraction of cells by the inflamed tissue. The biological objective of leucocyte–endothelial interactions is to direct circulating cells into their appropriate tissue sites with efficiency and specificity [56]. The total addition of various stimuli probably avoids the high degree of specificity in the interaction of HUVEC with circulating cells. It is likely that in vivo the final effect is the result of a series of synergistic and antagonistic responses determined by a cascade of events triggered by the inflammatory stimulus.
REFERENCES
- 1.Butcher EC. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;20:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Picker LJ, Warnock RA, Burns AR, Doerschuck CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP140. Cell. 1991;66:921–33. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 3.Von Andrian UH, Chambers JD, McEvoy L, Bargatze RF, Arfors KE, Butcher EC. A two step model of leukocyte–endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte b2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–42. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison DC, Betz SJ, Jacobs DM. Isolation of a lipid A bound polypeptide responsible for ‘LPS initiated’ mitogenesis in C3H/Hej spleen cells. J Exp Med. 1976;144:840–6. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whisler RL, Cornwell DG, Proctor KV, Downs E. Bacterial lipopolysaccharide acts on human endothelial cells to enhance the adherence of peripheral blood monocytes. J Lab Clin Med. 1989;114:708–16. [PubMed] [Google Scholar]
- 6.Yu CL, Haskard DO, Cavender D, Ziff M. Effects of bacterial lipopolysaccharides on the binding of lymphocytes to endothelial cell monolayer. J Immunol. 1986;136:569–73. [PubMed] [Google Scholar]
- 7.Schönbeck UH, Flad D, Rietschel TH, Brandt E, Loppnow H. S-form LPS induces leukocyte adhesion to human vascular endothelial cell as potent as IL-1: lipid A precursor Ia antagonises induction of adhesion by LPS. J End Res. 1994;1:4–13. [Google Scholar]
- 8.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967;50:109–19. [PMC free article] [PubMed] [Google Scholar]
- 9.McGovern VJ, Slavutin LJ. Pathology of Salmonella colitis. Am J Sug Pathol. 1979;3:483–90. doi: 10.1097/00000478-197912000-00001. [DOI] [PubMed] [Google Scholar]
- 10.McCormick BA, Miller SI, Carnes D, Madara JL. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–9. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galdiero F, de Cipollaro l'Ero G, Benedetto N, Galdiero M, Tufano MA. Release of cytokines (TNF, IL-1ß, IL-4, IL-6 and IFN-γ) by Salmonella typhimurium porins. Infect Immun. 1993;61:155–61. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero F, Sommese L, Scarfogliero P, Galdiero M. Biological activities-lethality, Shwartzman reaction and pyrogenicity of Salmonella typhimurium porins. Microb Pathog. 1994;16:111–9. doi: 10.1006/mpat.1994.1012. [DOI] [PubMed] [Google Scholar]
- 13.Galdiero F, Tufano MA, Sommese L, Folgore A, Tedesco F. Activation of the complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984;46:559–63. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galdiero F, Tufano MA, Galdiero M, Masiello S, De Rosa M. Inflammatory effects of Salmonella typhimurium porins. Infect Immun. 1990;58:3183–8. doi: 10.1128/iai.58.10.3183-3186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galdiero M, Cipollaro de L'Ero G, Donnarumma G, Marcatili A, Galdiero F. Interleukin-1 and Interleukin-6 gene expression in human monocytes stimulated with Salmonella typhimurium porins. Immunol. 1995;86:612–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Tufano MA, Rossano F, Catalanotti P, Liguori G, Marinelli A, Baroni A, Marinelli P. Properties of Yersinia enterocolitica porins: interference with biological functions of phagocytes, nitric oxide production and selective cytokine release. Res Microbiol. 1994;145:297–307. doi: 10.1016/0923-2508(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 17.Tufano MA, Rossano F, Catalanotti P, Liguori G, Capasso C, Ceccarelli T, Marinelli P. Immunobiological activities of Helicobacter pylori porins. Infect Immun. 1994;62:1392–9. doi: 10.1128/iai.62.4.1392-1399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–41. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnarumma G, Brancaccio F, de Cipollaro l'Ero G, Folgore A, Marcatili A, Galdiero M. Release of GM-CSF, sE-Selectin, and sICAM-1 by human vascular endothelium stimulated with Gram-negative and Gram-positive bacterial componentes. Endothelium. 1996;4:11–22. [Google Scholar]
- 20.Nurminen M. A mild procedure to isolate the 34 kDa, 35 kDa and 36 kDa porins of the outer membrane of Salmonella typhimurium. FEMS Microbiol. 1978;3:331. (Letter) [Google Scholar]
- 21.Leammli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;277:680–75. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1985;193:265–75. [PubMed] [Google Scholar]
- 23.Tsai CM, Frasch E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–9. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard DK, Klein TW, Friedman H, Stewart WE. Induction of gamma interferon by endotoxin in ‘aged’ murine splenocyte cultures. In: Sventivanyi A, Friedman H, Nowotny A, editors. Immunobiology and immunopharmacology of bacterial endotoxins. New York: Plenum Press; 1986. [Google Scholar]
- 25.Galanos C, Luderitz O, Westphal O. A new method for the extraction of R lipopolisaccharides. Eur J Biochem. 1969;9:245–9. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 26.Westphal O, Lüdertz O, Bister F. Über die Extraktion von Bakterien mit Phenol/Wasser. Z Naturforsch. 1952;7b:148–5. [Google Scholar]
- 27.Gimbrone MA. Culture of vascular endothelium. In: Theodore H, Spaet MD, editors. Progress in hemostasis and thrombosis. III. New York: Grune and Stratton; 1976. p. l. [PubMed] [Google Scholar]
- 28.Freudenberg MA, Keppler D, Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986;51:891–5. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte–endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505–9. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karplus TE, Ulevitch RJ, Wilson CB. A new method for reduction of endotoxin contamination from protein solutions. J Immunol Methods. 1987;105:211–20. doi: 10.1016/0022-1759(87)90268-7. [DOI] [PubMed] [Google Scholar]
- 31.Morzycki W, Sadowaska J, Issekutz AL. Interleukin-1 and tumour necrosis factor alpha induced polymorphonuclear leukocyte– endothelial cell adhesion and transendothelial migration in vitro. Immunol. 1990;25:331. doi: 10.1016/0165-2478(90)90204-4. (Letter) [DOI] [PubMed] [Google Scholar]
- 32.Bevilacqua MP. Endothelial leukocyte adhesion molecules. Ann Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 33.Dustin ML, Rothlein Bhan AK, Dinariello CA, Springer TA. Induction by IL-1 and interferon-γ tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–54. [PubMed] [Google Scholar]
- 34.Issekutz AC, Issekutz TB. The contribution of LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) to the in vivo migration of polymorphonuclear leukocytes to inflammatory reaction in the rat. Immunol. 1992;76:655–61. [PMC free article] [PubMed] [Google Scholar]
- 35.Hoepelman AT, Tuomanen EL. Consequences of microbial attachment: directing host cell functions with adhesions. Infect Immun. 1992;60:1729–33. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jellati TJ, Abrescia LD, Radolf JD, Furie MB. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–7. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee PK, Vercellotti GM, Deringer JR, Schlievert PM. Effects of staphylococcal toxic shock syndrome toxin 1 on aortic endothelial cells. J Infect Dis. 1991;164:711–9. doi: 10.1093/infdis/164.4.711. [DOI] [PubMed] [Google Scholar]
- 38.Noel RF, Jr, Sato TT, Mendez C, Johnson MC, Pohlman TH. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–53. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohlman TH, Harlan JM. Endotoxin–endothelial cell interactions. In: Morrison DC, Ryan J, editors. Bacterial endotoxic lipopolysaccharides. Boca Raton: CRC Press; 1992. p. 459. [Google Scholar]
- 40.Suttorp N, Buerke M, Otto ST. Stimulation of PAF-synthesis in pulmonary artery endothelial cells by Staphylococcus aureus alpha-toxin. Thromb Res. 1992;67:243–52. doi: 10.1016/0049-3848(92)90143-x. [DOI] [PubMed] [Google Scholar]
- 41.Morrison DC, Ulevitch RJ. The interaction of bacterial endotoxins with cellular and humoral mediation systems. Am J Pathol. 1978;92:527–18. [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison DC, Danner RL, Dinarello CA, et al. Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future direction. J End Res. 1994;1:71–73. [Google Scholar]
- 43.Sultzer BM, Craig JP, Castagna R. Endotoxin associated proteins and their polyclonal and adjuvant activities. In: Szentvananyi A, Friedman H, editors. Immunobiology and immunopharmacology of bacterial endotoxins. New York: Plenum; 1986. pp. 435–47. [Google Scholar]
- 44.Nikaido H, Vaara M. Outer membrane. In: Neidhardt FC, Curtiss R III, Ingraham JL, et al., editors. Escherichia coli and Salmonella cellular and molecular biology. Washington: American Society for Microbiology; [Google Scholar]
- 45.Mored JJ, Less A, Snapper LM. T cell-independent antigens Type 2. Ann Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 46.Seppälä I, Mäkelä O. Antigenicity of dextran protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J Immunol. 1989;143:1259–64. [PubMed] [Google Scholar]
- 47.Carroll AM, Palladino MA, Oettgen H, Sousa MD. In vivo localisation of cloned IL-2 dependent T cells. Cell Immunol. 1983;76:69–80. doi: 10.1016/0008-8749(83)90349-0. [DOI] [PubMed] [Google Scholar]
- 48.Dailey MO, Fathmann CG, Butcher EC, Pillemer E, Weissman IL. Abnormal migration of T cell clones. J Immunol. 1982;128:2134–6. [PubMed] [Google Scholar]
- 49.Lotze MT, Line B, Mathisen DJ, Rosenberg SA. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumours. J Immunol. 1980;125:1487–93. [PubMed] [Google Scholar]
- 50.Hamman A, Jablonski-Westrich D, Scholz KU, Duijvestijn A, Butcher EC, Thiele HG. Alterations in homing receptor expression and organ-specific high endothelial venule binding of lymphocytes upon activation. J Immunol. 1988;140:737–43. [PubMed] [Google Scholar]
- 51.Tufano MA, Berlingieri MT, Sommese L, Galdiero F. Immune response in mice and effects on cells by outer membrane porins from Salmonella typhimurium. Microbiologica. 1984;7:353–66. [PubMed] [Google Scholar]
- 52.Kishimoto TK, Jutila M, Berg E, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 53.Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilization of monocyte Mac-1 and p150, 95 adhesion protein from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987;80:535–44. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–50. [PubMed] [Google Scholar]
- 55.Lo SK, Lee S, Ramos R, Lobb R, Rosa M, Chi RG, Wright SD. Endothelial–leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, MAC-1, αMβ2) on human neutrophils. J Exp Med. 1991;173:1493–500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebnet K, Kaldscan EP, Anderson AO, Shaw S. Orchestrated information transfer underlying leukocyte endothelial interactions. Ann Rev Immunol. 1996;14:155–77. doi: 10.1146/annurev.immunol.14.1.155. [DOI] [PubMed] [Google Scholar]


