Abstract
We examined the effects of intravenous immunoglobulin (IVIG) on cytokine regulation in vivo using samples taken before and after replacement-dose (200–400 mg/kg) IVIG in a group of patients with common variable immunodeficiency (CVID) and X-linked agammaglobulinaemia (XLA). The intracellular cytokine content of CD4+ and CD8+ lymphocytes, and their CD28+/− subsets, were measured following in vitro activation with phorbol myristate acetate (PMA) and ionomycin. The cytokines IL-2, interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), and the early activation marker CD69, were assessed by four-colour flow cytometry of whole blood cultures taken before and after IVIG infusion. There was a significant increase in IL-2 expression in CD4+ (and CD4+28−) cells and an increase in TNF-α expression in CD8+28− cells following IVIG in CVID, but not in XLA patients. IFN-γ and CD69 expression were not affected by IVIG infusion. This increase in TNF-α and IL-2, combined with unchanged IFN-γ expression, is evidence against the putative ‘anti-inflammatory’ role of IVIG, and may explain the failure of resolution of granulomata in CVID patients treated with IVIG alone.
Keywords: intravenous immunoglobulin, intracellular cytokine, common variable immunodeficiency, X-linked agammaglobulinaemia, cytokine modulation
INTRODUCTION
Intravenous immunoglobulin (IVIG) therapy, consisting of pooled human immunoglobulin (mainly IgG) from several thousand donors, is now the treatment of choice for patients with antibody deficiency [1]. This replacement IVIG is typically given at doses of 200–400 mg/kg every 3–4 weeks. The mechanism of action of IVIG in these patients has not been clearly established, but is likely to operate in a number of overlapping fashions [2], including: neutralizing toxins, binding to microbes and facilitating opsonization, binding to Fc receptors and modulating cytokine synthesis and release (reviewed in [3]). In addition, other soluble components in commercial IVIG preparations, such as soluble CD8 and soluble HLA molecules, and the sugars used as stabilizing agents, may also be involved in the mechanisms of action [4].
In this study we investigate the effects of IVIG on modulating cytokine expression by subsets of T cells. The anti-proliferative actions of IVIG on T cells stimulated with mitogens have been well documented both in vitro [5,6] and in vivo [7]. The effects of IVIG on cytokine production have been harder to assess, but recent developments in cell permeabilization and fixation techniques, together with the production of directly conjugated anti-cytokine antibodies, mean that it is now possible to examine the cytokine production of individually identified lymphocytes under various culture conditions [8,9].
SUBJECTS AND METHODS
In the present study, the effects of replacement dose IVIG (200–400 mg/kg every 3–4 weeks) in vivo were assessed in lymphocytes cultured from patients with the primary immunodeficiency disorders, common variable immunodeficiency (CVID) and X-linked agammaglobulinaemia (XLA). Blood samples were drawn before and after routine IVIG therapy, and comparisons were made with cells from normal subjects, who did not receive IVIG. As we have previously established differences in interferon-gamma (IFN-γ) production between CD28− and CD28+CD8+ cells in CVID, and between CVID and normals [10], the effects of IVIG on these subpopulations were also examined in the CVID patients.
Subjects
This study involved nine patients with long-standing CVID (five male, four female, mean age 49 years) who were receiving replacement dose IVIG (200–400 mg/kg per 3 weeks), as detailed in Table 1, and three subjects with XLA (aged 29, 37 and 25 years). Five healthy controls (four male, one female, mean age 36.4 years) were used in the time-course experiment. All subjects gave informed consent, and the research carried the approval of our hospital's Ethics Committee.
Table 1.
Characteristics of common variable immunodeficiency (CVID) patients studied
In vivo effects of IVIG
The immunodeficient patients attended for routine IVIG therapy and venous blood (5 ml) was collected into lithium-heparin tubes immediately prior to IVIG administration. The immunoglobulin was given at standard rates (mean time for infusion 3 h). After the IVIG was administered, the drip was disconnected, approx. 10 ml of blood were drawn through the administration needle and discarded. A further 5-ml sample was collected into lithium-heparin tubes as before. Both pre- and post-IVIG samples were then analysed simultaneously. To control for any effects of the 3-h delay ex vivo before analysing the pre-IVIG specimens, blood from five normal volunteers was drawn on two occasions, 3 h apart, and blood from both time points was analysed simultaneously.
Assessment of intracellular cytokine production
A whole blood method was used, as previously described [11]. Briefly, 250 μl of whole blood were diluted 1:3 and cultured in the presence (‘stimulated’ cells) or absence (‘unstimulated’ cells) of phorbol myristate acetate (PMA; 15 ng/ml) with ionomycin 2 μmol/l. Monensin 3 μmol/l was present in both culture systems to block export of cytokine from the Golgi apparatus, increasing the sensitivity of the technique by holding newly synthesized cytokine within the cell. The cells were then incubated at 37°C for 2 h (4 h for the IL-2 assays). Erythrocytes were lysed using Optilyse C (Coulter, Luton, UK), and the remaining cells were fixed and permeabilized using commercial reagents (Leukoperm; Serotec, Oxford, UK). The cells were stained using directly conjugated anti-cytokine MoAbs, and directly conjugated anti-surface marker antibodies. The monoclonals used were: anti-IFN-γ/FITC (clone B-B1), anti-tumour necrosis factor-alpha (TNF-α)/FITC (clone B-D9), anti-IL-2/FITC (clone B-G5) (all Serotec), anti-CD8/ECD (Coulter), anti-CD3/PE-Cy5, and anti-CD28/PE (all Immunotech, Beckman Coulter UK, High Wycombe, UK). Following staining, the cells were washed and resuspended in paraformaldehyde (0.5% in PBS), prior to flow cytometry on a four-colour Coulter Epics XL/MCL flow cytometer.
Flow cytometry
For analysis, 10 000 events in a lymphocyte light scatter gate were acquired, without colour compensation for spectral overlap between the four fluorescence channels. Compensation was applied electronically after acquisition using the WinList Compensation Toolbox (Verity) using, as standards, single-colour anti-CD3 staining for each of the four fluorochromes (FITC, PE, ECD, PE-Cy5). Each four-colour tube contained an antibody against cytokine, an antibody for cell subset (CD28), and anti-CD3 and anti-CD8 to define major lymphocyte populations. Cells from within a tight lymphocyte light scatter gate were analysed using WinList 3.0 (Verity). Regions were defined for CD3+8− cells (‘CD4+’ cells) and CD3+8bright+ cells (‘CD8+’ cells). Further subsetting of cells was carried out by dividing CD4+ and CD8+ cells into CD28− and CD28+ populations. The cytokine production within each subpopulation was determined by defining cytokine-negative cells as those in the cultured but unstimulated (monensin only) cells. Stimulated cells showing cytokine fluorescence greater than that in the unstimulated cells were defined as cytokine-positive. Cell activation after culture was confirmed by staining for CD69 expression, a marker for early activation.
Statistical analysis
Comparisons were made between the level of cytokine expression before and after the 3 h ex vivo period (normals) or before and after the 3 h IVIG infusion (CVID, XLA) within a given subject. Since the number of subjects was small, and the results were not normally distributed, the analysis was performed using the sign test for paired samples, with significance set at the P < 0.05 level. For comparisons quoted in the text between donor groups, the Mann–Whitney U-test was employed.
RESULTS
Changes in cytokine expression with IVIG are not due to ex vivo changes in the pretreatment specimen (normal controls)
Cells treated with monensin only (unstimulated cultures) did not produce cytokine, and regions to define cytokine-positive cells were set using the monensin only cultures. To exclude any effect of sample age ex vivo prior to analysis of cytokine synthetic capacity, blood was taken from normal volunteers and left at room temperature for 3 h (the mean duration of IVIG administration in the patients). A second sample was then taken, and the 3-h-old sample and fresh sample were analysed simultaneously. As illustrated in Figs 1,2,3 and 4 and Table 2, there was no change in TNF-α, IFN-γ, IL-2 or CD69 expression in normal donors following a 3-h delay in analysis. Any change in cytokine levels in the patient groups could therefore be attributable to IVIG effects, rather than be due to changes in the specimen ex vivo prior to analysis.
Fig. 1.
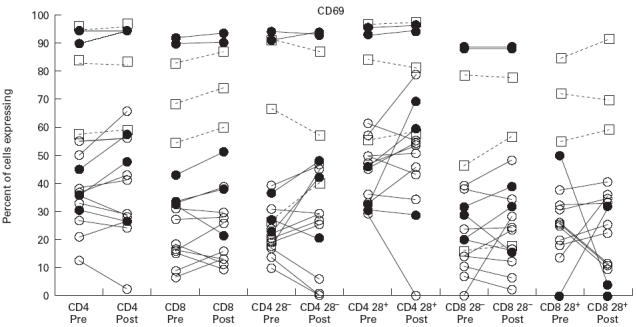
Expression of CD69 following activation with phorbol myristate acetate (PMA) and ionomycin in CD4 cells and CD8 cells and their CD28− and CD28+ subsets. ‘Pre’ indicates samples taken prior to intravenous immunoglobulin (IVIG) infusion, and ‘post’ indicates samples following IVIG infusion, in common variable immunodeficiency (CVID) patients (○) and X-linked agammaglobulinaemia (XLA) patients (□). Normal donors (•) are shown for comparison, with ‘pre’ samples taken 3 h before ‘post’ samples and left at room temperature prior to simultaneous analysis with ‘post’ samples.
Fig. 2.
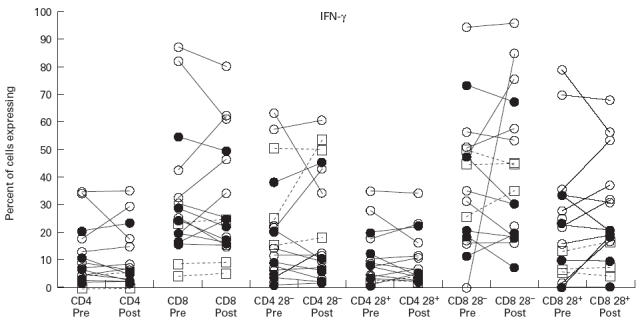
Expression of IFN-γ following activation with phorbol myristate acetate (PMA) and ionomycin in CD4 cells and CD8 cells and their CD28− and CD28+ subsets. Symbols and explanation as for Fig. 1.
Fig. 3.
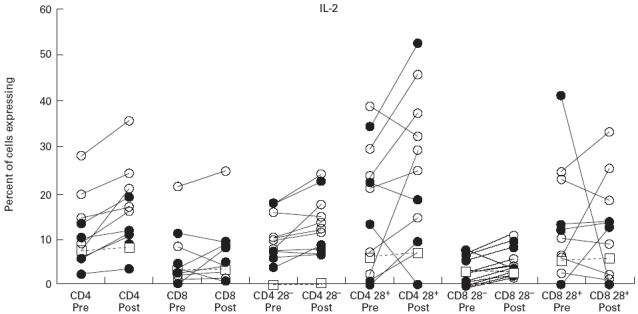
Expression of IL-2 following activation with phorbol myristate acetate (PMA) and ionomycin in CD4 cells and CD8 cells and their CD28− and CD28+ subsets. Symbols and explanation as for Fig. 1.
Fig. 4.
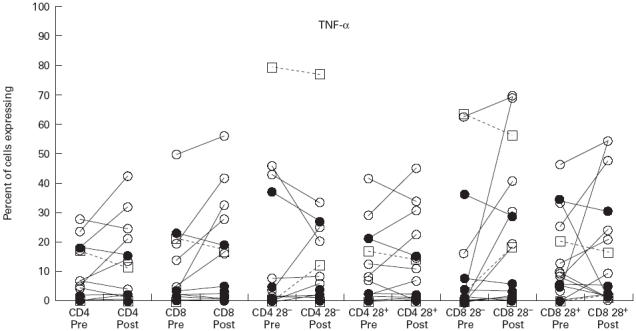
Expression of tumour necrosis factor-alpha (TNF-α) following activation with phorbol myristate acetate (PMA) and ionomycin in CD4 cells and CD8 cells and their CD28− and CD28+ subsets. Symbols and explanation as for Fig. 1.
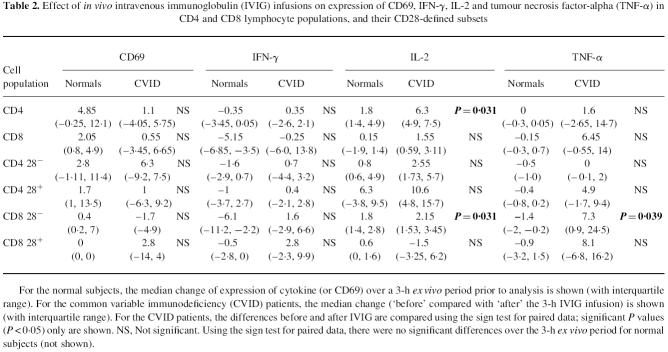
Table 2.
Effect of in vivo intravenous immunoglobulin (IVIG) infusions on expression of CD69, IFN-γ, IL-2 and tumour necrosis factor-alpha (TNF-α) in CD4 and CD8 lymphocyte populations, and their CD28-defined subsets
For the normal subjects, the median change of expression of cytokine (or CD69) over a 3-h ex vivo period prior to analysis is shown (with interquartile range). For the common variable immunodeficiency (CVID) patients, the median change (‘before’ compared with ‘after’ the 3-h IVIG infusion) is shown (with interquartile range). For the CVID patients, the differences before and after IVIG are compared using the sign test for paired data; significant P values (P < 0.05) only are shown. NS, Not significant. Using the sign test for paired data, there were no significant differences over the 3-h ex vivo period for normal subjects (not shown).
Fewer cells from CVID patients are activated by PMA and ionomycin than cells from XLA patients or normals
The expression of CD69 following 2 h of culture with PMA and ionomycin is shown in Fig. 1. In both CD4+ and CD8+ lymphocytes, the proportion of cells expressing CD69 was much lower in CVID than in either XLA or normals. This finding was most striking in CD4+28− and CD8+28− cells in CVID which showed very low CD69 expression. Interestingly, the exception to this pattern was in CD8+28+ cells, which in normals displayed very low levels of CD69 on activation (Fig. 1), yet high levels in XLA. IVIG had no effect on CD69 expression in either CD4+ or CD8+ cells, or their subsets delineated by CD28 in CVID patients (Table 2), or XLA patients (Fig. 1).
IFN-γ expression
Greater numbers of CD8+ cells than CD4+ cells expressed IFN-γ in all of the donor groups studied (Fig. 2). The highest levels of IFN-γ expression were in CVID. The findings of North et al. [10] were confirmed, with the expanded CD8+28− subset from CVID patients expressing high amounts of IFN-γ together with a significant overproduction of IFN-γ from the CD8+28+ subset, compared with normals. Although there was an upward trend in IFN-γ expression in CVID following IVIG therapy, this did not reach statistical significance (see Table 2).
IL-2 expression
IL-2 expression was greater in CD4+ than CD8+ cells in all subsets, and for all groups studied (Fig. 3). CVID subjects had greater percentages of CD4+ cells expressing IL-2 than normals, but this difference was not statistically significant. Most strikingly, a significantly greater proportion of CVID CD4+ cells expressed IL-2 following IVIG; but there was no significant change in IL-2 expression in total CD8+ cells (Table 2). However, subset analysis revealed that CD8+28− cells from CVID patients significantly increased IL-2 expression following IVIG, although the increase was small (see Table 2). IL-2 expression was not affected by IVIG in the single XLA subject in whom this cytokine was measured (Fig. 3).
TNF-α expression
Expression of TNF-α was similar in both CD4+ and CD8+ cells (Fig. 4), and there were no significant differences in levels of expression between patient groups. However, the proportion of CD8+ cells positive for TNF-α increased significantly following IVIG in the CVID patients, but only in the CD8+28− subset (Table 2). This effect was not apparent in the three XLA subjects studied (Fig. 4).
DISCUSSION
Until the development of the technology for intracellular cytokine measurement, any abnormalities of expression of cytokines in CVID were hard to relate to specific cell populations. As a consequence, studies of cytokine levels within the plasma or culture supernatants gave conflicting results [12,13], and were not able to account for the pathophysiology of CVID. Intracellular cytokine assays go a long way to addressing this problem, and using them we have previously reported high levels of IFN-γ expression in CVID [13] within the CD28+/− subsets of Ficoll-separated CD8+ cells [10].
Intracellular cytokine content has previously been measured by indirect immunofluorescence [14], which does not readily permit the identification of sufficient numbers of cells within the lymphocyte subsets being examined. However, improvements in flow cytometry have recently permitted the examination of single lymphocytes by subset and cytokine content in both separated cells [13,15] and whole blood [11,16]. It can be argued that whole blood techniques are more appropriate [17,18], since lymphocytes are cultured in a more physiological milieu, and the potential of cell activation by density gradient centrifugation [19] is avoided. Flow cytometry has the dual advantages of better definition of the association between cytokine expression in specific cell subpopulations, and more rapid sampling of larger numbers of cells. Alternative techniques assessing cytokine mRNA by in situ methods may not reflect actual cytokine protein expression, and only examine small numbers of cells.
Several previous studies have investigated the in vitro effects of IVIG on cytokine production, but the methods used (examination of cells by immunofluorescent microscopy or cell culture supernatants) were limited in their ability to distinguish cytokine production by specific cell populations, and may have been perturbed by the cell separation techniques employed. For example, commercial IVIG preparations were used at concentrations of 6 mg/ml in vitro in a series of experiments by Andersson's group [20,21]. Following stimulation with PMA and ionomycin, lymphocytes (subsets not defined) from normal donors cultured with IVIG for 96 h were shown to produce less IL-3, IL-4, IL-5, transforming growth factor-beta (TGF-β) and granulocyte- macrophage colony-stimulating factor (GM-CSF) as assessed by intracellular indirect immunofluorescence. However, levels of TNF-α and IFN-γ were unchanged. When streptococcal exotoxin A was used to stimulate lymphocytes cultured with IVIG, there was a reduction in IFN-γ expression, and reduced TGF-β production [22].
Another in vitro study showed that IL-2 secretion into culture supernatants is reduced by IVIG preparations [23]. Although there was no effect on the level of IL-2 mRNA, the IVIG inhibited intracellular IL-2 levels, suggesting that IVIG modulates IL-2 synthesis at the post-transcriptional level.
We now turn to the in vivo effects of IVIG. The anti-proliferative effects of IVIG have been demonstrated in vivo in immunodeficient children receiving replacement-dose IVIG [24]. Pokeweed mitogen (PWM)-stimulated immunoglobulin production was reduced following therapy with IVIG. The effects of single doses of IVIG were also studied in vivo in hypogammaglobulinaemic patients, and were shown to increase plasma levels of TNF-α and soluble TNF receptors [25]. In a separate study [26], Farber showed that plasma TNF-α concentrations increase during adverse reactions to IVIG but become undetectable prior to the next treatment. Uneventful infusions were not associated with elevations in TNF-α.
Increases in TNF-α production by lipopolysaccharide (LPS)-stimulated lymphocytes were noted during a clinical trial of high-dose IVIG for rheumatoid arthritis. In three out of four patients, there was an increased TNF-α production following IVIG [27]. However, the dose of IVIG was five times higher than in the present study.
Plasma levels of cytokines have also been measured in a group of children receiving IVIG for recurrent epilepsy [28]. Samples taken immediately before, and 20 min after IVIG infusions did not show any change in IL-2, IL-4 or IL-6 over 6 months of treatment. However, there was a statistically significant increase in plasma IFN-γ and IL-6 immediately following each infusion. No increase in activation markers on lymphocytes or monocytes was detected, but there was an increase in CD64 (FcγRI) expression on monocytes. The authors attributed the mechanism to an IVIG-mediated increase in lymphocyte-produced IFN-γ causing monocyte activation and subsequent IL-6 production.
The mechanism of the changes in cytokines mediated by IVIG is not clear. It must be borne in mind that IVIG preparations may themselves contain cytokines which could influence the production of other cytokines: significant quantities of TGF-β1 and TGF-β2, but not IL-6, IL-10 or TNF-α, were recently demonstrated in a number of commercial IVIG preparations [29]. Some of the antibodies in IVIG may also be anti-cytokine in specificity [30]; antibodies to IL-1α, TNF [31] and IL-8 [32] have been detected in the serum of normal individuals. Other, non-immunoglobulin components of commercial IVIG preparations may also be immunomodulatory; both maltose and sucrose, at the concentrations present in IVIG, can have an anti-proliferative effect [4].
There is some evidence that one mechanism for the effect of IVIG on cytokine expression may be Fc-mediated, since intact immunoglobulin in vitro (but not F(ab′)2) could induce TNF-α expression in culture supernatants [33]. However, the effects of IVIG are complex, since the same group [34] demonstrated that both the production of IFN-γ and TNF-α could be inhibited by IVIG, and that this effect was due to anti-IFN-γ (but not anti-TNF-α) antibodies present within the IVIG. This effect could be duplicated by addition of anti-IFN-γ instead of IVIG. Such anti-IFN-γ antibodies were shown to be present at higher concentrations in IVIG preparations high in specific antibodies to hepatitis B and cytomegalovirus (CMV) [35], suggesting that they are an autoantibody response to viral infection.
It is possible that the effects of the immunoglobulin on cytokine production by lymphocytes are indirect, since it has recently been shown that the in vitro reduction of cytokine production by lymphocytes cultured with IVIG is dependent upon accessory cells [36]; we are currently investigating this effect in vivo.
We have now shown directly in CVID, for the first time, that IVIG in vivo increases the potential of a subset of CD8+ lymphocytes to make TNF-α and CD4+ lymphocytes to make IL-2. The increase in TNF synthetic capacity by IVIG in vivo is supported clinically by the finding that concurrent infections in IVIG recipients, or excessively rapid administration, results in an adverse reaction [37], and that increases in plasma TNF have been noted following adverse reactions to IVIG [26]. In these cases, IVIG may be reducing the TNF secretion threshold of lymphocytes already partially activated by the infection. In contrast, IVIG has been shown to down-regulate TNF-α expression in lymphocytes from patients infected with HIV, and to increase membrane-bound p75 TNF receptors [38]. However, patients with HIV may have an increased amount of TNF-α secretion compared with normals, and the effects of IVIG in this context may not be found in other clinical situations.
It is notable from our own work that the increases in IL-2 and TNF-α expression following IVIG occurred mostly in the CD28− subset. This population of cells is expanded in CVID [39] and may represent ‘end-stage’ T cells entering the apoptotic process [40]. We have shown here that following stimulation with PMA and ionomycin, the CD28− population is activated to a greater extent than the CD28+ population (as shown by CD69 expression) in normals, but not in CVID. This failure of CVID CD8+28− cells to be activated despite a high cytokine production may be fundamental to the disease state. The high levels of CD69 expression, but low levels of cytokine expression in XLA is interesting, but unexplained.
Finally, there is a general clinical perception that IVIG is ‘anti-inflammatory’. However, in our studies there was no decrease in IFN-γ expression, but there was an increase in TNF-α (a proinflammatory cytokine) expression following IVIG. This suggests that there is a theoretical downside to IVIG therapy, although such effects are likely to be transient. It is likely that the regulation of TNF-α in CVID differs from that in either XLA or normals, since the TNF allele +488A is over-represented in CVID patients with granulomatous disease [41]. The effect of this increased TNF-α may not be cytotoxic in vivo, since IVIG has been shown to protect cell cultures against TNF-α-induced cell death. This occurs with both Fab and Fc portions of immunoglobulin [42].
The increase in IL-2 expression, although small, was unexpected and appears to be limited to the CD4+ subset. This may be an advantage of IVIG therapy, since previously reported ‘deficiencies’ of IL-2 in CVID have been shown to be due to a reduction in the number of IL-2-secreting CD4+ cells [13].
Our results cast doubt on an anti-inflammatory role for IVIG, although its effect on macrophage function needs to be tested to obtain a better picture of the overall effect. Since XLA patients show a different pattern of response to IVIG, our findings in CIVD may be specific for this disease, and may provide clues to an underlying immunomodulatory defect involving TNF-α production. Finally, we need to consider the possibility that IVIG could exacerbate the common occurrence of granulomatous complications requiring steroid therapy in CVID, which usually progress, despite IVIG therapy.
Acknowledgments
W.A.C.S. was supported in part by the Peter Samuels Royal Free Fund. Anti-cytokine monoclonal antibodies were kindly provided by Dr Andrew Lane, Serotec, UK. We would like to thank Sisters Cilla Freud and Annie Ryan for their kind help with obtaining blood samples.
REFERENCES
- 1.Buckley RH, Schiff RI. The use of intravenous immune globulin in immunodeficiency disease. New England J Med. 1991;325:110–7. doi: 10.1056/NEJM199107113250207. [DOI] [PubMed] [Google Scholar]
- 2.Kaveri SV, Mouthon L, Kazatchkine MD. Immunomodulating effects of intravenous immunoglobulin in autoimmune and inflammatory diseases. J Neurol, Neurosurg Psychiatry. 1994;57(Suppl.):6–8. doi: 10.1136/jnnp.57.suppl.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouthon L, Kaveri SV, Spalter SH, et al. Mechanism of action of intravenous immune globulin in immune mediated diseases. Clin Exp Immunol. 1996;104(Suppl. 1):3–9. [PubMed] [Google Scholar]
- 4.Alder LBA, Morgan LA, Spickett GP. Contribution of stabilizing agents present in intravenous immunoglobulin preparations to modulation of mononuclear cell proliferation in vitro. Scand J Immunol. 1996;44:585–91. doi: 10.1046/j.1365-3083.1996.d01-350.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Schaik IN, Lundkvist I, Vermeulen M, Brand A. Polyvalent immunoglobulin for intravenous use interferes with cell proliferation in vitro. J Clin Immunol. 1992;12:325–34. doi: 10.1007/BF00920789. [DOI] [PubMed] [Google Scholar]
- 6.Van Schaik IN, Vermeulen M, Brand A. In vitro effects of polyvalent immunoglobulin for intravenous use. J Neurol, Neurosurgery Psychiatry. 1994;57(Suppl.):15–17. doi: 10.1136/jnnp.57.suppl.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballow M, White W, Desbonnet C. Modulation of in vitro synthesis of immunoglobulin and the induction of suppressor activity by therapy with intravenous immunoglobulin. J All Clin Immunol. 1989;84:595–601. doi: 10.1016/0091-6749(89)90196-6. [DOI] [PubMed] [Google Scholar]
- 8.Schauer U, Jung T, Krug N, Frew A. Measurement of intracellular cytokines. Immunol Today. 1996;17:305–6. doi: 10.1016/0167-5699(96)30020-0. [DOI] [PubMed] [Google Scholar]
- 9.Carter LL, Swain SL. Single cell analyses of cytokine production. Curr Opin Immunol. 1997;9:177–82. doi: 10.1016/s0952-7915(97)80132-x. [DOI] [PubMed] [Google Scholar]
- 10.North ME, Webster ADB, Farrant J. Primary defect in CD8+ lymphocytes in the antibody deficiency disease (common variable immunodeficiency): abnormalities in intracellular production of interferon-gamma (IFN-γ) CD28+ (‘cytotoxic’) CD28− (‘suppressor’) CD8+ subsets. Clin Exp Immunol. 1998;111:70–75. doi: 10.1046/j.1365-2249.1998.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sewell WAC, North ME, Webster ADB, Farrant J. Determination of intracellular cytokines by flow-cytometry following whole-blood culture. J Immunol Methods. 1997;209:67–74. doi: 10.1016/s0022-1759(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 12.Paganelli R, Capobianchi MR, Ensoli B, D’Offizi GP, Facchini J, Dianzani F, Aiuti F. Evidence that defective gamma interferon production in patients with primary immunodeficiencies is due to intrinsic incompetence of lymphocytes. Clin Exp Immunol. 1988;72:124–9. [PMC free article] [PubMed] [Google Scholar]
- 13.North ME, Ivory K, Funauchi M, Webster ADB, Lane AC, Farrant J. Intracellular cytokine production by human CD4+ and CD8+ T cells from normal and immunodeficient donors using directly conjugated anti-cytokine antibodies and three-colour flow cytometry. Clin Exp Immunol. 1996;105:517–22. doi: 10.1046/j.1365-2249.1996.d01-795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1995;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Prussin C, Metcalf DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–28. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 16.Jason J, Larned J. Single-cell cytokine profiles in normal humans: comparisons of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997;207:13–22. doi: 10.1016/s0022-1759(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 17.Maino VC, Ruitenberg JJ, Suni MA. Flow cytometric method for analysis of cytokine expression in clinical samples. Clin Immunol Newsletter. 1996;16:95–98. [Google Scholar]
- 18.de Groot D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1-beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 19.Maino VC, Suni MA, Ruitenberg JJ. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995;20:127–33. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- 20.Andersson UG, Björk L, Skansen-Saphir U, Andersson JP. Down-regulation of cytokine production and interleukin-2 receptor expression by pooled human IgG. Immunol. 1993;79:211–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson UG, Björk L, Skansen-Saphir U, Anderssson J. Pooled human IgG modulates cytokine production by lymphocytes and monocytes. Immunol Rev. 1994;139:21–42. doi: 10.1111/j.1600-065x.1994.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 22.Saphir-Skansén U, Andersson J, Björk L, Andersson U. Lymphokine production induced by streptococcal pyrogenic exotoxin-A is selectively down-regulated by pooled human IgG. Eur J Immunol. 1994;24:916–22. doi: 10.1002/eji.1830240420. [DOI] [PubMed] [Google Scholar]
- 23.Modiano JF, Amran D, Lack G, Bradley K, Ball C, Domenico J, Gelfand EW. Posttranscriptional regulation of T-cell IL-2 production by human pooled immunoglobulin. Clin Immunol Immunopathol. 1997;83:77–85. doi: 10.1006/clin.1997.4329. [DOI] [PubMed] [Google Scholar]
- 24.Ballow M, White W, Desbonnet C. Modulation of in vitro synthesis of immunoglobulin and the induction of suppressor activity by therapy with intravenous immunoglobulin. J Allergy Clin Immunol. 1989;84:595–601. doi: 10.1016/0091-6749(89)90196-6. [DOI] [PubMed] [Google Scholar]
- 25.Aukrust P, Frøland SS, Libakk N-B, Müller F, Nordøy I, Haug C, Espevik T. Release of cytokines, soluble cytokine receptors, and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo. Blood. 1994;84:2136–43. [PubMed] [Google Scholar]
- 26.Farber CM, Crusiaux A, Schandené L, van Vooren JP, Goldman M, Dupont E, Tasiaux N. Tumor necrosis factor and intravenous gamma globulins in common variable immunodeficiency. Clin Immunol Immunopathol. 1994;72:233–6. doi: 10.1006/clin.1994.1136. [DOI] [PubMed] [Google Scholar]
- 27.Maksymowych WP, Avina-Zubieta A, Loung M, Russel AS. High dose intravenous immunoglobulin (IVIG) in severe refractory rheumatoid arthritis: no evidence for efficacy. Clin Exp Rheumatol. 1996;14:657–60. [PubMed] [Google Scholar]
- 28.Ling Z-D, Yeoh E, Webb BT, Farrell K, Doucette J, Matheson DS. Intravenous immunoglobulin induces interferon-gamma and interleukin-6 in vivo. J Clin Immunol. 1993;13:302–9. doi: 10.1007/BF00920238. [DOI] [PubMed] [Google Scholar]
- 29.Kekow J, Reinhold D, Pap T, Asorge S. Intravenous immunoglobulins and transforming growth factor β. Lancet. 1998;351:184–5. doi: 10.1016/S0140-6736(05)78212-X. [DOI] [PubMed] [Google Scholar]
- 30.Abe Y, Horiuchi A, Miyake M, Kimura S. Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol Rev. 1994;139:5–19. doi: 10.1111/j.1600-065x.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 31.Abe Y, Miyake M, Segawa T, Kimura S. Enzyme-linked immunosorbent assay for human tumor necrosis factor (hTNF) Clin Chim Acta. 1988;176:213–7. doi: 10.1016/0009-8981(88)90210-0. [DOI] [PubMed] [Google Scholar]
- 32.Reitamo S, Remitz A, Varga J, Ceska M, Effenberger F, Jimemez S, Uitto J. Demonstration of interleukin-8 and autoantibodies to interleukin-8 in the serum of patients with systemic sclerosis and related disorders. Arch Dermatol. 1993;129:189–93. [PubMed] [Google Scholar]
- 33.Toungouz M, Denys CH, de Groote D, Dupont E. In vitro inhibition of tumor necrosis factor-alpha and interleukin-6 production by intravenous immunoglobulins. Br J Haematol. 1995;89:698–703. doi: 10.1111/j.1365-2141.1995.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 34.Toungouz M, Denys C, Dupont E. Blockade of proliferation and tumor necrosis factor-alpha production occurring during mixed lymphocyte reaction by interferon-gamma-specific natural antibodies contained in intravenous immunoglobulins. Transplantation. 1996;62:1292–6. doi: 10.1097/00007890-199611150-00020. [DOI] [PubMed] [Google Scholar]
- 35.Denys C, Toungouz M, Dupont E. Increased in vitro immunosuppressive action of anti-CMV and anti-HBs intravenous immunoglobulins due to higher amounts of interferon-gamma specific neutralising antibodies. Vox Sang. 1997;72:247–50. doi: 10.1046/j.1423-0410.1997.7240247.x. [DOI] [PubMed] [Google Scholar]
- 36.Skansén-Saphir U, Andersson J, Björk L, Ekberg C, Fehniger TE, Henter J-I, Andersson U. Down-regulation of lymphokine synthesis by intravenous gammaglobulin is dependent upon accessory cells. Scand J Immunol. 1998;47:229–35. doi: 10.1046/j.1365-3083.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 37.Jolles S, Hill H. Management of aseptic meningitis secondary to intravenous immunoglobulin. Br Med J. 1998;316:936. [PMC free article] [PubMed] [Google Scholar]
- 38.Aukrust P, Hestdal K, Lien E, Bjerkeli V, Nordoy I, Espevik T, Muller F, Froland SS. Effects of intravenous immunoglobulin in vivo on abnormally increased tumor necrosis factor-alpha activity in human immunodeficiency virus type 1 infection. J Infect Dis. 1997;176:913–23. doi: 10.1086/516510. [DOI] [PubMed] [Google Scholar]
- 39.North ME, Akbar AN, Borthwick N, et al. Co-stimulation with anti-CD28 (Kolt-2) enhances DNA synthesis by defective T cells in common variable immunodeficiency. Clin Exp Immunol. 1994;95:204–8. doi: 10.1111/j.1365-2249.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borthwick NJ, Bofill M, Hassan I, Panayiotidis P, Janossy G, Salmon M, Akbar AN. Factors that influence activated CD8+ T-cell apoptosis in patients with acute herpesvirus infections: loss of costimulatory molecules CD28, CD5 and CD6 but relative maintenance of Bax and Bcl-X expression. Immunology. 1996;88:508–15. [PMC free article] [PubMed] [Google Scholar]
- 41.Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-α polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159:6236–41. [PubMed] [Google Scholar]
- 42.Stangel M, Schumacher HC, Ruprecht K, Boegner F, Marx P. Immunoglobulins for intravenous use inhibit TNF alpha cytotoxicity in vitro. Immunol Invest. 1997;26:569–78. doi: 10.3109/08820139709088541. [DOI] [PubMed] [Google Scholar]