Abstract
The reduced incidence of graft versus host disease following the use of human cord blood as a source of stem cells for bone marrow reconstitution challenges our understanding of the immunocompetence of newborn T cells. Newborn CD4+ T cells express mainly the CD45RA phenotype and have been considered to respond comparably to adult CD4+ T cells exhibiting the CD45RA phenotype. We compared the in vitro kinetics of phenotypic conversion of newborn and adult CD4+CD45RA+ T cells to CD4+CD45RO+ T cells. The cytokine profile and B cell helper activity of the converted CD4+CD45RO+ T cell population were also determined. Newborn CD4+CD45RA+ T cells were converted to CD4+CD45RO+ with significantly faster time kinetics than adult CD4+CD45RA+ T cells, following either phytohaemagglutinin (PHA) or anti-CD2 activation. Freshly purified newborn naive T cells did not produce IL-2, IL-4 or interferon-gamma (IFN-γ) following stimulation, whereas adult naive T cells secreted IL-2 and adult-derived CD4+CD45RO+ T cells secreted all three cytokines under the same stimulatory conditions. However, newborn and adult CD4+CD45RA+ T cells, following primary stimulation and maturation in vitro, acquired the ability to secrete a Th1-type cytokine profile of IL-2 and IFN-γ after secondary stimulation. Newborn CD4+ naive T cells that acquired the CD45RO phenotype in vitro also gained B cell helper activity equivalent to that of adult in vitro matured CD4+ naive T cells. These findings suggest that newborn and adult CD4+CD45RA+ T cell subsets are differentially responsive to various stimuli. They show that newborn CD4+CD45RA+ naive T cells can transform more quickly than their adult counterparts into functionally equivalent CD4+CD45RO+ T cells, a process that may be important to counteract the immature immune environment which exists in the newborn.
Keywords: newborn, CD4+CD45RA+, CD4+CD45RO+, in vitro conversion
INTRODUCTION
Expression of CD45 isoforms in human T cells distinguishes naive (CD45RA+) from memory (CD45RO+) T cells [1,2]. This definition is based on the ability of CD45RO+ T cells, but not CD45RA+T cells, to respond to recall antigen in vitro [1–3], and the fact that the majority of CD4+T cells in cord blood express the CD45RA antigen [4,5]. Furthermore, upon appropriate stimulation, naive CD4+CD45RA+ T cells can be phenotypically converted in vitro into memory type CD4+CD45RO+ T cells and acquire memory cell characteristics such as helper activity for B cells, production of IL-4 and interferon-gamma (IFN-γ) and expression of increased levels of adhesion molecules [1,6–11]. However, it is not clear if this differentiation process is reversible, as the CD45RA antigen can be re-expressed by a minority of cloned CD45RA− human T cells [12] and co-expressed with CD45RO in a cyclic fashion in human T cell lines [13]. Interconversion of CD45 isoforms in vivo has also been shown not to be unidirectional in rats [14], but rat memory and naive CD45 T cell fractions are considered not to be equivalent with human CD45 T cell subsets [15,16].
A greater understanding of the human newborn immune system is an important area of immunology, especially in respect of the high frequency of neonatal sepsis [17–19] and the recent uses of umbilical cord blood as a source of stem cells for bone marrow reconstitution [20–22], which show a reduced incidence of graft versus host disease (GVHD) compared with using adult bone marrow. Compared with adult T cells, newborn T cells exhibit decreased B cell helper activity [23–25] and secrete less IL-2, IFN-γ and IL-4 in response to a variety of stimuli [26–30]. Cord blood T cells proliferate normally following activation by alloantigen [31]. However, following repeated allogeneic stimulation, little or no cytotoxic T cell activity is generated [31], and tolerance is induced after restimulation of cord T cell primary cultures with allogeneic cells as measured by proliferative unresponsiveness [32]. Previous published studies of newborn T cell responses used unfractionated T cell populations, and have compared responses of newborn CD45RA+T cells with both adult CD45RA+ plus CD45RO+ T cells [33,34]. It may be that newborn CD4+CD45RA+ T cells are truly ‘naive’ with regard to prior foreign antigen exposure, whereas adult CD4+CD45RA+ T cells that develop in a different immune environment are in a more developed stage of ontogeny.
The purpose of this study was to directly compare the kinetics of the phenotypic in vitro conversion of newborn and adult CD4+CD45RA+ T cells with CD4+CD45RO+ T cells and to characterize the effector responses of the resultant populations.
MATERIALS AND METHODS
Culture media, cytokines and MoAbs
Mitogenic anti-CD2 antibodies, AICD2M1 and AICD2M2, were a gift from Dr S. Meuer (Deutsches Krebforschungszentrum, Heidelberg, Germany) and used at a concentration of 0.25 μg/ml. Phorbol myristate acetate (PMA; 10 μg/ml) and pokeweed mitogen (PWM; 1:1000 dilution) were purchased from Gibco (Paisley, UK). Ionomycin (1 μg/ml) and phytohaemagglutinin (PHA; 5 μg/ml) were obtained from Sigma (Poole, UK). Human rIL-2 (sp. act. 2.0 × 106 U/mg; Boehringer Mannheim, Lewes, UK) was used at a concentration of 10 U/ml. All cell cultures were carried out in complete medium consisting of RPMI 1640 medium (Gibco) supplemented with l-glutamine 2 mm, gentamycin 50 μg/ml, fungizone 2 μg/ml and 10% heat-inactivated fetal calf serum (FCS; Gibco).
Purification of lymphocyte subpopulations
Adult CD4+CD45RA+ and CD4+CD45RO+ T cell subsets were isolated from adult peripheral blood and newborn CD4+CD45RA+ T cell subsets from human umbilical cord blood as described [35]. Briefly, mononuclear cells were isolated by Ficoll–Hypaque density gradient centrifugation and depleted of adherent cells by plastic adherence followed by nylon wool adherence. CD4+CD45RA+ T cells were obtained from the non-adherent T cell population by negative selection, using Dynabeads coated with a range of specific MoAbs (Dynal A.S., Oslo, Norway). At each of the three depletion cycles used, the cell/bead mixture was centrifuged five times at 100 g for 5 min at 25°C and left for a further 7 min. To obtain CD4+CD45RA+ T cells, anti-CD45RO-coated beads were added to cycle 3 in the procedure. The resultant CD4+CD45RA+ populations were > 99% viable and > 98% CD3+, CD4+ and CD45RA+. They contained < 1.0% CD8+, CD16+, CD19+, CD14+, HLA-DR+ and double-positive CD45RA/RO+cells as determined by flow cytometry [35]. Purity was initially defined by non-responsiveness to PHA at a final concentration of 5 μg/ml. To obtain CD4+CD45RO+ T cells, anti-CD45RA-coated beads were added to cycle 3 instead of anti-CD45RO-coated beads.
As a source of accessory cells, adherent mononuclear cells (1 × 107 cells/ml) were removed from the plastic surface of the tissue culture flask and treated with mitomycin-C (25 μg/ml) (Sigma) for 1 h at 37°C. The number of CD14+ monocytes present was determined by FACScan analysis. To obtain B cell preparations, adult peripheral blood mononuclear cells (PBMC) were treated with 1 volume of 20 mm L-Leu methyl ester solution (Sigma) for 45 min to deplete monocytes. T cells were separated from non-T cells by E-rosette formation using aminoethylisothiouronium bromide-treated sheep erythrocytes (Becton Dickinson, San Jose, CA) [36]. E-rosette-forming cells (E+) were separated from non-rosetting cells (E−) by centrifugation over Ficoll–Hypaque. E− cells were recovered from the interface, treated again with sheep erythrocytes, and used as a source of B cells.
Phenotypic conversion of CD4+CD45RA+ T cells to CD4+CD45RO+ T cells
Ten millilitres of adult CD4+CD45RA+, newborn CD4+CD45RA+ or adult CD4+CD45RO+ T cells (1 × 106 cells/ml) were added to 75-cm2 flasks (Nunclon, Roskilde, Denmark) (day 1). Either 2% monocytes plus PHA or 4% monocytes plus anti-CD2 MoAbs were added. On days 4, 6, 8, 10 and 12 post-activation, aliquots of the T cell subsets were removed in order to determine viability, growth (by ethidium bromide/acridine orange staining) and phenotype (by flow cytometry (FCM) analysis). At all time points the viability of the cell populations was > 97%. The percentage of the in vitro matured T cell subsets expressing the CD45RA and CD45RO cell surface antigens on specific days was analysed by FCM. The concentration of the remaining cells was adjusted to 1 × 106 cells/ml and IL-2 was added on days 4, 8 and 12, to maintain cell viability and promote propagation.
Measurement of B cell helper activity of T cell subsets
Fifty microlitres of a range of cell concentrations (0.2–10 × 104 cells/well) of either fresh or in vitro matured newborn CD4+CD45RA+, adult CD4+CD45RA+ or adult CD4+CD45RO+ T cell subsets were added in triplicate to 96-well round-bottomed microtitre plates (Nunc). Adult E− cells (100 μl; 6 × 104 cells/well) and 5 μl of PWM were added to appropriate wells, and the plate was incubated at 37°C. The optimum time kinetics for the induction of helper function was found to vary but occurred in all experiments from day 8–10 after PHA activation or from day 10–12 after anti-CD2 activation. The concentration of IgG present was measured by a standard sandwich ELISA. In brief, 96-well microtitre plates (Nunclon) were coated with 100 μl of a solution containing goat anti-human anti-IgG antibody (10 μg/ml; Cappel, Durham, NC) in 50 mm sodium carbonate buffer pH 9.6. Cell supernatants, diluted in PBS pH 7.2 containing 0.05% Tween-20, were next added. The amount of bound antibody was determined by the addition of horseradish peroxidase (HRP)-conjugated mouse anti-IgG antibody (Tago Immunologicals, Burlingame, CA), followed by orthophenylenediamine (OPD; Sigma) and hydrogen peroxide as chromogen and substrate, respectively. The average baseline value for IgG production by E− cells stimulated with PWM from which the value obtained for E− cells alone was subtracted was 50 ± 40 ng/ml. The relative response for each experiment was calculated as: (IgG (ng/ml) in sample well/IgG (ng/ml) in control wells (no modulator cells)).
Measurement of cytokines
Two hundred microlitres of either freshly prepared or in vitro matured T cell subsets containing adult CD4+CD45RA+, newborn CD4+CD45RA+ or adult CD4+CD45RO+ T cell subsets (2 × 105 cells/well) were co-cultured in 96-well flat-bottomed microtitre plates (Nunclon) with PMA and ionomycin. The plates were incubated for 48 h at 37°C, after which 180 μl of supernatant were removed. Levels of IFN-γ, IL-4 or IL-2 in the supernatant were measured using solid-phase enzyme amplified sensitivity immunoassay (EASIA) techniques as described by the manufacturers (Medgenix, Watford, UK). These kits use several MoAbs directed against distinct epitopes of the cytokine being measured, giving rise to highly sensitive assays with extended standard range and short incubation times.
Statistical analysis
The Mann–Whitney Wilcoxan rank order test using the statistical computer program, Statworks, was used to determine significant differences between values of adult and newborn samples. P < 0.05 was considered significant.
RESULTS
Kinetics of expression of CD45RA and CD45RO antigens on newborn and adult T cells during prolonged culture in vitro
Adult and newborn CD4+CD45RA+ T cells were activated by PHA or anti-CD2 MoAbs and propagated in IL-2 over a 10–12-day time period. Adult CD4+CD45RO+ T cells were similarly treated, and as expected, the phenotype of these cells (CD45RA−RO+) did not alter over the time period examined (data not shown).
Both adult and newborn PHA-activated CD4+CD45RA+ T cells lost expression of CD45RA antigen on their cell surface after 10 days (Fig. 1a), while CD45RA antigen expression gradually, but not completely, decreased on anti-CD2-activated CD4+CD45RA+ T cells (Fig. 1d). The kinetics of loss of newborn CD45RA antigen was significantly faster than that of adults (P < 0.01). For example, by day 4 post-PHA activation, 11 ± 3% of newborn CD4+ T cells expressed CD45RA, compared with 37 ± 7% of adult CD4+ T cells. Similarly, by day 4, 51 ± 7% and 76 ± 7% of newborn and adult anti-CD2-activated CD4+ T cells were CD45RA+, respectively.
Fig. 1.
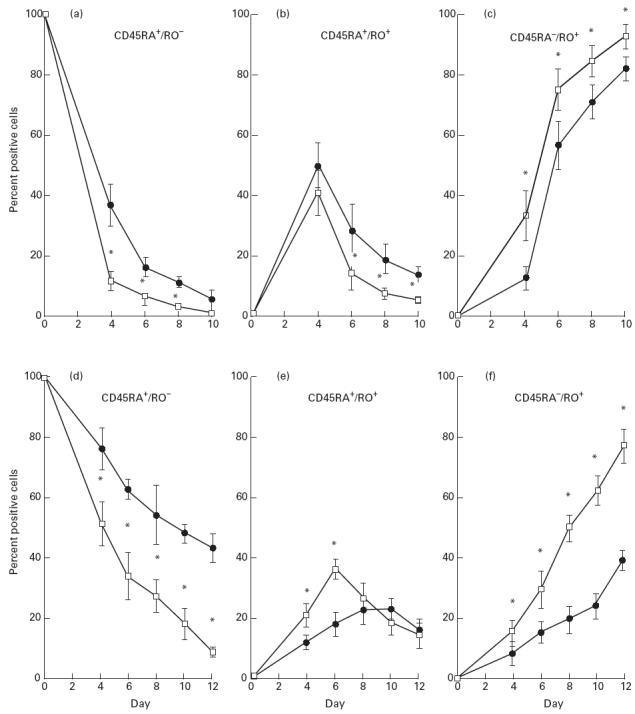
Kinetics of expression of CD45RA and CD45RO antigens on newborn and adult T cells during prolonged phytohaemagglutinin (PHA) and anti-CD2 MoAb activation. Adult (•, n = 7) and newborn (□, n = 6) CD4+CD45RA+ T cells (1 × 106 cells/ml) were co-cultured with PHA plus 2% accessory cells (a,b,c) or anti-CD2 MoAbs plus 4% accessory cells (d,e,f), and cultures were propagated in IL-2. On specified days, cell aliquots were removed, washed thoroughly, and double-stained with FITC-labelled anti-CD45RA MoAbs and PE-labelled anti-CD45RO MoAbs (Dakopatts, High Wycombe, UK). Flow cytometric analysis was performed on a Becton Dickinson FACScan. Data are presented as the average percentage (+ 1 s.d.) of cells which were CD45RA+RO− (a,d), CD45RA+RO+ (b,e), or CD45RA−RO+ (c,f) at a given time point. *P < 0.05 compared with adult values.
A transient population of double-positive CD45RA+RO+ cells was observed for both newborn and adult activated CD4+ T cells (Fig. 1b,e). A decrease in this double-positive population paralleled a rapid increase in the percentage of newborn and adult CD45RA−RO+ T cells (Fig. 1c,f). By day 10, only 5 ± 2% newborn and 14 ± 3% adult PHA-activated CD4+ T cells were double-positive (CD45RA+RO+), whereas 93 ± 4% newborn and 83 ± 4% adult T cells expressed CD45RO alone (CD45RA−RO+).
Newborn CD4+ naive T cells, co-cultured with anti-CD2 MoAbs plus IL-2, lost the naive CD45RA+RO− phenotype (9%) and acquired the memory phenotype CD45RA−RO+ (92%) after 12 days. In contrast, 43% of adult CD4+ T cells still retained the CD45RA antigen after 12 days and only 46% expressed the CD45RA−RO+ phenotype.
Growth kinetics of newborn and adult CD4+CD45RA+ T cells and adult CD4+CD45RO+ T cells
No cell growth had occurred 4 days after PHA stimulation, after which time exogenous IL-2 was added to the culture medium. By day 6, an increase in cell growth from 1 × 107 cells to 1.7 ± 0.3 × 107 and to 1.6 ± 0.2 × 107 for adult and newborn CD4+CD45RA+ T cells, respectively, was measured (Fig. 2). In contrast, only 1.2 ± 0.1 × 107 of adult CD4+CD45RO+ T cells were present by day 6. No significant difference in the growth kinetics of adult and newborn CD4+CD45RA+ T cells was found. However, the overall rate of CD4+CD45RO+ T cell growth was significantly lower on days 6, 8 and 10 compared with CD4+CD45RA+ T cells (P < 0.02).
Fig. 2.
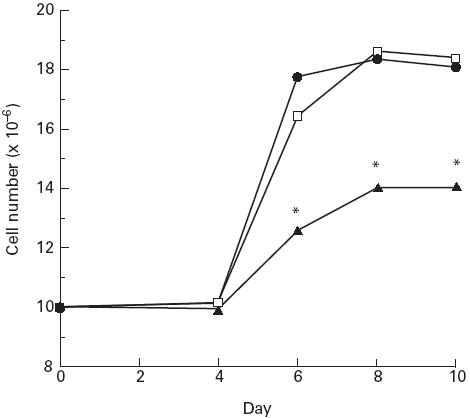
Growth kinetics of newborn and adult CD4+CD45RA+ T cells and adult CD4+CD45RO+ T cells. Ten million newborn CD4+CD45RA+ (n = 5), adult CD4+CD45RA+ (n = 5) or adult CD4+CD45RO+ (n = 5) T cell subsets were cultured with phytohaemagglutinin (PHA) plus 2% accessory cells and propagated in IL-2. On specified days, cell aliquots were removed and the overall cell number present was estimated using EB/AO staining and microscopic analysis. Data are presented as the average cell number for each cell type, at each time point. *P < 0.05 compared with adult CD4+CD45RA+ values. •, Adult CD45RA+/RO−; □, newborn CD45RA+/RO−; ▴, adult CD45RA−/RO+.
B cell helper activity of freshly purified and in vitro matured newborn and adult T cell subsets
The ability of freshly purified and in vitro matured newborn and adult CD4+CD45RA+ T cells to augment B cell IgG production in a PWM-driven system was examined. Neither newborn nor adult CD4+CD45RA+ T cells were capable of inducing IgG secretion from B cells at any cell concentration examined. In contrast, as few as 0.2 × 104 adult CD4+CD45RO+ T cells enhanced IgG secretion 10 ± 0.5-fold, and addition of 4 × 104 of these T cells resulted in an optimum increase (21 ± 3-fold) of B cell IgG production (Fig. 3a). After prolonged co-culture in vitro with either PHA or anti-CD2 MoAbs, both adult and newborn CD4+CD45RA+ T cell subsets acquired the ability to increase B cell immunoglobulin production (Fig. 3b,c). For example, 4 × 104 PHA-preactivated CD4+CD45RA+ T cells caused a 5.5 ± 2.5 and 5.8 ± 2.7-fold increase in IgG production for newborns and adults, respectively, compared with when no modulator cells were added. Similarly, 4 × 104 anti-CD2 preactivated CD4+CD45RA+ T cells augmented IgG production by 6.5 ± 2.7-fold for newborns and by 5.9 ± 2.9-fold for adults. CD4+CD45RO+ T cells retained their ability to augment IgG production after ongoing culture with PHA or with anti-CD2 MoAbs. There were no significant differences found between either the activities of adult and newborn CD4+CD45RA+ T cells or between these subsets and adult CD4+CD45RO+ T cells at any cell concentration examined (P > 0.05).
Fig. 3.
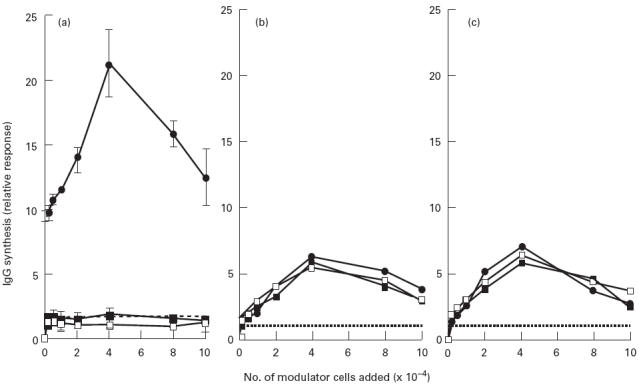
Helper activities of freshly purified and in vitro matured T cell subsets. A range of concentrations (0.2–10 × 104 cells/well) of newborn and adult CD4+CD45RA+ T cells and adult CD4+CD45RO+ T cells that were either freshly purified (a), preactivated using phytohaemagglutinin (PHA) plus 2% accessory cells and propagated in IL-2 for 8–10 days (b), or preactivated using anti-CD2 MoAbs and propagated in IL-2 for 10–12 days (c), were co-cultured with 6 × 104 allogeneic E− cells/well and pokeweed mitogen (PWM). After 7 days culture, supernatants were harvested for measurement of IgG production by ELISA. The values represent the mean from triplicate cultures in eight experiments. To avoid confusion in data presentation, error bars have been omitted from (b) and (c). S.d. ranged between 0.1 and 0.4 for each data point. •, Adult CD45RA−/RO+; ▪, Adult CD45RA+/RO−; □, newborn CD45RA+/RO−.
Cytokine production by freshly purified and matured in vitro newborn and adult T cell subsets
The ability of newborn and adult CD4+ T cell subsets to produce IL-2, IFN-γ or IL-4 was examined (Table 1). After primary stimulation with PMA plus ionomycin, freshly purified adult CD4+CD45RO+ T cells secreted IL-2, IL-4 and IFN-γ. Under similar conditions, adult CD4+CD45RA+ T cells produced only IL-2, and no cytokines were detected using newborn CD4+CD45RA+ T cells. After primary stimulation in vitro with PHA plus IL-2 for 6 days, followed by stimulation with PMA plus ionomycin, newborn CD4+CD45RA+ T cells acquired the ability to secreted IL-2. These in vitro matured newborn and adult CD4+CD45RA+ T cells also secreted IFN-γ, which was significantly lower (P < 0.05) with newborn cells, but they did not produce detectable levels of IL-4 under these conditions.
Table 1.
Cytokine production by T cell subsets
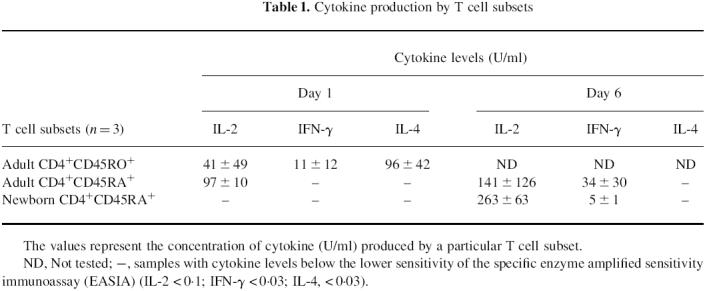
The values represent the concentration of cytokine (U/ml) produced by a particular T cell subset.
ND, Not tested; –, samples with cytokine levels below the lower sensitivity of the specific enzyme amplified sensitivity immunoassay (EASIA) (IL-2 < 0.1; IFN-g < 0.03; IL-4, < 0.03).
DISCUSSION
We have previously hypothesized that newborn CD4+CD45RA+ T cells represent precursors of adult CD4+CD45RA+ T cells that can further develop into CD4+CD45RO+ T cells upon appropriate activation [34]. It may be that the time span needed to convert newborn CD4+CD45RA+ T cells in vitro into CD4+CD45RO+ T cells differs form that needed for adult CD4+CD45RA+ T cells. This study confirms a previous report showing that newborn CD4+CD45RA+ naive T cells, activated for a prolonged time in vitro, are converted into CD4+CD45RO+ effector T cells [37]. Interestingly, two recent reports have shown that expression of CD45RO+ on neonatal blood T cells is induced in vivo, early in infection, suggesting that there is rapid conversion of the CD45RA+ to CD45RO+ phenotype on newborn CD4 T cells [38,39]. In this study, by directly comparing newborn and adult CD45RA+ T cells we have shown that the kinetics of this phenotypic transformation is significantly faster for newborn naive T cells than for adult naive T cells. Furthermore, in vitro matured newborn and adult naive T cells were found to acquire equivalent effector function, characteristic of memory T cells, such as B cell helper function and production of IFN-γ [6–11].
The extent of phenotypic transformation of cells is related to their rate of proliferation, since presynthesized CD45RA molecules are diluted as stimulated cells divide [8]. The incomplete phenotypic conversion of adult anti-CD2-activated naive T cells seen in this study may be attributed to a lower sensitivity of these cells to anti-CD2 MoAbs compared with newborn naive T cells [35]. Moreover, adult CD4+CD45RA+ T cells may be more dependent on accessory cell costimulatory signals than newborn CD4+CD45RA+ T cells [35]. In support of this, it has been shown that the Fc receptors on accessory cells can cross-link anti-CD2 MoAbs [40,41]. However, addition of a range of concentrations of anti-CD2 MoAbs and IL-2 did not have any discernible effect on phenotype conversion (data not shown), indicating that these were not limiting factors.
One possible explanation for the phenotypic conversion could be that a small number of CD4+CD45RO+ T cells contaminated the freshly isolated CD4+CD45RA+ subset and preferentially responded to PHA or IL-2, or both, so that their relative frequency was dramatically increased during the in vitro culture periods. However, CD4+CD45RA+ T cells, and not CD4+CD45RO+ T cells, preferentially respond to PHA [37]. Secondly, the growth rate of CD4+CD45RA+ T cells in IL-2 alone is greater than that of CD4+CD45RO+ T cells in IL-2 [6], and as shown in this study the growth rate of PHA-activated adult and newborn CD4+CD45RA+ T cells is faster compared with adult CD4+CD45RO+ T cells (Fig. 2).
We confirmed that adult CD4+CD45RO+ T cells induce B cell IgG secretion in a PWM-driven system, whereas neither adult nor newborn naive CD4+CD45RA+ T cells possess B cell helper activity (Fig. 3a). Adult CD4+CD45RO+ T cells that were matured in vitro maintained their capacity to augment PWM-mediated IgG production, albeit at a lower level of activity (Fig. 3b,c). This result has been previously noted [37], although the reason is not understood.
These results support a view that CD45RO phenotype on CD4 T cells is associated with ‘helper’ T cell function. Twelve days after anti-CD2 activation, 92% of newborn and 46% of adult CD4+CD45RA+ T cells expressed the CD45RO antigen, but despite this phenotypic difference, ‘helper’ activity of the converted cells was equivalent over a wide range of cell concentrations. A number of possible explanations arise to explain this result. First, adult and newborn anti-CD2-activated CD4+ CD45RA+ T cells may have expressed comparable phenotypes after 7 days culture in the PWM-driven ‘helper’ assay system, as PWM can induce the phenotypic conversion of CD4+CD45RA+ T cells into CD4+CD45RO+ T cells after continuous culture [11]. Second, the adult anti-CD2-preactivated CD4+CD45RA+ T cell subset (46% CD45RO+), may contain, on a cell to cell basis, more potent ‘helper’ T cells than their newborn counterparts (92% CD45RO+). Finally, the expression of a particular CD45 isoform might not always be associated with a particular function. For example, Rothstein et al. [9] activated CD4+CD45RA+ T cells with concanavalin A (Con A), and found that although a proportion of cells became CD45RA−and CD45RO+, this subset retained its original B cell suppressor activity and did not acquire B cell helper activity when isolated.
Freshly purified newborn CD4+CD45RA+ T cells did not secrete IL-2, unlike adult CD4+CD45RA+ T cells that were stimulated under the same experimental conditions. This lack of IL-2 may reflect differential sensitivity to the stimuli used or differential kinetics of cytokine secretion between newborn and adult CD4+CD45RA+ T cells, as has been previously suggested [35].
These findings indicate that newborn and adult CD4+CD45RA+ naive T cells acquired equivalent CD4+CD45RO+ effector T cell function after in vitro activation, such as B cell helper activity and polarization into production of Th1-type cytokines. The rapid conversion of newborn CD4+CD45RA+ T cells into memory CD4+CD45RO+ T cells demonstrated in this study, may parallel a fast, efficient response in vivo, which would be advantageous to the newborn within an otherwise immature immune environment.
REFERENCES
- 1.Akbar AN, Terry L, Timms A, Beverley PCL, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–6. [PubMed] [Google Scholar]
- 2.Sanders ME, Makogoba MW, Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988;9:195–9. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- 3.Plebanski M, Saunders M, Burtles SS, Crowe S, Hooper DC. Primary and secondary human in vitro T-cell responses to soluble antigens are mediated by subsets bearing different CD45 isoforms. Immunol. 1992;75:86–91. [PMC free article] [PubMed] [Google Scholar]
- 4.De Paoli P, Battistin S, Santini G. Age related changes in human lymphocyte subsets: progressive reduction of the CD4/CD45R (suppressor inducer) population. Clin Immunol Immunopathol. 1988;48:290–6. doi: 10.1016/0090-1229(88)90022-0. [DOI] [PubMed] [Google Scholar]
- 5.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, De Bruyene M. Development and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13:215–8. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 6.Clement LT, Yamashita N, Martin AM. The functionally distinct subpopulations of human helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988;141:1464–70. [PubMed] [Google Scholar]
- 7.Serra HM, Krowka JF, Ledbetter JA, Pilarski LM. Loss of CD45R (Lp220) represents a post-thymic T cell differentiation event. J Immunol. 1988;140:1441–5. [PubMed] [Google Scholar]
- 8.Kristensson K, Dohlsten M, Fischer H, Ericsson PO, Hedlund G, Sjogren HO, Carlsson R. Phenotypical and functional differentiation of CD4+/CD45RA+ human T cells following polyclonal activation. Scand J Immunol. 1990;32:243–53. doi: 10.1111/j.1365-3083.1990.tb02917.x. [DOI] [PubMed] [Google Scholar]
- 9.Rothstein DM, Sohen S, Daley JF, Schlossman SF, Morimoto C. CD4+CD45RA+ and CD4+CD45RA− T cell subsets in man maintain distinct function and CD45RA expression persists on a subpopulation of CD45RA+ cells after activation with ConA. Cell Immunol. 1990;129:449–67. doi: 10.1016/0008-8749(90)90220-l. [DOI] [PubMed] [Google Scholar]
- 10.Wallace L, Beverley PCL. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunol. 1990;69:460–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer JM, Plaza A, Kreisler M, Diaz-Espada F. Differential interleukin secretion by in vitro activated human CD45RA and CD45RO CD4+ T cell subsets. Cell Immunol. 1992;141:10–20. doi: 10.1016/0008-8749(92)90123-7. [DOI] [PubMed] [Google Scholar]
- 12.Brod SA, Rudd CE, Purvee M, Hafler DA. Lymphokine regulation of CD45R expression on human T cell clones. J Exp Med. 1989;170:214–51. doi: 10.1084/jem.170.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothstein DM, Yamada A, Schlossman SF, Morimoto C. Cyclic regulation of CD45 isoform expression in a long-term human CD4+CD45RA+ T cell line. J Immunol. 1991;146:1175–82. [PubMed] [Google Scholar]
- 14.Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163–6. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 15.Merkenschlager M. Confusion over CD45 iosform. Nature. 1991;352:28. doi: 10.1038/352028a0. [DOI] [PubMed] [Google Scholar]
- 16.Clement LT. Isoforms of the CD45 common leucocyte antigen family: markers for human T-cell differentiation. J Clin Immunol. 1992;12:1–10. doi: 10.1007/BF00918266. [DOI] [PubMed] [Google Scholar]
- 17.LaGamma EF, Drusin LM, Mackles AW, Machalek S, Auld PAM. Neonatal infections. An important determinant of late NICN mortality in infants less than 1,000 g at birth. Am J Dis Child. 1983;137:831–8. doi: 10.1001/archpedi.1983.02140350016005. [DOI] [PubMed] [Google Scholar]
- 18.Baley JE, Goldfarb J. Neonatal infections. In: Klaus MH, Fanaroff AA, editors. Care of the high-risk neonate. 4. Philadelphia: W.B. Saunders, Co.; 1993. pp. 323–44. [Google Scholar]
- 19.Vesikari T, Janas M, Gronroos P, et al. Neonatal septicemia. Arch Dis Child. 1985;60:542–6. doi: 10.1136/adc.60.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable haematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:328–32. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluckman E, Broxmeyer HE, Aurbrach A, et al. Haematopoietic reconstitution in a patient with Fanconi''s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 22.Van Bekkum DW. In: The biology of bone marrow transplantation. Gale RP, Fox CE, editors. San Diego: Academic Press; 1980. p. 175. [Google Scholar]
- 23.Andersson U, Bird AG, Britton S, Palacios R. Humoral and cellular immunity in human studies at the cell level from birth to two years of age. Immunol Rev. 1991;57:37. doi: 10.1111/j.1600-065x.1981.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 24.Splawaski JB, Jelinek DF, Lipsky PE. Delineation of the functional capacity of human neonatal lymphocytes. J Clin Invest. 1991;87:545–50. doi: 10.1172/JCI115029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno Y, Ichihara M, Hasuig M, Maruyama H, Miyawaki T, Taniguchi N, Komiyama A. T-cell dependent production of IgG by human cord blood B cells in reconstituted SCID mouse. Scand J Immunol. 1992;35:415–22. doi: 10.1111/j.1365-3083.1992.tb02876.x. [DOI] [PubMed] [Google Scholar]
- 26.Bertotto A, Gerli R, Lanfrancone L, Crupi S, Arcangeli C, Cernetti C, Spinozzi F, Rambotti P. Activation of cord T lymphocytes. II. Cellular and molecular analysis of the defective response induced by anti-CD3 monoclonal antibody. Cell Immunol. 1990;127:247–53. doi: 10.1016/0008-8749(90)90130-j. [DOI] [PubMed] [Google Scholar]
- 27.Miyawaki T, Seki H, Taga K, Sato H, Taniguchi N. Dissociated production of interleukin-2 and immune (γ) interferon by phytohaemagglutinin stimulated lymphocytes in healthy infants. Clin Exp Immunol. 1985;59:505–10. [PMC free article] [PubMed] [Google Scholar]
- 28.Wakasugi N, Virelizier J, Arenzana-Seisdedos F, Rothhut B, Heurta JM, Russo-Marie F, Fiers WE. Defective IFN-γ production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985;134:172–8. [PubMed] [Google Scholar]
- 29.Lewis DB, Yu CC, Meyer J, English BK, Kahn SJ, Wilson CB. Cellular and molecular mechanism for reduced interleukin 4 and interferon-γ production by neonatal T cells. J Clin Invest. 1991;87:194–8. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson CB. The ontogeny of T lymphocyte maturation and function. J Pediatr. 1991;118:S4. doi: 10.1016/s0022-3476(05)82182-1. [DOI] [PubMed] [Google Scholar]
- 31.Risdon G, Gaddy J, Stehman FB, Broxmeyer HE. Proliferation and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cell Immunol. 1994;154:14–24. doi: 10.1006/cimm.1994.1053. [DOI] [PubMed] [Google Scholar]
- 32.Risdon G, Gaddy J, Broxmeyer HE. Allogeneic responses of human umbilical cord blood. Blood Cells. 1994;20:566–72. [PubMed] [Google Scholar]
- 33.Gerli R, Bertotto A, Crupi S, et al. Activation of cord T lymphocytes. I. Evidence for a defective T cell mitogenesis induced through the CD2 molecule. J Immunol. 1989;142:2583–9. [PubMed] [Google Scholar]
- 34.Hassan J, Reen DJ. Neonatal CD4+CD45RA+ T cells. Precursors of adult CD4+CD45RA+ T cells? Res Immunol. 1993;144:87–92. doi: 10.1016/0923-2494(93)80064-6. [DOI] [PubMed] [Google Scholar]
- 35.Early EM, Reen DJ. Antigen-independent responsiveness to interleukin-4 demonstrates differential regulation of newborn human T cells. Eur J Immunol. 1996;26:2885–9. doi: 10.1002/eji.1830261212. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan ME, Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974;5:131–9. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- 37.Clement LT, Vink PE, Bradley GE. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990;145:102–8. [PubMed] [Google Scholar]
- 38.Hodge S, Hodge G, Flowe R, Han P. Surface activation markers of T lymphocytes: role in the detection of infection in neonates. Clin Exp Immunol. 1998;113:33–38. doi: 10.1046/j.1365-2249.1998.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tezuka T, Sugita K, Mizobe N, et al. Transient increase in CD45RO expression on T lymphocytes in infected newborns. Pediatr Res. 1998;43:282–90. doi: 10.1203/00006450-199802000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Huet S, Wakasugi H, Sterkers G, Gilmours J, Turz T, Boumseil L, Bernard A. T cell activation via CD2 (T, gp50): the role of accessory cells in activating resting T cells via CD2. J Immunol. 1986;137:1415–20. [PubMed] [Google Scholar]
- 41.Brottier P, Bounsell L, Gelin C, Bernard A. T cell activation via CD2 (T, gp50) molecules: accessory cells are required to trigger T cell activation via CD2–D66 plus CD2-9.6/T11 epitopes. J Immunol. 1985;135:1624–31. [PubMed] [Google Scholar]


