Abstract
Kawasaki disease (KD) is an acute febrile illness of early childhood, in which the activation of monocytes/macrophages plays a central role in the development of vasculitis during the acute stage of disease. In this study we investigated peripheral blood T cells of 10 patients with KD, focusing on the Th1 and Th2 imbalance, using intracellular cytokine staining and analysis of the cytokine-producing T cells by flow cytometry. We observed a decrease in the numbers of IFN-γ-producing, but not IL-4-producing, CD3+ T cells, during the acute stage. Our results suggest that there is an imbalance of Th1 and Th2 subsets during the acute stage of KD.
Keywords: Kawasaki disease, Th1/Th2 T cell
INTRODUCTION
Kawasaki disease (KD) is an acute febrile illness of unknown aetiology, which occurs primarily during infancy and early childhood. Histopathological findings in KD indicate panvasculitis with endothelial necrosis, and infiltration of mononuclear cells into small and medium-sized blood vessels. Less than 1% of patients with KD may die due to aneurysms and/or thrombosis caused by coronary arteritis.
Activation of monocytes/macrophages plays a central role in the development of vasculitis during acute KD. At this stage of disease, we have reported an increase in the CD14+ monocyte/macrophage count [1,2], and increased serum levels of tumour necrosis factor-alpha (TNF-α) [1–3], soluble TNF receptor [4], and soluble intercellular adhesion molecule-1 (ICAM-1) [5]. We have also demonstrated activation of monocytes/macrophages by electron microscopy and immunohistochemistry [6].
Infiltration of activated T cells expressing HLA-DR antigen in biopsy skin lesions [7] and coronary vascular lesions at autopsy has been reported [8]. However, it is still uncertain whether peripheral blood T cells are activated in acute KD, as some reports have provided evidence of T cell activation [9,10], whereas others have not [2,11–14]. Leung et al. reported that peripheral blood T cell activation with expansion of T cell receptor (TCR) Vβ2 and Vβ8 is evident during the acute stage, which is caused by the production of toxic shock syndrome toxin-1 superantigens by Staphylococcus aureus [15–17]. However, others have been unable to provide similar evidence [18–20].
On the basis of the cytokine secretion pattern, two functional classes of murine T helper cells have been identified. T helper 1 (Th1) cells predominantly produce IL-2 and IFN-γ and play a major role in cell-mediated immunity, largely mediated by IFN-γ. Th2 cells produce IL-4, IL-5 and IL-10, and are responsible for antibody-dominated immunity. Th1 and Th2 subsets have been implicated in the regulation of many immune responses [21].
Recently a method for intracellular cytokine staining has been developed, which enables the detection of cytokine-producing cells by single laser flow cytometry [22,23]. We have applied this technique to study the intracellular levels of IFN-γ and IL-4 in circulating CD3+ T cells obtained from KD patients to determine whether peripheral blood T cells are activated during acute KD.
PATIENTS AND METHODS
Patients and control subjects
We studied peripheral blood obtained from 10 patients with KD who were seen at our hospital between July 1997 and April 1998, and eight healthy children. Informed consent was obtained from the subjects' parents before participation in the study.
Kawasaki disease
The 10 patients with KD comprised four boys and six girls (aged 0.3–4.1 years, mean 1.7 years), who met the diagnostic criteria for KD [24]. All patients received standard treatment with intravenous gammaglobulin (IVGG) 400 mg/kg per day (Venilon, Teijin Co.) for 5 days, and oral aspirin (30 mg/kg per day). The onset of illness was defined as the day on which fever appeared. Blood samples were obtained on days 3–7 (4.9 ± 1.3 (mean ± s.d.)) prior to treatment (acute stage), and on days 34–52 (42.9 ± 8.3, convalescent stage). In the five out of 10 patients, samples were obtained on days 9–12 (10.2 ± 1.6, subacute stage) after IVGG treatment. Two-dimensional echocardiography was used to detect the presence of coronary artery lesions (CAL). Coronary arteries with diameters of ≥ 4 mm were classified as abnormal, in accordance with the KD Cardiovascular Lesion Diagnostic Criteria of the Research Committee on KD. None of the patients developed CAL.
Control subjects
We also studied eight healthy children (three boys and five girls, aged 0.6–4 years; mean 1.8 years) as a control group. Control samples were tested in parallel with the patients' samples.
Detection of intracellular cytokines by flow cytometry
We detected intracellular cytokines by flow cytometry as reported previously [22,23]. Briefly, whole blood (500 μl) was cultured in tubes for 4 h at 37°C under 7% CO2 in RPMI 1640 medium. Cells were stimulated with 25 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma Chemical Co, St Louis, MO) and 1 mg/ml ionomycin (Sigma) in the presence of 10 mg/ml brefeldin A (Sigma).
Cultured cells were stained with PerCP-conjugated anti-CD3 MoAb (Becton Dickinson, Mountain View, CA). Erythrocytes were lysed by adding 2 ml of lysing solution (Becton Dickinson) for 10 min. After washing with washing buffer containing PBS with 0.5% bovine serum albumin (BSA) and 0.1% NaN3, leucocytes were permeabilized using the FACS Permeabilizing Solution (Becton Dickinson) for 10 min. Cells were stained with FITC-conjugated anti-IFN-γ MoAb and PE-conjugated anti-IL-4 MoAb for 30 min. As a last step, the cells were washed with the washing buffer and resuspended in 1% paraformaldehyde.
Stained cells were analysed using a FACScan flow cytometer (Becton Dickinson) equipped with a 15-mW argon ion laser and filter settings for FITC (530 nm), PE (585 nm) and PerCP (677 nm).
Statistical analysis
Statistical analyses were performed using the Mann–Whitney U-test and paired Wilcoxon signed rank test for comparison of means.
RESULTS
Figure 1 shows a typical dot plot of IFN-γ and IL-4 staining in CD3+ T cells derived from the peripheral blood of a female KD patient aged 4 years. In this case, there was a decreased percentage of IFN-γ-producing CD3+ T cells at the acute stage in comparison with the convalescent stage. As shown in Fig. 2, the numbers of CD3+ T cells producing IFN-γ were lower in the subjects with acute KD (5.0 ± 3.6% (mean ± s.d.)) than in those at the convalescent stage (14.3 ± 4.8%; P < 0.01) and normal subjects (15.9 ± 3.6%; P < 0.01). After IVGG treatment during the subacute stage, the percentage of IFN-γ-producing CD3+ T cells was increased (9.8 ± 5.2%, n = 5), but was not as high as in the control subjects and the KD patients at the convalescent stage. The percentage of CD3+ T cells producing IL-4 was very low. There were no significant differences in the percentages of CD3+ T cells staining for IL-4 among KD patients during the acute, subacute and convalescent stages, and in normal subjects (1.3 ± 1.0%, 1.8 ± 1.4%, 3.1 ± 3.5%, and 1.3 ± 1.1%, respectively), as shown in Fig. 3. One KD patient whose condition was complicated by atopic dermatitis showed an increase of IL-4-producing CD3+ T cells at the convalescent stage.
Fig. 1.
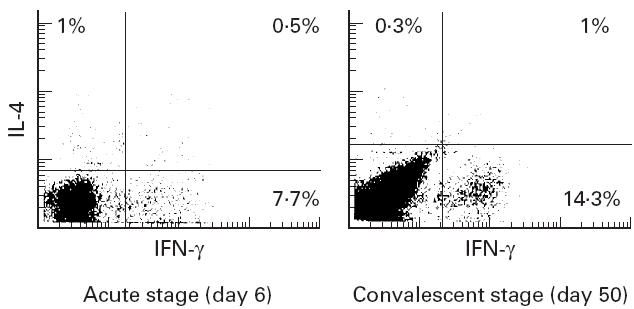
A typical dot plot of IFN-γ and IL-4 staining in CD3+ T cells derived from peripheral blood of a female Kawasaki disease (KD) patient aged 4 years. The numbers show the percentage of cytokine-producing CD3+ T lymphocytes gated on CD3+ T lymphocytes.
Fig. 2.
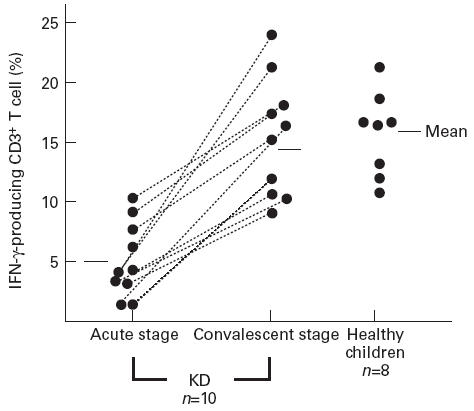
The percentage of IFN-γ-producing CD3+ T lymphocytes gated on CD3+ T lymphocytes in Kawasaki disease (KD) patients and control subjects.
Fig. 3.
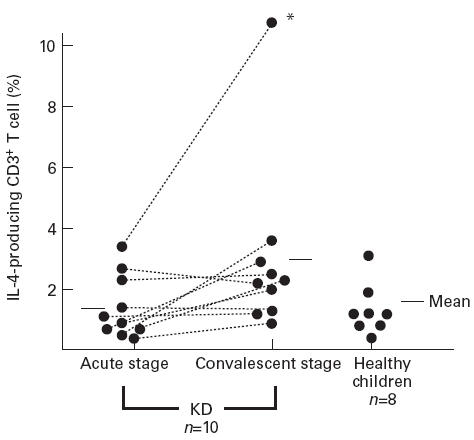
The percentage of IL-4-producing CD3+ T lymphocytes gated on CD3+ T lymphocytes in Kawasaki disease (KD) patients and control subjects. *This case complicated by atopic dermatitis.
DISCUSSION
This is the first investigative report of T cell activation in KD focusing on Th1 and Th2 imbalance using flow cytometry for detection of intracellular cytokines. Using this method, several previous studies have demonstrated Th1 and Th2 imbalance in various immunological diseases. These studies revealed a greatly increased proportion of IL-2- and IFN-γ-producing T cells in bronchoalveolar lavage fluid from asthmatic patients [23], reduced production of IFN-γ- and IL-2-producing T cells in atopic patients [25], and a decreased percentage of T cells capable of secreting IFN-γ, but not IL-4, in patients with multiple sclerosis [26]. When T cells are stimulated with PMA and ionomycin, CD4 molecules on the cell surface become difficult to analyse using anti-CD4 MoAb. Therefore we investigated only CD3+ T cells in the present study.
We found that the number of IL-4-producing CD3+ T cells was much lower than that of IFN-γ-producing CD3+ T cells in both patients with KD and control subjects, as reported previously. There was a decrease of IFN-γ-producing CD3+ T cells, but not IL-4-producing CD3+ T cells, during the acute stage of KD. These findings suggest that there is an imbalance of Th1 and Th2 in the peripheral blood during acute KD. We have already reported that only 30% of KD patients show slightly increased serum levels of IFN-γ [3]. We have also demonstrated that peripheral blood CD4+ and CD8+ T cell counts are decreased [2], and that the cells are not activated, at least in terms of cell surface activation markers such as HLA-DR [12] and LFA-1 [13], and the serum level of soluble CD2 [14].
The decrease of IFN-γ-producing CD3+ T cells may reflect the hypofunction of a proportion of peripheral blood T cells during acute KD. It is not unlikely that this hypofunction of peripheral blood T cells is responsible for the unresponsiveness observed during acute KD. It is not completely clear how T cells acquire their functional unresponsiveness, although it has been reported that a lack of CD28 costimulation through the CD80 and CD86 molecules on antigen-specific cells might play a causative role [27]. It has also been reported that Th1 helper T cells are completely inhibited by prostaglandin E2 (PGE2), whereas Th2 cells are largely unaffected [28]. We have already demonstrated that plasma PGE2 levels are markedly increased during the acute stage of KD [29]. Since we found that IFN-γ-producing CD3+ T cells were decreased, whereas the number of IL-4-producing CD3+ T cells was unchanged, the increased production of PGE2 by activated monocytes/macrophages might be responsible for the decreased activation of Th1-type T cells.
Another possible explanation for the decrease of IFN-γ-producing CD3+ T cells during acute KD is the infiltration of activated T cells expressing the HLA-DR antigen, which has been observed in biopsied skin [7] and coronary vascular lesions at autopsy [8]. It is possible that IFN-γ-producing CD3+ T cells are temporarily withdrawn from the peripheral circulation during acute KD, and that sequestration of IFN-γ-producing CD3+ T cells may be a feature of this disease. A decrease of IFN-γ-producing CD3+ T cells may therefore simply reflect a shift of Th1-type T cells into the vascular tissue compartment.
With regard to Th2-type T cells, we demonstrated that the numbers of IL-4-producing CD3+ T cells were not decreased during acute KD. One patient who had large numbers of IL-4-producing CD3+ T cells at the convalescent stage developed atopic dermatitis. Th2-type T cells are involved in the production of IgE and the development of allergic diseases, and are also responsible for antibody-dominated immunity [21]. It has been reported that the serum levels of IL-4 and IL-10 are increased [30] and that polyclonal B cell activation is evident during acute KD [9]. In addition, we have also reported that KD patients have a background of allergy [31]. Taken together, these findings suggest that Th2-type T cells might be activated during acute KD.
In conclusion, we have demonstrated a decrease in the number of Th1-type CD3+ T cells in the peripheral blood of patients with acute KD. Our results suggest the importance of studying the function of peripheral blood T cells in terms of Th1-type and Th2-type in KD at the acute stage.
REFERENCES
- 1.Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, Yabuta K, Kato H. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–51. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa S, Matsubara T, Yabuta K. Mononuclear cell subsets and coronary artery lesions in Kawasaki disease. Arch Dis Child. 1992;67:706–8. doi: 10.1136/adc.67.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-γ in Kawasaki disease involved coronary artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa S, Matsubara T, Umezawa Y, Okumura K, Yabuta K. Serum levels of p60 soluble tumor necrosis factor receptor during acute Kawasaki disease. J Pediatr. 1994;124:721–5. doi: 10.1016/s0022-3476(05)81361-7. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa S, Imai K, Matsubara T, Yone K, Yachi A, Okumura K, Yabuta K. Increased levels of circulating intercellular adhesion molecule-1 in Kawasaki disease. Arthritis Rheum. 1992;35:672–7. doi: 10.1002/art.1780350611. [DOI] [PubMed] [Google Scholar]
- 6.Koga M, Ishihara T, Takahashi M, Umezawa Y, Furukawa S. Activation of peripheral blood monocytes and macrophages in Kawasaki disease: ultrastructual and immunohistochemical investigation. Pathol Int. 1998;48:512–7. doi: 10.1111/j.1440-1827.1998.tb03942.x. [DOI] [PubMed] [Google Scholar]
- 7.Sugawara T, Hattori S, Hirose S, Furukawa S, Yabuta K, Shirai T. Immunopathology of the skin lesions of Kawasaki disease. In: Schulman ST, editor. Kawasaki disease. New York: Alan R Liss; 1987. pp. 185–92. [PubMed] [Google Scholar]
- 8.Terai M, Kohno Y, Namba M, Umemiya T, Niwa K, Nakajima H, Mikata A. Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol. 1990;21:231–4. doi: 10.1016/0046-8177(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 9.Leung DYM, Chu ET, Wood N, Grady S, Meade R, Geha RS. Immunoregulatory T cell abnormalities in mucocutaneous lymph node syndrome. J Immunol. 1983;130:2002–4. [PubMed] [Google Scholar]
- 10.Barron K, DeCunto C, Montalvo J, Orson F, Lewis D. Abnormalities of immunoregulation in Kawasaki syndrome. J Rheumatol. 1988;15:1243–9. [PubMed] [Google Scholar]
- 11.Terai M, Kohno Y, Niwa K, Toba T, Sakurai N, Nakajima H. Imbalance among T-cell subsets in patients with coronary arterial aneurysms in Kawasaki disease. Am J Cardiol. 1987;60:555–9. doi: 10.1016/0002-9149(87)90304-3. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Matsubara T, Tsuji K, Motohashi T, Okumura K, Yabuta K. Serum soluble CD4 and CD8 levels in Kawasaki disease. Clin Exp Immunol. 1991;86:134–9. doi: 10.1111/j.1365-2249.1991.tb05785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa S, Matsubara T, Tsuji K, Okumura K, Yabuta K. Transient depletion of T cells with bright CD11a/CD18 expression from peripheral circulation during acute Kawasaki disease. Scand J Immunol. 1993;37:377–80. doi: 10.1111/j.1365-3083.1993.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa S, Matsubara T, Obara T, Imai K, Okumura K, Yabuta K. Soluble CD2 levels in serum during acute Kawasaki disease and infectious mononucleosis. J Infect Dis. 1993;167:778–9. doi: 10.1093/infdis/167.3.778. [DOI] [PubMed] [Google Scholar]
- 15.Abe J, Kotzin BL, Jujo K, Melish ME, Glode MP, Kohsaka T, Leung DYM. Selective expansion of T cells expressing T-cell receptor variable regions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–70. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe J, Kotzin BL, Meissner C, et al. Characterization of T-cell repertoire changes in acute Kawasaki disease. J Exp Med. 1993;177:791–6. doi: 10.1084/jem.177.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung DYM, Meissner HC, Fulton DR, Murray DL, Kotzin BL, Schlievert PM. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki disease. Lancet. 1993;342:1385–8. doi: 10.1016/0140-6736(93)92752-f. [DOI] [PubMed] [Google Scholar]
- 18.Pietra BA, De Inocencio J, Giannini EH, Hirsch R. TCRVβ family repertoire and T cell activation markers in Kawasaki disease. J Immunol. 1994;153:1881–8. [PubMed] [Google Scholar]
- 19.Terai M, Miwa K, Williams T, Kabat W, Fukuyama M, Okajima Y, Igarashi H, Schulman ST. The absence of evidence of staphylococcal toxin involvement in the pathogenesis of Kawasaki disease. J Infect Dis. 1995;172:558–61. doi: 10.1093/infdis/172.2.558. [DOI] [PubMed] [Google Scholar]
- 20.Choi J-H, Chwae Y-J, Shim W-S, Kim D-S, Kwon D-H, Kim J-D, Kim S-J. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–6. [PubMed] [Google Scholar]
- 21.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 22.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intercellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 23.Krung N, Madden J, Redington AE, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–26. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki T, Kosaki G, Osawa S, Shigematsu I, Yanagawa S. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6. [PubMed] [Google Scholar]
- 25.Jung T, Lack G, Schauer U, Uberuck W, Rentz H, Gelfand EW, Rieger CHL. Decreased frequency of interferon-γ- and interleukin-2-producing cells in patients with atopic disease measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–27. doi: 10.1016/s0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 26.Crucian B, Dunne P, Friededman H, Ragsdale R, Pross S, Widen R. Detection of altered T helper 1 and T helper 2 cytokine production by peripheral blood mononuclear cells in patients with multiple sclerosis utilizing intracellular cytokine detection by flow cytometry and surface marker analysis. Clin Diagn Lab Immunol. 1996;3:411–6. doi: 10.1128/cdli.3.4.411-416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qun ZY, Lorre K, de Boer M, Ceuppens JL. B7-blocking agents, alone or in combination with cyclosporin A, induce antigen-specific anergy of human memory T cells. J Immunol. 1997;158:4734–40. [PubMed] [Google Scholar]
- 28.Gold KN, Weyand CM, Goronzy JJ. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;6:925–33. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- 29.Lee T, Furukawa S, Fukuda Y, Yabuta K, Kato H. Plasma prostaglandin E2 levels in Kawasaki disease. Prostaglandins Leukot Essent Fatty Acids. 1988;31:53–57. doi: 10.1016/0952-3278(88)90076-2. [DOI] [PubMed] [Google Scholar]
- 30.Hirao J, Hibi S, Andoh T, Ichimura T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int Arch Allergy Immunol. 1996;112:152–6. doi: 10.1159/000237447. [DOI] [PubMed] [Google Scholar]
- 31.Matsubara T, Fujita Y, Sato T, Sasai K, Furukawa S. The prevalence of allergy in Kawasaki disease. Allergy. 1998;53:815–6. doi: 10.1111/j.1398-9995.1998.tb03983.x. [DOI] [PubMed] [Google Scholar]


