Abstract
Primary infections with EBV are rarely observed after the age of 20. Some individuals even remain seronegative all their lives. Previously, a lack of EBV receptors on B cells of persistently EBV− adults was described as a reason for long-term EBV-seronegativity. The present study examined the CD21 receptor status of 20 repeatedly EBV− healthy adults and 32 EBV+ volunteers by means of flow cytometry. CD21 molecules on the surface of CD19+ B cells were quantified using anti-IgG-coated microbeads. The percentage of CD19+/CD21+ B lymphocytes was slightly lower in the peripheral blood of EBV− donors, but the CD21 antibody binding capacity on CD19+ B cells showed no significant differences between EBV− and EBV+ adults. In vitro studies showed an equally good EBV transformability of peripheral B lymphocytes of EBV− and EBV+ donors. Since HLA-DR was recently described as a co-receptor for EBV infection of B cells, we also determined HLA-DRB1 alleles in the EBV− group. We found a significant negative association of EBV-seronegativity with HLA-DR13 in comparison with 111 healthy blood donors. In summary, a biologically significant lack of the EBV receptor CD21 on peripheral B lymphocytes of persistently EBV− adults was excluded as a reason for long-term EBV-seronegativity.
Keywords: Epstein–Barr virus, Epstein–Barr virus receptors, resistance, CD21, HLA-DR
INTRODUCTION
The human complement receptor type 2 (CR2), designed as CD21, is a membrane glycoprotein and serves as a receptor for the C3d, C3dg, and iC3b proteins of complement. Structurally, it consists of 15 or 16 extracellular short consensus repeats (SCR) followed by a transmembrane domain and an intracytoplasmic region [1]. CD21 was described as a receptor for the envelope glycoprotein gp350/220 of the EBV [2] and also as a receptor for interferon-alpha (IFN-α) [3]. It is found on mature B lymphocytes and follicular dendritic cells [1]. Moreover, CD21-related antigens were suggested to be present on a minority of thymocytes and peripheral T cells [4]. Its presence on epithelial cells of the oropharynx also remains unclear, but it was shown that infection of the epithelium by EBV involves a receptor structurally homologous to CD21 [5]. The CD21 molecule is a functional ligand for CD23 and is involved in IgE and IgG4 production of human B lymphocytes [6]. Binding of the EBV antigen gp350/220 leads to capping of EBV–receptor complexes on the surface of B cells and mediates subsequent internalization of EBV into the cell [7].
EBV belongs to the human herpesvirus family and is ubiquitously distributed all over the world. It is the causative agent of infectious mononucleosis (IM) and is associated with several malignancies in man, such as African endemic Burkitt's lymphoma, nasopharynx carcinoma of the undifferentiated form, B cell lymphomas in immunosuppressed individuals, and Hodgkin's disease [8]. Infection with EBV commonly occurs during childhood in an asymptomatic form, leading to a rapid increase in EBV antibodies. Seroepidemiological data from various regions of the world demonstrate a prevalence of anti-EBV antibodies of 85–95% at the age of 20 years [9]. Thereafter, primary EBV infections are a rare event, but are still observed [10]. This suggests that some individuals remain EBV− due to natural resistance. In a previous study, we searched for immunological explanations for the phenomenon of long-term EBV-seronegativity and suggested a higher monokine production [11].
However, in 1981 Gervais et al. described a relative lack of EBV receptors on B cells of persistently EBV− adults [12]. In that study, B lymphocytes of 45.6% of the EBV− individuals were hardly able to bind EBV. This result was obtained by means of EBV-coupled, rosette-forming erythrocytes. Since this procedure is dependent on the researcher's subjective evaluation and is nowadays obsolete, we evaluated the expression of the meanwhile identified EBV receptor on B cells, CD21, of 20 long-term EBV− adults in comparison with 32 EBV+ individuals by means of flow cytometry. The functionality of the detected CD21 receptor was assessed by EBV transformation studies. EBV infection of B cells also involves HLA-DR as a co-receptor [13]. To analyse the possible implications of different HLA-DR molecules in susceptibility to EBV, HLA-DRB1 genotyping was performed in the EBV− group by means of a polymerase chain reaction (PCR) method with sequence specific primers [14].
SUBJECTS AND METHODS
Study and control population
A total of 1769 healthy blood donors had been screened for EBV-specific antibodies by means of a commercially available ELISA (Enzygnost Anti-EBV/IgG; Behring, Marburg, Germany) and by standard immunofluorescence technique (IFT; Fresenius, Oberursel, Germany). Of these, 30 adults lacking EBV-specific antibodies were investigated for their immunological properties as recently described [11]. After this first study, 20 of these EBV− adults (six women, 14 men, between 21 and 59 years old, median age 31 years) were reinvestigated in the present study and again found to be EBV− by IFT. Sera were screened for IgG antibodies to EB-viral capsid antigen (VCA) and EB nuclear antigen (EBNA) as well as for IgM antibodies to EB-VCA. EBV-seronegativity was defined with EB-VCA IgG < 1:40, EB-VCA IgM < 1:10, and EBNA IgG < 1:10. Blood samples from 32 volunteers (eight women, 24 men, between 20 and 52 years with a median age of 33 years) who were EBV+ by IFT with EB-VCA ≥ 1:40, EB-VCA IgM < 1:10, and EBNA IgG ≥ 1:10 served as positive controls. ELISA and IFT were performed according to the manufacturer's recommendations.
Analysis of B cells by flow cytometry
EDTA anti-coagulated peripheral blood was drawn from the volunteers after informed consent. Differential blood counts for total numbers of leucocytes were obtained by automatic determination on a Coulter MAX-M cell counter (Coulter, Krefeld, Germany). After incubation of 100 μl blood each with 10 μl of anti-CD14/anti-CD45 (Mo2-RD1/KC56-FITC), 10 μl of anti-CD3/anti-CD19 (CD3(IgG1)-FITC/B4-RD1), and 10 μl of anti-CD3, anti-CD16, anti-CD56 (CD3-ECD, CD16-FITC, NKH-1-RD1) for 15 min, respectively, the samples were prepared for flow cytometry by an automatic workstation (Multi-Q-Prep; Coulter). Non-specific-binding isotype-specific MoAbs were used as controls (MsIg G2b-ECD/MsIgG1-RD1/MsIgG1-FITC, MsIgG2a-RD1, MsIgG1-FITC). All MoAbs were purchased from Immunotech/Coulter (Hamburg, Germany). For analysis we used a flow cytometer (EPICS XL-MCL; Coulter) equipped with an air-cooled argon laser (excitation wavelength 488 nm). After calculation of the total number of B cells per μl, the EDTA anti-coagulated blood was adjusted to 100 B lymphocytes/μl by the use of PBS pH 7.2 (Gibco, Paisley, UK). Blood/PBS was then stained with 10 μl of anti-CD19 and anti-CD21 each (B4-RD1, CD21-FITC), incubated for 15 min and afterwards prepared with the Multi-Q-Prep workstation. Anti-CD21 MoAb was from clone BL13 [15]. IgG1-FITC/IgG1-PE was used as an isotype-specific control. The samples were assayed at a flow rate of 1000 particles/s, for an analysis of 5000 B cells per sample. The lymphocyte population was gated in an electronic bit map by using the forward scatter versus the log90° scatter (side scatter log10). The gated population was further analysed in a correlation plot of CD19-PE versus CD21-FITC. B lymphocyte-specific antibody binding was calculated by electronic subtraction of non-specific binding of the isotypic control from the binding of the test sample and expressed as a percentage of antibody-positive cells and as mean channel fluorescence.
Quantification of the antibody binding capacity
We quantified the B lymphocyte-specific CD21 antibody binding after calibration with goat anti-mouse IgG-coated microbeads (Quantum Simply Cellular; FCSC Europe, Leiden, The Netherlands) following a recently described protocol [16]. Similar protocols are now widely used for quantification of surface markers on leucocytes [17–20]. The microbead suspension contained four bead populations with different defined capacities to bind murine MoAbs and a blank control. The beads were all of the same size (7–10 μm) according to the size of lymphocytes. Briefly, 50 μl of microbead suspension were incubated with 20 μl of FITC-conjugated anti-CD21 MoAb. Incubation and flow cytometric analysis were carried out under the same instrument settings as for the B lymphocyte analysis. Data analysis was performed with special software enclosed with the microbeads (QuickCal Program; FCSC). By determination of the peak fluorescence signal for each microbead population (Fig. 1), a calibration curve with the relative level of fluorescence per binding site was calculated (Fig. 2). The number of binding sites on the investigated B cells was then calculated from the mean channel fluorescence signals obtained with the anti-CD21 staining of B cells.
Fig. 1.
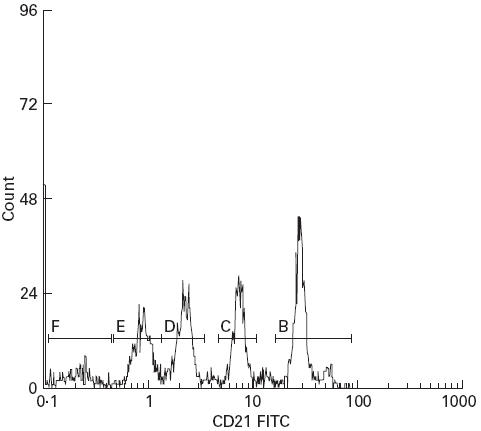
Fifty microlitre microbead suspensions were incubated with 20 μl of anti-CD21(FITC) MoAb. Four different populations and a blank control are distinguishable (B–F).
Fig. 2.
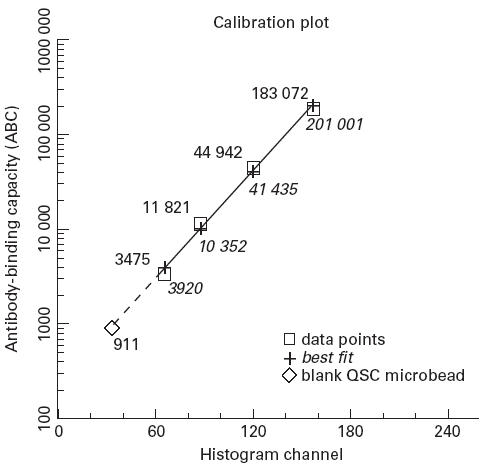
Relationship between the amount of anti-mouse IgG bound to the microbeads (antibody-binding capacity) and the mean channel fluorescence signal of each microbead population and the blank control incubated with anti-CD21(FITC) (calculated antibody-binding capacity); correlation r2 = 0.995.
Preparation of infective EBV
B95-8 marmoset cell line was grown in culture medium (IMDM; BioWhittaker, Verviers, Belgium; supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), l-glutamine 2 mm (Gibco), penicillin 100 U/ml, streptomycin 100 μg/ml (Gibco), 24 ml minimal essential medium-alpha, (Gibco), 5 ml OPI media supplement (Sigma, Deisenhofen, Germany)) for 10 days at a density of 5 × 105 cells/ml. Cells and medium were then frozen at −20°C for 2 h to release intracellular virus particles. Afterwards, cell suspension was filtrated through a sterile 0.45-μm filter, aliquoted in 1-ml portions and stored at −80°C. The virus preparation had a titre of 10−3 transforming units as determined on human umbilical cord blood lymphocytes (HUCBL). Ten replicate aliquots each containing 2 × 105 HUCBL were infected with a 10-fold dilution series of the EBV stock solution. Efficiency of transformation was defined as the negative log to the base 10 of the virus dilution which induced immortalization in at least 50% of the cultures after 4 weeks.
EBV transformation
Peripheral blood mononuclear cells (PBMC) of six persistently EBV− and six age- and sex-matched EBV+ adults were isolated from buffy coats by standard density centrifugation (Ficoll Separation Solution; Biochrom, Berlin, Germany). After two wash steps in PBS (10 min at 400 g) cells were resuspended in wash medium (RPMI 1640 (Biochrom), 2% FCS, l-glutamine 2 mm, penicillin 100 U/ml, streptomycin 100 μg/ml) and adjusted to a concentration of 1 × 107/ml. To eliminate lysosome-rich, inhibiting cell populations, l-leucyl-l-leucine-methylester (2.5 mm; Bachem, Heidelberg, Germany) was added to 1 × 108 PBMC. Following 15 min of incubation at room temperature and two wash steps with wash medium, cells were resuspended in 2 ml of the EBV stock solution. A negative control consisting of culture medium was included in every experiment. After removing excess virus by another wash step, 10 replicate aliquots containing 2 × 106 cells in 2 ml were dispensed into 24-well tissue culture plates (Becton Dickinson, Lincoln Park, NJ) and incubated for 4 weeks in a humidified atmosphere with 5% CO2. Cultures were fed weekly by carefully removing half of the supernatant and adding 1 ml of fresh culture medium. Immortalization was assessed visually by means of an inverted microscope and expressed as the percentage of cultures showing typical lymphoblastoid cell clumps.
DNA typing of HLA-DRB1
DNA was extracted from PBMC by proteinase K digestion and a salting-out procedure. DNA was adjusted to a concentration of 100 μg/ml and used at a final concentration of 50 ng per reaction. We used our protocol for rapid DNA typing with nested PCR and sequence specific primers as published by Bein et al. [14]. This protocol dissolves HLA-DRB1 alleles at the serologically determinable level (low resolution). The results were compared with frequencies of HLA-DRB1 alleles in our community [21].
Statistical analysis
Statistical analysis was performed with commercially available software for personal computers (SPSS for Windows 5.01). Data are expressed either as mean ± s.d. or as median. Comparison of EBV− and EBV+ individuals regarding flow cytometry was carried out with the Mann–Whitney U-test. HLA association was calculated with the χ2 test. P < 0.05 was considered significant.
RESULTS
Cell counts
With regard to the blood cell counts, we found a significantly higher percentage of monocytes in the peripheral blood of EBV− adults (P < 0.05). All other leucocyte values were within the normal range (Table 1). The distribution of the lymphocyte subpopulations showed no significant differences between the two study populations.
Table 1.
Differential blood cell counts and lymphocyte subpopulations of EBV− and EBV+ adults
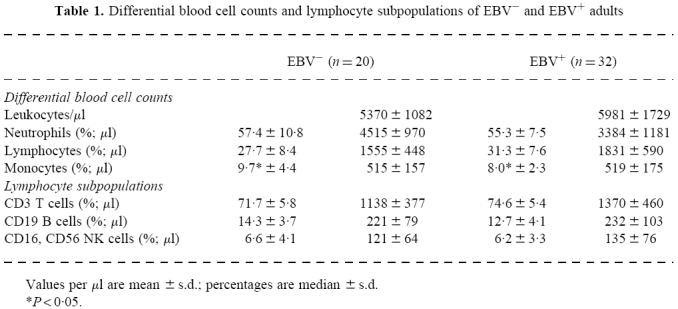
Values per µl are mean ±s.d.; percentages are median ±s.d.
*P < 0·05.
Determination of CD21+ cells
After adjusting the peripheral blood to 100 B cells/μl, we determined the absolute number of CD19+/CD21+ cells/μl and the percentage of CD21+ B lymphocytes in relation to all lymphocytes as well as in relation to all B lymphocytes (CD19+). As shown in Table 2, we observed differences neither in total numbers of CD21+ B cells nor in relation to all lymphocytes. However, a significantly lower percentage of CD21-expressing B cells relative to all B cells was found. By means of the anti-CD21 MoAb, clone BL13 [15], no expression of CD21 on peripheral T cells was seen (data not shown).
Table 2.
CD21+ B lymphocytes of long-term EBV− and EBV+ adults
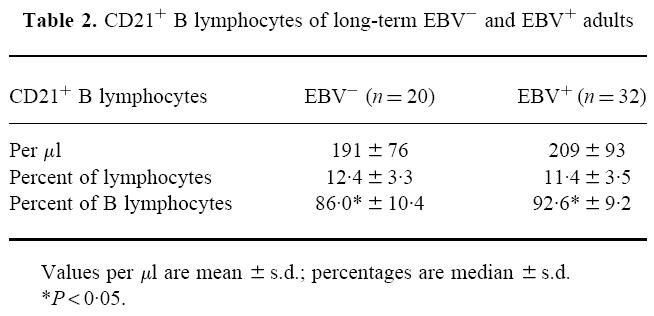
Values per µl are mean ±s.d.; percentages are median ±s.d.
*P < 0·05.
Quantification of the CD21 binding capacity on B cells
Determination of CD21 binding sites on peripheral B cells from EBV− and EBV+ donors was carried out within a period of 3 weeks. A new calibration curve was created each day by means of the microbead suspension. We found a significantly lower CD21-FITC mean fluorescence signal (P < 0.01) on B cells of EBV− individuals (Fig. 3). However, the calculated CD21 antibody binding capacity on peripheral CD19+ B cells did not differ significantly between EBV− and EBV+ adults after calibration with the anti-IgG-coated microbeads.
Fig. 3.
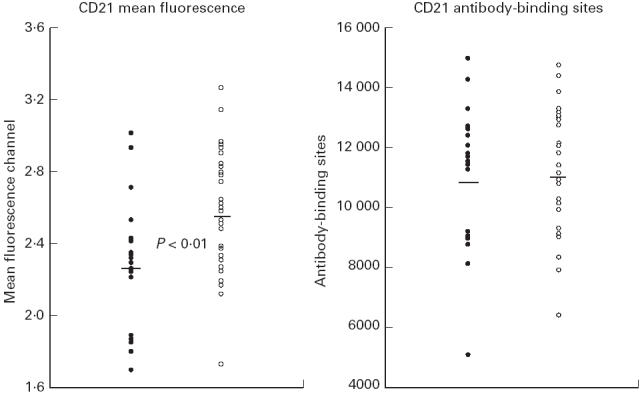
Mean channel fluorescence signals of CD21-FITC-stained B cells as well as the CD21 antibody-binding capacity on B cells of 20 EBV− and 32 EBV+ adults. Antibody binding was calculated by calibrating the fluorescence signals against the microbead standard. •, EBV−, n = 20; ○, EBV+, n = 32.
EBV-induced transformation
To assess the susceptibility of EBV− adults to EBV infection, we determined the in vitro transformability of peripheral B cells of six persistently EBV− donors and six age- and sex-matched EBV+ controls. As given in Table 3, cultures of either group showed an overall good transformability with a median efficiency of transformation of 80% in either group.
Table 3.
EBV-induced transformation in long-term cultures of EBV− and EBV+ adults
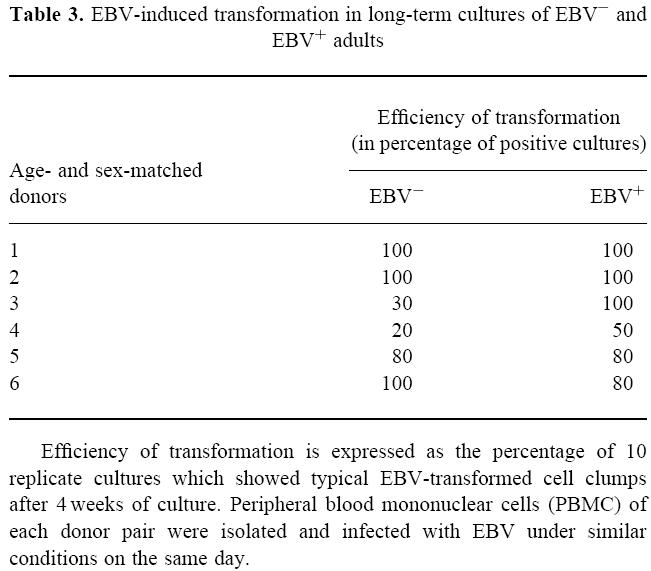
Efficiency of transformation is expressed as the percentage of 10 replicate cultures which showed typical EBV-transformed cell clumps after 4 weeks of culture. Peripheral blood mononuclear cells (PBMC) of each donor pair were isolated and infected with EBV under similar conditions on the same day.
HLA-DRB1 frequencies
We performed a low resolution HLA-DRB1 genotyping of 20 EBV− adults. The absolute as well as the relative HLA-DRB1 antigen frequencies are given in Table 4. The results were compared with data from a large group of healthy blood donors regardless their EBV serostatus (n = 111) who were analysed by high-resolution HLA-DR typing [21]. A significant negative association was observed for HLA-DR13 and EBV-seronegativity (χ2 = 6.42; P < 0.05). No other HLA-DR molecule revealed a positive or negative association with EBV-seronegativity.
Table 4.
Frequencies of HLA-DRB1 antigens
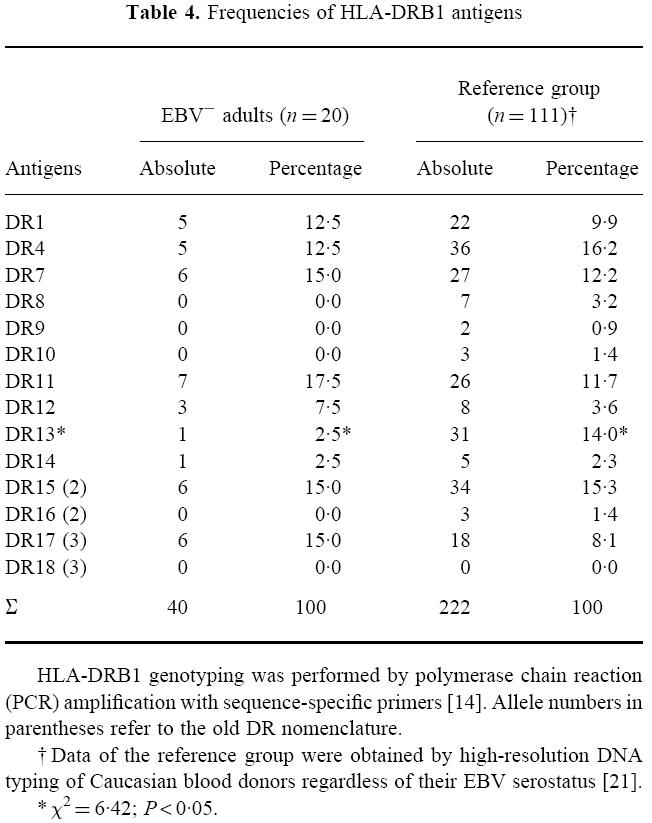
HLA-DRB1 genotyping was performed by polymerase chain reaction(PCR) amplification with sequence-specific primers [14]. Allele numbers in parentheses refer to the old DR nomenclature.
†Data of the reference group were obtained by high-resolution DNA typing of Caucasian blood donors regardless of their EBV serostatus [21].
*x2=6·42;P <0·05.
DISCUSSION
A relative lack of EBV receptors on B cells of persistently EBV− adults as a reason for long-term EBV-seronegativity was suggested in a previous study [12]. That study examined the presence of EBV receptors in 66 EBV− or EBV+ adults by means of EBV-coupled, rosette-forming erythrocytes and found a relative lack of EBV receptors in 45.8% of the EBV− group (11 of 24), but in no EBV+ individual, i.e. the percentage of B cells able to bind EBV was < 2% in the EBV− group. The lack of EBV receptors showed an overall presence of < 0.5% in relation to the whole population of > 2000 individuals. However, these data have not been confirmed yet. Therefore, we examined the presence of the now known EBV receptor CD21 on B lymphocytes of repeatedly EBV− adults by means of flow cytometry and MoAbs.
Peripheral blood cell analysis revealed a significantly higher monocyte count in EBV− adults. This is consistent with our recent findings about immunological properties of EBV− adults [11]. The result underlines our consideration of higher monocyte activity in maintenance of EBV-seronegativity.
Determination of the number of CD21-expressing B lymphocytes in our study showed a significantly lower percentage in EBV− individuals. Besides follicular dendritic cells, CD21 is expressed only on mature B lymphocytes [1]. This might suggest that EBV− persons contain more immature B lymphocytes in their blood. However, it is rather unlikely that the observed difference in the number of CD21-expressing B cells between EBV− and EBV+ adults in fact reflects the relative lack of EBV receptors described by Gervais et al. [12]. Since EBV− adults still show a high number of CD21+ B cells (86.0%), it is doubtful whether this ‘lack’ has any relevance regarding the prevention of EBV infection. At least our transformation experiments demonstrated in principle the infectability of B lymphocytes of EBV− adults. The biological relevance of the significant reduction in CD21+ B cells of EBV− individuals is therefore questionable.
Moreover, quantification of the mean CD21 binding capacity of B lymphocytes showed no significant differences between EBV− and EBV+ individuals. The findings by Gervais et al. [12] are thus probably not due to a significant lack in surface CD21 molecules of EBV− adults' B cells. Interestingly, we found a significantly lower CD21-FITC mean channel fluorescence signal for EBV− in comparison with EBV+ adults. This underlines the importance of calibrating the fluorescence signal against a known standard if the experiments aim at a quantification of surface molecules, and investigations are carried out over a period of several days.
One might argue that the CD21 MoAb used in our study only detects an EBV receptor which is unable to bind EBV or to mediate virus penetration into the cell. The extracellular part of the CD21 molecule consists of 15 or 16 SCR domains [1]. The binding sites for C3d, EBV and IFN-α are all localized in the first two SCR [1,3]. A deletion of this region would presumably lead to a CD21 molecule unable to bind EBV. The anti-CD21 MoAb we used does not bind within the first two SCR domains [15]. The possible expression of a truncated form of CD21 lacking the first two SCR is thus not completely ruled out in persistently EBV− individuals. However, two observations might argue against this consideration: (i) the only splicing variant of CD21 described so far is the lack of exon 11 resulting in 15 instead of 16 SCR [22]; (ii) peripheral B lymphocytes from EBV− adults were as easily infectable by EBV as those from EBV+ donors, suggesting the presence of an active CD21 receptor. Nevertheless, a CD21 molecule exhibiting a mutation within the EBV binding region—comparable to the effect of chemokine receptor 5 gene mutation in resistance to HIV-1 [23]—is still conceivable for some persistently EBV− adults and needs further investigation.
Because EBV is known to bind to HLA-DR [24] which is constitutively present on B lymphocytes, and because HLA-DR serves as a cofactor for EBV infection of B cells [13], an association for HLA-DR molecules and the susceptibility to EBV infection appeared possible. Data concerning HLA-molecules of EBV− adults was published in 1981 [12,25]. These groups only investigated the expression of HLA class I alleles, since determination of class II alleles was highly inaccurate at that time. They described inconsistent and contradictory associations regarding HLA-A and -B alleles, neither of which could be confirmed by us (data not shown). However, HLA-DRB1 typing in 20 repeatedly EBV− adults for the first time showed a significant negative association for HLA-DR13 and EBV-seronegativity. Binding of EBV to HLA-DR involves an outer envelope glycoprotein of EBV, gp42 [24]. EBV lacking gp42 is not able to infect B cells [26]. Although gp42 probably binds to the constant part of the HLA-DR β-chain [13], it might be possible that EBV has a higher affinity to HLA-DR13 which is of relevance for infection of B cells.
In conclusion, the presented data reject the suggested lack of EBV receptors on B cells of persistently EBV− adults. A negative association was found between EBV-seronegativity and presence of HLA-DR13. The impact of this finding has to be studied further, but its importance is doubtful, since it concerns only a minor fraction of EBV− individuals.
Acknowledgments
We would like to thank A. Reil for her helpful discussion and U. Doherty for critical reading the manuscript.
REFERENCES
- 1.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 2.Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Bird PA, Fearon DT. Epstein–Barr virus receptor of human B lymphocytes is the C3d receptor, CR2. Proc Natl Acad Sci USA. 1984;81:4510–4. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delcayre AX, Salas F, Mathur S, Kovats K, Lotz M, Lernhardt W. Epstein Barr virus/complement C3d receptor is an interferon α receptor. EMBO J. 1991;10:919–26. doi: 10.1002/j.1460-2075.1991.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsoukas CD, Lambris JD. Expression of EBV/C3d receptors on T cells: biological significance. Immunol Today. 1993;14:56–59. doi: 10.1016/0167-5699(93)90059-T. [DOI] [PubMed] [Google Scholar]
- 5.Birkenbach M, Tong X, Bradbury LE, Tedder TF, Kieff E. Characterization of an Epstein–Barr virus receptor on human epithelial cells. J Exp Med. 1992;176:1405–14. doi: 10.1084/jem.176.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnefoy JY, Pochon S, Aubry JP, Graber P, Gauchat JF, Jansen K, Flores-Romo L. A new pair of surface molecules involved in human IgE regulation. Immunol Today. 1993;14:1–2. doi: 10.1016/0167-5699(93)90313-A. [DOI] [PubMed] [Google Scholar]
- 7.Tanner J, Weiss J, Fearon D, Whang Y, Kieff E. Epstein–Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates absorption, capping, and endocytosis. Cell. 1987;50:203–13. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- 8.Rickinson AB, Kieff E. Fields BN Virology. New York: Raven Press; 1995. Epstein–Barr virus; pp. 2397–446. [Google Scholar]
- 9.de-Thé G, Day NE, Geser A, et al. De-Thé G, Epstein H. Oncogenesis and herpes viruses II. Vol. 11. Lyon: IARC Scientific Publication; 1975. Seroepidemiology of the Epstein–Barr virus: preliminary analysis of an international study—a review; pp. 3–18. [PubMed] [Google Scholar]
- 10.Schmader KE, van der Horst CM, Klotman ME. Epstein–Barr virus and the elderly host. Rev Infect Dis. 1989;11:64–73. doi: 10.1093/clinids/11.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Jabs WJ, Wagner HJ, Neustock P, Klüter H, Kirchner H. Immunologic properties of Epstein–Barr virus-seronegative adults. J Infect Dis. 1996;173:1248–51. doi: 10.1093/infdis/173.5.1248. [DOI] [PubMed] [Google Scholar]
- 12.Gervais F, Willis A, Leyritz M, Lebrun A, Joncas JH. Relative lack of Epstein Barr virus (EBV) receptors on B cells from persistently EBV seronegative adults. J Immunol. 1981;126:897–900. [PubMed] [Google Scholar]
- 13.Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, Hutt-Fletcher LM. Epstein–Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–62. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bein G, Gläser R, Kirchner H. Rapid HLA-DRB1 genotyping by nested PCR amplification. Tissue Antigens. 1992;39:68–73. doi: 10.1111/j.1399-0039.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JHM, Fischer E, Kazatchkine MD, Brochier J, Revillard JP. Characterization of monoclonal antihuman-B-cell antibody BL13 as an anti-C3d-receptor (CR2) antibody. Scand J Immunol. 1986;23:279–85. doi: 10.1111/j.1365-3083.1986.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 16.Klüter H, Schlenke P, Müller-Steinhardt M, Paulsen M, Kirchner H. Impact of buffy coat storage on the generation of inflammatory cytokines and platelet activation. Transfusion. 1997;37:362–7. doi: 10.1046/j.1537-2995.1997.37497265335.x. [DOI] [PubMed] [Google Scholar]
- 17.Fay SP, Posner RG, Swann WN, Sklar LA. Real-time analysis of the assembly of ligand, receptor, and G protein by quantitative fluorescence flow cytometry. Biochem. 1991;30:5066–75. doi: 10.1021/bi00234a033. [DOI] [PubMed] [Google Scholar]
- 18.Ginaldi L, Farahat N, Matutes E, De Martinis M, Morilla R, Catovsky D. Differential expression of T cell antigens in normal peripheral blood lymphocytes: a quantitative analysis by flow cytometry. J Clin Pathol. 1996;49:539–44. doi: 10.1136/jcp.49.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denny TN, Stein D, Mui T, Scolpino A, Holland B. Quantitative determination of surface antibody binding capacities of immune subsets present in peripheral blood of healthy blood donors. Cytometry. 1996;26:265–74. doi: 10.1002/(SICI)1097-0320(19961215)26:4<265::AID-CYTO5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Ginaldi L, De Martinis M, D’Ostilio A, Di Gennaro A, Marini L, Quaglino D. Altered lymphocyte antigen expressions in HIV infection. A study by quantitative flow cytometry. Am J Clin Pathol. 1997;108:585–92. doi: 10.1093/ajcp/108.5.585. [DOI] [PubMed] [Google Scholar]
- 21.Reil A, Bein G, Machulla HKG, Sternberg B, Seyfarth M. High-resolution DNA typing in immunoglobulin A deficiency confirms a positive association with DRB1*0301, DQB1*02 haplotypes. Tissue Antigens. 1997;50:501–6. doi: 10.1111/j.1399-0039.1997.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 22.Illges H, Braun M, Peter HH, Melchers I. Analysis of the human CD21 transcription unit reveals differential splicing of exon 11 in mature transcripts and excludes alternative splicing as the mechanism causing solubilization of CD21. Mol Immunol. 1997;34:683–93. doi: 10.1016/s0161-5890(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 23.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 24.Spriggs MK, Armitage RJ, Comeau MR, et al. The extracellular domain of the Epstein–Barr virus BZLF2 protein binds the HLA-DR β chain and inhibits antigen presentation. J Virol. 1996;70:5557–63. doi: 10.1128/jvi.70.8.5557-5563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edmond E, Steel CM, Ennis M, Cameron F, Dick H. HLA antigens in adults negative for antibody to Epstein–Barr virus (EBV) Tissue Antigens. 1981;18:252–7. doi: 10.1111/j.1399-0039.1981.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Hutt-Fletcher LM. Epstein–Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J Virol. 1998;72:158–63. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


