Abstract
This study presents the immunophenotypic and functional analysis of lymphocyte subsets obtained from peripheral blood and lymphoid tissue from HIV+ individuals treated with highly active anti-retroviral therapy (HAART) alone or in combination with 6 million units international (MUI) s.c. IL-2. Before treatment, the HIV+ patients had reduced CD4 and increased CD8 values in the peripheral blood and lymphoid tissue and impaired cytokine production by peripheral blood mononuclear cells (PBMC). After 24 weeks of treatment, all the HIV+ patients demonstrated increased CD4 values in peripheral blood and lymphoid tissue. The use of IL-2 did not promote an additional CD4 expansion compared with HAART alone; increased ‘naive’ and CD26+ CD4 cells and reduced CD8 cells were found in the peripheral blood and lymphoid tissue of the IL-2-treated, but not of the HAART-treated patients. Both types of treatment induced a significant reduction of the CD8/CD38+ cells. While HAART alone had negligible effects on cytokine production by PBMC, the combined use of HAART + IL-2 was unable to increase the endogenous production of IL-2, but caused an increase of IL-4, IL-13 and interferon-gamma (IFN-γ) and a reduction of monocyte chemoattractant protein-1 (MCP-1) production. These data suggest that, although in this schedule IL-2 has minimal efficacy on CD4 recovery when compared with HAART alone, it produces an increase of ‘naive’ and CD26+CD4 cells and a partial restoration of cytokine production. These data may be used to better define clinical trials aiming to improve the IL-2-dependent immunological reconstitution of HIV-infected subjects.
Keywords: HIV infection, IL-2 injections, lymphocyte subsets, lymphoid tissue, peripheral blood, cytokines
INTRODUCTION
HIV causes immunodeficiency through the progressive destruction of CD4+ lymphocytes in the peripheral blood and in the lymphoid tissue [1–6].
Among CD4 cells, the CD45RA+/CD62L+ subset defines the ‘naive’ cells, whose appearance after depletion of the immune system is considered to be a marker predictive of host immune reconstitution [7–10]. In contrast, CD45RO+ lymphocytes represent mature ‘memory’ pre-existing cells. CD4 cells can be further phenotypically defined by the measurement of costimulatory molecule expression, such as CD26 and CD28 [11–14]. HIV infection affects also the CD8+ lymphocyte subpopulations by inducing the expression of activation markers and of cytolytic-specific and non-cytolytic markers and activities that may be involved in the control of HIV replication and ultimately in HIV disease progression [14–20]. These phenotypically defined CD4 and CD8 subsets appear to be deeply perturbed by ongoing HIV infection and their measurements are used to establish the degree of immune reconstitution after anti-retroviral therapies [5,10,15]. This description, however, represents an over-simplification of a complex phenomenon, where functional abnormalities of the immune system, such as reduced cytokine production and decreased lymphocyte proliferation to HIV- or non-HIV-specific antigens, can be present in association with normal CD4 counts [21,22].
The data available in literature agree with the fact that the most recent anti-retroviral therapies allow a significant suppression of HIV replication and a reversal of immune activation, but do not permit a complete reconstitution of the immune system [21,22]. In addition, the information available on this complicated topic is still incomplete, because most of the previously obtained data refer to treatments with reverse transcriptase inhibitors, are limited to peripheral blood, and the immunophenotypic investigations restricted to some markers or functional assays have not been performed [3,5,6,10,15,23].
Because of the limited activity of anti-retroviral regimens, immune-based therapies have been attempted to improve the efficacy of these drugs; in particular, the peculiar ability of IL-2 to directly expand the CD4 T cell pool has been used to ameliorate immune reconstitution in HIV-infected patients [24–26].
Based on the above-mentioned considerations, we aimed in this study to investigate the dynamic changes of selected CD4 and CD8 subpopulations in the lymphoid tissue and in the peripheral blood of HIV+ patients receiving HAART and to observe the efficacy of the addition of IL-2 to this therapeutic regimen in order to reconstitute phenotypically defined CD4 and CD8 lymphocyte subsets and to improve the in vitro production of selected cytokines.
PATIENTS AND METHODS
Patient population
Since January 1997, the patients seen at the Division of Medical Oncology and AIDS at the Aviano Cancer Institute, Italy, were enrolled to participate in a mono-institutional randomized study for the evaluation of clinical, immunological and virological effects of HAART plus IL-2 versus HAART alone. Patients were included in the study if they met the following criteria: documented HIV infection, stage Al, A2, B1, B2 according to the 1993 CDC classification [27], naive for anti-retroviral therapy or pretreated only with the combination of two reverse transcriptase inhibitors (RTIs), CD4 counts > 200/mm3 and HIV viraemia > 500 RNA copies/ml in naive patients or two consecutive HIV viraemia values 1 log above the nadir value observed during RTI therapy in pretreated patients; no previous IL-2 therapy, at least 18 years of age, granulocytes < 1000/mm3, platelets < 100 000/mm3, haemoglobin < 10 g/dl, GOT, GPT, FAL and γGT no more than three times the normal values, bilirubin ≤ 2 mg/dl, creatinine ≤ 1.5 mg/dl. Thyroid abnormal function and significant cardiac, pulmonary and central nervous system (CNS) impairment were considered as additional exclusion criteria.
The study protocol was approved by the Institutional review board and informed consent was obtained from the study participants. Patients were randomized to receive RTI plus Indinavir or two RTIs plus Indinavir plus IL-2: IL-2 was subcutaneously administered with the following schedule: 6 million units international (MUI) of Proleukin (Chiron, Emeryville, CA) at days 1–5 and 8–12 of a 28-day-cycle for a total of six cycles (overall 24 weeks duration). IL-2 was administered once a day and just before injection patients received 1 g of paracetamol as premedication. This therapeutic schedule was chosen because of the excellent immunological and clinical results obtained in previous trials where RTIs have been used in association with IL-2 [26,28]. Toxicity was evaluated according to NCI criteria, as described [28]. In cases of toxicity greater than grade 2, IL-2 administration was delayed until toxicity became lower than grade 1. When toxicity grade 2 or greater persisted for more than 2 weeks, an IL-2 half reduction was planned. In cases of toxicity or intolerance to Indinavir, patients were allowed to switch to Ritonavir or Saquinavir. Peripheral blood samples and lymphoid tissue samples for immunological analysis were obtained just before (t = 0) and after therapy (t = 24 weeks). Plasma viraemia was determined at the same time points. As controls, we used 10 consenting HIV− persons from the same age group of HIV+ patients, attending at the ENT clinic for non-inflammatory problems.
Immunophenotypic analysis
Peripheral blood samples were obtained in EDTA and evaluated by a whole blood lysing technique, as previously described [26,28]. Briefly, 100 μl of blood were added to the appropriate MoAb combination and incubated for 15 min: after this incubation the samples were lysed and fixed by a commercial preparation (Immunoprep; Coulter, Milan, Italy). Mononuclear cells were also isolated from a sample of the lymphatic tissue (LT) of the posterior nasopharyngeal wall; this type of sample has been shown to be representative of the immunological and virological phenomena occurring during HIV infection [29]. The nasopharyngeal biopsy was taken under local anaesthesia, passing through the nose a biopsy forceps and a flexible nasopharyngoscope. Each LT biopsy was minced and filtered through a 40-μm nylon mesh; cells were then separated by density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). Lymphocyte yield was from 5 to 10 million cells per biopsy and, to exclude epithelial cell contamination, lymphocytes were gated on the basis of scatter properties and CD45 reactivity. The following MoAb combinations were used to stain peripheral blood or LT cellular preparations: CD3–FITC/CD19–PE, CD4–Cy5/CD45RA PE/CD62L–FITC, CD4–FITC/CD45RO–PE, CD4–FITC/CD28–PE, CD4–FITC/CD26–PE, CD3–Cy5/CD8–FITC/CD38–PE, CD3–Cy5/CD8–FITC/CD28–PE, CD3–Cy5/CD8–FITC/C1.7.1–PE (C1.7 is a MoAb from Immunotech, Marseille, France, that stains cytotoxic effector cells) [30] and CD45–FITC.
In vitro cytokine production in culture supernatants
In vitro cytokine production was measured as previously described [26]. Briefly, peripheral blood mononuclear cells (PBMC) were separated by centrifugation on Ficoll–Hypaque (Nycomed, Milan, Italy), washed and incubated in RPMI 1640, 10% fetal calf serum (FCS), at 3 × 106 cells/ml in the presence or absence of 1% phytohaemagglutinin (PHA; Gibco Labs, Milan, Italy). Culture supernatants were harvested after 48 h, centrifuged, filtered and frozen until cytokine measurements by commercial ELISA assays (IL-2, IL-4, IL-13, interferon-gamma (IFN-γ) from Biosource, Celbio, Milan, Italy, and monocyte chemoattractant protein-1 (MCP-1) from Endogen, Tema Ricerca, Bologna, Italy) had been performed.
Plasma viraemia measurements
Plasma viraemia was measured by the commercial bDNA system (Chiron Corp., Emeryville, CA) following the manufacturer's instructions.
Statistical analysis
Since the variables under study were not normally distributed, non-parametric statistical tests were chosen. The Mann–Whitney test was used to compare the distributions of variables between two groups, and Wilcoxon rank sum test was used to analyse paired values in the same group [31].
RESULTS
Clinical and virological data
Ten patients were evaluated for the immunological analysis in the peripheral blood and in the LT before and after 24 weeks of therapy; five were treated with HAART alone and five with HAART plus IL-2. The five patients treated with HAART were three males and two females, mean age 29 years (range 28–54 years), four were naive for anti-retroviral therapy and the pretreated one switched to new RTIs. The median plasma HIV viraemia before treatment was 31 000 copies/ml (range 1000–204 000, mean 52 030 ± 76 000 copies/ml) and resulted in < 500/copies/ml in all the patients after 24 weeks of treatment. All the patients were fully adherent to HAART and toxicity was limited to grade 1. In the HAART plus IL-2 group, four patients were males and one a female, mean age was 31 years (range 23–49 years), three were naive for anti-retroviral therapy and the two pretreated switched to new RTIs. None of the patients interrupted therapy and toxicity was limited to grade 2, except for two episodes of grade 3 hyperbilirubin that remained asymptomatic without an increase of serum transaminase levels. It remains to be established whether IL-2 can potentiate the hepatotoxicity induced by the use of Indinavir.
The median plasma HIV viraemia was 21 500 copies/ml (range 8700–141 500, mean 46 300 ± 49 000 copies/ml) before treatment and became < 500 copies/ml after 24 weeks of therapy.
CD4+ and CD8+ lymphocytes and CD4/CD8 ratios in lymphoid tissue and peripheral blood before and after therapy
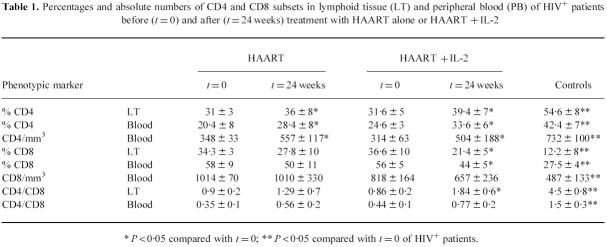
The percentage and absolute numbers of CD4 and CD8 subsets did not differ, before therapy, in the patients who were treated with HAART alone or HAART in combination with IL-2. All the HIV+ patients considered in this study had reduced CD4 and increased CD8 values when compared with controls (Table 1, P < 0.05).
Table 1.
Percentages and absolute numbers of CD4 and CD8 subsets in lymphoid tissue (LT) and peripheral blood (PB) of HIV+ patients before (t = 0) and after (t = 24 weeks) treatment with HAART alone or HAART + IL-2
* P < 0·05 compared with t = 0; ** P < 0·05 compared with t = 0 of HIV+ patients.
After therapy, both groups of HIV+ patients demonstrated a superimposable increase in the percentage and absolute numbers of CD4 cells. In contrast, only IL-2-treated subjects demonstrated a significant reduction of the percentage of CD8+ lymphocytes and an increase of the CD4/CD8 ratio reaching statistical significance in the LT (P < 0.05).
Based on this findings, we suggest that the addition of IL-2 to HAART in the treatment of HIV+ patients does not have significant effects on the percentage and absolute numbers of CD4+ cells in comparison with HAART alone. However, because of the reduction of the CD8 subset numbers, this therapeutical combination was associated with a preferential increase of the CD4/CD8 ratios in LT and peripheral blood of HIV+ patients.
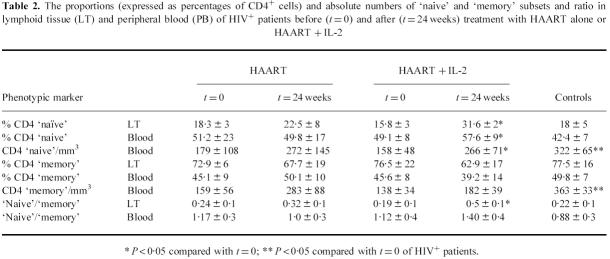
‘Naive’ and ‘memory’ CD4+ lymphocytes in lymphoid tissue and peripheral blood before and after therapy
Table 2 shows the data regarding the proportion of CD4 ‘naive’ (as defined by the percentages of CD4 cells expressing the CD45RA/CD62L+ phenotype) and ‘memory’ (as defined by the percentages of CD4 cells expressing the CD45RO+ phenotype) lymphocyte subsets in lymphoid tissue and peripheral blood of HIV+ patients and controls. Because the CD4 subset in the lymphoid tissue was mostly constituted by ‘memory’ cells, the ‘naive’ to ‘memory’ ratio in this site was < 0.25 both in patients and controls; in contrast, in the peripheral blood the two subpopulations were equally represented (‘naive’ to ‘memory’ ratios around 1). The data shown in Table 2 suggest that, while treatment with HAART did not modify the ratio because of an increase of both subsets, the use of IL-2 caused an increase of the ‘naive’ to ‘memory’ ratios by preferentially expanding the ‘naive’ cells.
Table 2.
The proportions (expressed as percentages of CD4+ cells) and absolute numbers of ‘naive’ and ‘memory’ subsets and ratio in lymphoid tissue (LT) and peripheral blood (PB) of HIV+ patients before (t = 0) and after (t = 24 weeks) treatment with HAART alone or HAART + IL-2
* P < 0·05 compared with t = 0; ** P < 0·05 compared with t = 0 of HIV+ patients.
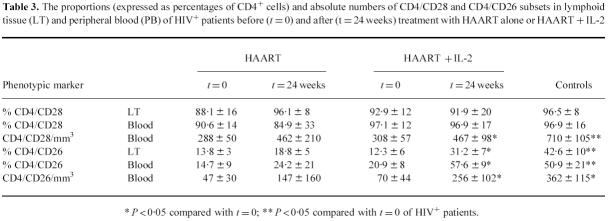
CD4+/CD28+ and CD4+/CD26+ lymphocytes in lymphoid tissue and peripheral blood before and after therapy
We also measured the proportions (expressed as the percentages of CD4 cells expressing the CD28+ or the CD26+ phenotype) and absolute numbers of the CD4/CD28+ and CD4/CD26+ subsets before and after treatments (Table 3). The CD4+ cells expanded after treatment both in the LT and peripheral blood were consistently CD28+ in all the patients studied. In contrast, the CD4/CD26+ subset, that was depleted by ongoing HIV infection, was statistically significantly increased in the peripheral blood and LT only in IL-2-treated patients. From these data, we can suggest that the treatments with HAART alone or with IL-2 produced peculiar modifications of discrete CD4 lymphocyte subsets.
Table 3.
The proportions (expressed as percentages of CD4+ cells) and absolute numbers of CD4/CD28 and CD4/CD26 subsets in lymphoid tissue (LT) and peripheral blood (PB) of HIV+ patients before (t = 0) and after (t = 24 weeks) treatment with HAART alone or HAART + IL-2
* P < 0·05 compared with t = 0; ** P < 0·05 compared with t = 0 of HIV+ patients.
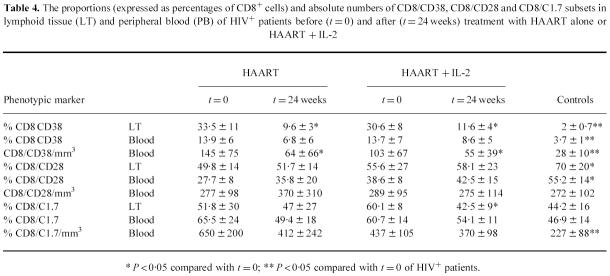
Phenotype of CD8+ lymphocytes in the lymphoid tissue and peripheral blood before and after therapy
An immunophenotypic evaluation of the CD8 subset was also performed (Table 4). Irrespective of the type of treatment used, after 24 weeks of therapy the proportions (expressed as percentages of CD8+ cells expressing CD38) and the absolute numbers of CD8/CD38+ activated cells were reduced in LT and peripheral blood of the patients. This observation suggests that the suppression of viral replication induced by HAART is probably sufficient to reduce the expression of CD38. The proportions and absolute numbers of CD8/CD28+ lymphocytes, a subset that has been suggested to play a role in the control of HIV replication through a non-cytotoxic response [20], were not modified after either type of treatment. The proportion of CD8+ cells expressing the C1.7 38-kD activation antigen was reduced in the LT by IL-2 treatment.
Table 4.
The proportions (expressed as percentages of CD8+ cells) and absolute numbers of CD8/CD38, CD8/CD28 and CD8/C1.7 subsets in lymphoid tissue (LT) and peripheral blood (PB) of HIV+ patients before (t = 0) and after (t = 24 weeks) treatment with HAART alone or HAART + IL-2
* P < 0·05 compared with t = 0; ** P < 0·05 compared with t = 0 of HIV+ patients.
In conclusion, our results suggest that the additional therapeutic use of IL-2 did not significantly modify the composition of the CD8+ subset in comparison with HAART alone.
Pattern of cytokine production before and after therapy
In all the experiments, spontaneous cytokine production was < 10% of the values obtained from stimulated cultures. The data in Table 5 show stimulated minus spontaneous cytokine production (pg per ml) in culture supernatants. Baseline production of IL-2 and IFN-γ were reduced, while the production of IL-13 and of MCP-1 were increased in HIV+ patients before treatment compared with controls. While HAART alone did not produce significant effects on the cytokine production of treated patients, the combined use of HAART + IL-2 produced a statistically significant increase of IL-4 and IFN-γ production and a significant decrease of IL-13 and MCP-1 production after 24 weeks of treatment.
Table 5.
Cytokine production (pg per ml) in culture supernatants from peripheral blood phytohaemagglutinin (PHA)-stimulated mononuclear cells from HIV+ patents before (t = 0) and after (t = 24 weeks) treatment with HAART alone or HAART + IL-2
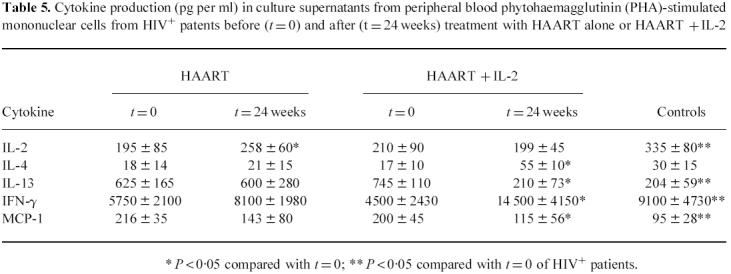
* P < 0·05 compared with t = 0; ** P < 0·05 compared with t = 0 of HIV+ patients.
DISCUSSION
The objective of this study was to assess whether treatment of HIV+ subjects for a 6-month period with HAART alone or in combination with IL-2 would produce significant immunophenotypic and functional improvements of the immune system
Our data show that in these patients CD4 lymphocytes were depleted in LT and peripheral blood before treatment and that the CD4/CD45RA/CD62L+ and the CD4/CD26+ were the most affected subpopulations; an expansion of activated CD8+ cells expressing CD38+ and the 30-kD antigen was also present in these subjects, while both the CD28+ and CD28−CD8+ populations were equally increased.
Therapy with HAART alone produced an augmentation of CD4+ cells without altering the proportion of ‘naive’ and ‘memory’ lymphocytes, while it did not affect CD8 percentages and cell numbers. These modifications were observed in peripheral blood and lymphoid tissue, suggesting that 6 months of anti-retroviral therapies produced true benefits on the patients' immune system. The data obtained in this study suggest also that the same therapeutic regimen including IL-2 that we have demonstrated to powerfully increase CD4 T cell numbers in HIV+ patients treated in association with transcriptase inhibitors [26,28] is less active when used in combination with HAART. Due to problems regarding patients' enrolment and obtaining tissue samples, other therapeutical trials have investigated a limited number of HIV+ subjects when using peripheral blood and/or lymphoid tissues [4,5,32–34]; therefore, although the number of patients included in this study does not allow definitive conclusions, our data may be useful to compare the immunological effects obtained in this study with those of previous clinical trials [32–34]. We here propose that, when used in combination with HAART, the specific effects of IL-2 consisted of an increase of the ‘naive’, of the CD26+ CD4 subset and of the CD4/CD8 ratios in the lymphoid tissue and the peripheral blood of treated individuals. Based on the data obtained in HIV− patients during bone marrow regeneration after chemotherapy, it was postulated that the expansion of the ‘naive’ population after therapy in HIV+ patients could also be considered as an index of a renewal mechanism of thymic or extrathymic origin [7–9]. How active the thymus is before and after treatment for HIV disease remains to be determined, however: in fact, on one hand, Walker et al. [35] suggested that the CD45RA+ lymphocytes emerging in HIV+ patients after anti-retroviral treatments can derive also from pre-existing memory phenotype cells reverting in vivo to assume the ‘naı¨ve’ phenotype; on the other hand, Douek et al. [36] demonstrated that in HIV+ patients treated with HAART there is a sustained increase in thymic output in most subjects. Irrespective of the origin of ‘naive’ cells appearing after treatments, and in view of their inherent resistance to productive HIV infection [37,38], the use of an immune-based therapy able to expand the CD4 ‘naive’ population that is probably not infected with HIV represents, in our opinion, a result that may contribute to delaying the progression of HIV disease. The correlation recently observed to occur between a reduction of HIV proviral load and the emergence of CD4/CD45RA+ cells in HIV+ patients treated with RT inhibitors and IL-2 further substantiates this hypothesis [39].
HIV infection is able not only to produce a depletion of CD4+ lymphocytes, but also to reduce immune functions sharply, as measured by cytokine production and lymphocyte proliferation. In this study we provide evidence that HAART alone did not modify cytokine production after 24 weeks, with the exception of a (moderate) increase of in vitro IL-2 secretion. We have previously shown that IL-2 treatment produced a sustained increase of IL-2, IL-4 and IFN-γin vitro production when associated with RTIs [26] and the present study suggests that IL-2 plus HAART is equally able to increase the production of those cytokines with the notable exception of IL-2, that is not modified after this type of treatment. We have preliminary evidence that this may be associated with an early inability of CD4 cells to produce endogenous IL-2 upon stimulation and we are currently investigating the possibility that longer periods of therapy (> 1 year) may reverse this abnormality in patients treated with this cytokine.
MCP-1 is a chemokine normally produced upon monocyte-macrophage cell stimulation [40]; it has been suggested that in vitro infection with HIV superinduces its production in human cells and that this phenomenon can be modulated by cytokines [41]. We have found in this study that the in vitro production of MCP-1 is in fact increased in PBMC from HIV+ subjects before treatment [42] and that the suppression of viral replication by HAART alone does not influence such a production. In contrast, the combined use of HAART plus IL-2 reduced MCP-1 secretion in treated patients. More extensive investigations are needed to clarify whether IL-2 may also influence the interactions between HIV and cells of the monocyte-macrophage lineage.
Recently published data have provided important information about the use of s.c. IL-2 in HIV+ subjects by showing that the s.c. route has an equivalent efficacy when compared with the i.v. route [43], and that the effect of IL-2 on immunological recovery is dose-dependent [44]. Since we have previously shown that this 6 MUI s.c. IL-2 therapeutic schedule did result in a powerful additional cell recovery of CD4 T cell numbers and functions when used in association with transcriptase inhibitors [26], the limited immunophenotypic and functional effects obtained when associating IL-2 with HAART suggest that the immunological efficacy of s.c. IL-2 is influenced also by the type of anti-retroviral treatment used.
In conclusion, our data may improve the current knowledge of the therapeutic use of s.c. IL-2 and contribute to the definition of clinical trials inducing a better immunological reconstitution of HIV+ patients. For these reasons, we suggest that an increased dosage or prolonging therapy for longer periods should be attempted to improve the beneficial effects of IL-2 in HIV-infected subjects.
REFERENCES
- 1.Hellerstein MK, McCune JM. T cell turnover in HIV disease. Immunity. 1997;7:583–9. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 2.Rosok B, Bostad L, Voltersvik P, Bjerknes R, Oloffson J, Asjo B, Brinchmann J. Reduced CD4 counts in blood do not reflect CD4 cell depletion in tonsillar tissue in asymptomatic HIV infection. AIDS. 1996;10:F35–F38. [PubMed] [Google Scholar]
- 3.Landay A, Bethel J, Schnittman S. Phenotypic variability of lymphocyte populations in peripheral blood and lymphnodes from HIV-infected individuals and the impact of antiretroviral therapy. AIDS Res Hum Retrovir. 1998;14:445–51. doi: 10.1089/aid.1998.14.445. [DOI] [PubMed] [Google Scholar]
- 4.Hufert FT, van Lunzen J, Janossy G, Bertram S, Schmitz J, Haller O, Racz P, von Laer D. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 1997;11:849–57. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Notermans D, Sedgewick G, et al. Kinetics of CD4 repopulation of lymphoid tissues after treatment of HIV infection. Proc Natl Acad Sci USA. 1998;95:1154–9. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lederman M, Connick E, Landay A, et al. Immunologic response associated with 12 weeks combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of ACTG protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 7.Mackall C, Hakim F, Gress R. T cell regeneration: all repertoires are not created equal. Immunol Today. 1997;18:245–511. doi: 10.1016/s0167-5699(97)81664-7. [DOI] [PubMed] [Google Scholar]
- 8.Mackall C, Fleisher T, Brown M, et al. Distinctions between CD8+ and CD4+ regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–7. [PubMed] [Google Scholar]
- 9.Watanabe N, De Rosa S, Cmelak A, Hoppe R, Herzemberg LA, Herzemberg LA, Roederer M. Long term depletion of naive T cells in patients treated for Hodgkin's disease. Blood. 1997;90:3662–72. [PubMed] [Google Scholar]
- 10.Autran B, Carcelain G, Li T, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Duke-Dohan JS, Kameoka J, Yaron A, Shlossman SF, Morimoto C. Enhancement of antigen-induced T cell proliferation by soluble CD26/DPPIV. Proc Natl Acad Sci USA. 1994;91:3082–6. doi: 10.1073/pnas.91.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz T, Underwood R, Khiroya R, Bachovchin WW, Huber B. Potentiation of the immune response in HIV+ individuals. J Clin Invest. 1996;97:1545–9. doi: 10.1172/JCI118577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.June C, Bluestone J, Nadler L, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 14.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzemberg LA, Herzemberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–6. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafeuillade A, Chouraqui M, Hittinger G, Poggi C, Delbeke E. Lymph node expansion of CD4+ lymphocytes during antiretroviral therapy. J Infect Dis. 1997;176:1378–82. doi: 10.1086/517326. [DOI] [PubMed] [Google Scholar]
- 16.Giorgi JV, Majchrowicz M, Johnson T, Hultin P, Matud J, Detels R. Immunologic effects of combined protease inhibitor and reverse transcriptase inhibitor therapy in previously treated chronic HIV-1 infection. AIDS. 1998;12:1833–44. doi: 10.1097/00002030-199814000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Azuma M, Phillips JH, Lanier LL. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 18.Caruso A, 5 Licenziati S, Canaris AD, et al. Contribution of CD4+, CD8+CD28+, and CD8+CD28- T cells to CD3+ lymphocyte homeostasis during the natural course of HIV-1 infection. J Clin Invest. 1998;101:137–44. doi: 10.1172/JCI195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinaldo C, Huang XL, Fan Z, et al. High levels of anti HIV-1 memory cytotoxic T lymphocyte activity and low viral load are associated with lack of disease in HIV-1 infected long term non-progressors. J Virol. 1995;69:5938–42. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy JA, Mackevicz CE, Barker E. Controlling HIV pathogenesis: the role of noncytotoxic anti HIV activity of CD8+ cells. Immunol Today. 1996;17:217–24. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 21.Emery S, Lane H. Immune reconstitution in HIV infection. Curr Opin Immunol. 1997;9:568–72. doi: 10.1016/s0952-7915(97)80112-4. [DOI] [PubMed] [Google Scholar]
- 22.Kelleher A, Al-Harti L, Landay A. Immunological effects of antiretroviral and immune therapies for HIV. AIDS. 1997;11(Suppl. A):S149–s155. [PubMed] [Google Scholar]
- 23.Pakker N, Notermans D, De Boer R, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz D, Skowron G, Merigan TC. Safety and effects of IL-2 plus zidovudine in asymptomatic individuals infected with HIV. J Acquir Immune Defic Syndr. 1991;4:11–23. [PubMed] [Google Scholar]
- 25.Kovacs JA, Vogel S, Albert JM, et al. Controlled trial of IL-2 infusions in patients with HIV. New Engl J Med. 1996;335:1350–6. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 26.De Paoli P, Zanussi S, Simonelli C, et al. Effects of subcutaneous IL-2 therapy on CD4 subsets and cytokine production in HIV+ subjects. J Clin Invest. 1997;100:2737–43. doi: 10.1172/JCI119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;4:1–19. [PubMed] [Google Scholar]
- 28.Simonelli C, Zanussi S, Sandri S, et al. Concomitant therapy with s.c. IL-2 and zidovudine plus didanosine in patients with early stage HIV infection. J Acquir Immune Defic Syndr. 1999;20:20–27. doi: 10.1097/00042560-199901010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Barzan L, Carbone A, Saracchini S, Vaccher G, Tirelli U, Comoretto R. Nasopharyngeal lymphatic tissue hypertrophy in HIV infected patients. Lancet. 1989;1:42–43. doi: 10.1016/s0140-6736(89)91695-4. [DOI] [PubMed] [Google Scholar]
- 30.Valiante N, Trinchieri G. Identification of a novel signal transduction molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397–406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armitage P, Berry G. Statistical methods in medical research. Oxford: Blackwell Scientific Publications; 1987. pp. 110–47. [Google Scholar]
- 32.Kelleher AD, Roggensack M, Emery S, Carr A, French MA, Cooper DA. Effects of IL-2 therapy in asymptomatic HIV-infected individuals on proliferative responses to mitogens, recall antigens and HIV-related antigens. Clin Exp Immunol. 1998;113:85–91. doi: 10.1046/j.1365-2249.1998.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson J, Fehninger T, Patterson B, Pottage J, Agnoli M, Jones P, Behbahani M, Landay A. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS. 1998;12:F123–f129. doi: 10.1097/00002030-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Lafeuillade A, Tamalet C, Poggi C, Pellegrino P, Tourres C, Izopet J. Antiretroviral effect of AZT-ddI combination on blood and lymph nodes. AIDS. 1997;11:67–72. doi: 10.1097/00002030-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Walker R, Carter C, Muul L, et al. Peripheral expansion of pre existing mature T cells is an important means of CD4+ T cell regeneration in HIV-infected adults. Nat Med. 1998;4:852–6. doi: 10.1038/nm0798-852. [DOI] [PubMed] [Google Scholar]
- 36.Douek D, McFarland R, Keiser P, et al. Changes in thymic function with age during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 37.Woods TC, Roberts BD, Butera ST, Folks TM. Loss of inducible virus in CD45RA naive cells after HIV-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–41. [PubMed] [Google Scholar]
- 38.Spina CA, Prince HE, Richman DD. Preferential replication of HIV 1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1555–64. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanussi S, Simonelli C, Bortolin MT, D'Andrea M, Comar M, Tirelli U, Giacca M, De Paoli P. Dynamics of provirus load and lymphocyte subsets after IL-2 treatment in HIV-infected patients. AIDS Res Hum Retrovir. 1999;15:97–103. doi: 10.1089/088922299311529. [DOI] [PubMed] [Google Scholar]
- 40.Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein 1. Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- 41.Mengozzi M, De Filippi C, Biswas P, et al. HIV infection superinduces MCP-1 production in human macrophages and in U937 promonocytic cells. 3. ECEAR: Munich; 1998. pp. 97–P3. Abstr. [Google Scholar]
- 42.Weiss L, Si-Mohammed A, Giral P, Castiel P, Ledur A, Blondin C, Kazatchkine M, Haefner-Cavaillon N. Plasma levels of MCP-1, but not those of MIP-lα and RANTES correlate with virus load in HIV infection. J Infect Dis. 1997;176:1621–4. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- 43.Levy Y, Capitani C, Houhou S, Carriere I, Gaustauld JA, Viard JP, Aboulker JP. 12th International Conference on AIDS. Geneva: 1998. IL-2 in HIV patients: a randomized trial comparing sc, PEG, CIV IL-2 with AZT + ddI; p. 41229. Abstr. [Google Scholar]
- 44.Davey RT, Chaitt DG, Piscitelli SC, et al. Subcutaneous administration of IL-2 in HIV type 1-infected persons. J Infect Dis. 1997;175:781–9. doi: 10.1086/513971. [DOI] [PubMed] [Google Scholar]