Abstract
Rheumatoid factor (RF) is the most common autoantibody found in patients with Sjögren's syndrome (SS). To study the genetic origin and the mechanisms acting behind its generation we have characterized and sequenced the immunoglobulin VH genes used by 10 IgM RF MoAbs derived from peripheral blood of six female patients with pSS. We compared the structure of the RF immunoglobulin VH genes with those obtained previously from rheumatoid arthritis (RA) patients and healthy immunized donors (HID). VH1 and VH4 were each used by four RF clones, one clone was encoded by VH3 family gene and one by VH2 family gene. This distribution frequency was different from that observed in RA, where VH3 was the dominant family, followed by VH1. Eight different germ-line (GL) genes encoded the clones and all of these genes were seen previously in RA and/or HID RF. Five clones rearranged to JH6, four rearranged to JH4 and one to JH5, in contrast to RF from RA and HID, where JH4 was most frequently used. D segment use and CDR3 structure were diverse. Interestingly, three out of four VH4 clones used the GL gene DP-79 that was seen frequently in RA RF. The degree of somatic mutation in the pSS RF was very much lower than seen in RA and HID RF. All the pSS RF clones except three were in or very close to GL configuration. This indicates that there is little role for somatic hypermutation and a germinal centre reaction in the generation of RF from peripheral blood in pSS.
Keywords: Sjögren's syndrome, rheumatoid factor, heavy chain variable region gene
INTRODUCTION
Sjögren's syndrome (SS) is an autoimmune disorder characterized by chronic inflammation of the exocrine glands, mainly the salivary and lachrymal glands leading to progressive destruction of these glands resulting in dryness of the mouth and conjunctiva (sicca syndrome) [1–3]. The disease can occur alone as pSS or it can be associated with other connective tissue diseases such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA), in which case it is referred to as secondary Sjögren's syndrome.
The main immunological feature of SS is mononuclear cell infiltration of the exocrine glands. Most of these cells are T cells (75–85%) and B cells (5–10%) [1,3]. A B cell activation results in hypergammaglobulinemia with elevated levels of circulating immune complexes and production of autoantibodies. Among the autoantibodies seen are rheumatoid factors (RF), anti-Ro/SSA and anti-La/SSB [1–4]. RF is found in the serum and saliva of 60–80% of pSS patients [5,6]. RF is also found in other autoimmune conditions such as RA in about 70% of patients [7–9], SLE as well as healthy donors following certain infections and immunization [10–12], and finally as a monoclonal component (MC) in lymphoproliferative diseases.
The structure and the genetic origins of RF are well characterized in RA and healthy immunized donors (HID) [12–26]. IgM RF from RA are polyclonal and highly mutated with relatively high affinity. This suggests that they have been through a germinal centre reaction with affinity maturation. However, evidence from HID suggests that high-affinity RF B cells are eliminated by the mechanism of peripheral tolerance [18]. The aims of this study were to determine the genetic origin and the structure of RF from pSS patients. This might help to understand the mechanism behind generation of RF in pSS and to determine whether they show evidence of being antigen-driven. For this purpose we derived 10 RF MoAbs from the peripheral blood of pSS patients using Epstein–Barr virus (EBV)/hybridoma technology. These MoAbs have diverse V gene usage. Most of the germ-line (GL) genes used were already identified in RF from RA but were used in different frequencies. Interestingly, seven out of 10 clones have very few somatic mutations, indicating that they have not been activated through a germinal centre reaction. Three other clones have moderate to high number of mutations, indicating a germinal centre process.
PATIENTS AND METHODS
Lymphocyte isolation and EBV transformation
Peripheral blood samples were obtained from six female patients with pSS aged between 36 and 77 years, fulfilling at least four of the preliminary European Classification Criteria for pSS [27]. They were either RF+ by Waaler agglutination test and/or latex test measured by standard nephelometry assay [28]. Mononuclear cells were isolated by high-density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway). The cells were incubated with 5 ml of supernatant from the B958 cell line containing EBV for 1 h at 37°C with 5% CO2. After 1 h, Dulbecco's modified Eagle's medium (DMEM; Sigma, St Louis, MO) + Hams F-12 + 10% fetal calf serum (FCS) was added to keep the number of cells at a concentration of 106 cells/ml, and the cells were incubated at 37°C and 5% CO2. Phytohaemagglutinin (PHA; Sigma) was added after 24 h at a concentration of 10 μg/ml.
Generation of antibody-secreting hybridomas
After 2 weeks the EBV-transformed B lymphocytes were fused with a mouse plasmacytoma cell line according to the method described before [29]. In brief, the fusions were performed with transformed B lymphocytes and the mouse plasmacytoma OURI in the ratio 1:1 in the presence of polyethylene glycol 45% (Sigma) in PBS. The fusion products were suspended in a DMEM culture medium + 10% FCS supplemented with hypoxanthine/aminopterin/thymidine (HAT; Sigma) at dilution of 1:50 and Ouabain at 1 μm.
ELISA and cloning
The supernatants from cell lines obtained from the fusions were screened after 2 weeks by ELISA for IgM and IgA RF activities. Briefly, 96-well ELISA plates (Maxisorp; Nunc-immuno plate, Roskilde, Denmark) were coated with human IgG (γ-kabi; Pharmacia, Uppsala, Sweden) 10 μg/ml in PBS. Plates were blocked with 1% bovine serum albumin (BSA). Following washing, supernatants were added and the plates were incubated at 37°C for 1–2 h. The plates were washed again and goat anti-human IgM (μ-chain specific) or goat anti-human IgA (α-chain specific) alkaline-phosphatase conjugate (Sigma) were added and incubated at 37°C for 2 h. Finally, the plates were washed as before, and Sigma 104 phosphatase substrate (1 mg/ml in diethanolamine buffer pH 9.8) was added and the absorbance was measured after 20 min at 405 nm. Uncoated trays blocked with BSA were used to eliminate antibodies giving unspecific binding. RF-secreting cell lines were cloned repeatedly by limiting dilution as described before [17].
Positive RF clones were tested in ELISA against Fc fragments of human IgG, rabbit IgG (10 μg/ml each; Sigma), Fab and F(ab′)2 of human IgG (5 μg/ml each; Calbiochem-Novabiochem, La Jolla, CA), tetanus toxoid (5 μg/ml; NBCI, Cambridge, UK), single-stranded DNA (ssDNA) (50 μg/ml; gift from Professor O. Mellbye, University of Oslo, Norway), and human IgG subclasses (10 μg/ml; a gift from Professor T. Michalsen, University of Oslo).
Preparation of mRNA, cDNA and sequencing
Total mRNA was extracted as described previously [12,30] and reverse transcribed to cDNA as described before [12,18]. VH region genes were amplified by polymerase chain reaction (PCR) using specific VH leader primers and cμ constant region primer as before [19,20], except for clones RF SN2, RF SN4 and RF VR1 (HY4) leader primer was used (5′ CTGGTGGCAGCTCCCAGATG 3′) and for RF EF2 (HY1) leader primer was used (5′ GCTCCAGGTGCCCACTCCCA 3′) and for RF SN1 (HM3R) (5′ TCGGACCGTATCCGACGGG GAATTCTCACA 3′) constant region primer was used. The PCR products were gel purified and directly sequenced in both directions [14] using the same primers as for PCR. The closest GL genes were identified by search in the V BASE and DNAPLOT sequence directory (I. Tomlinson, H. H. Althaus et al., MRC Centre for Protein Engineering, Cambridge, UK, and University of Cologne, Germany).
Statistical analysis
Mann–Whitney U-test was used to analyse the differences between the mean numbers of mutations of the three groups of RF (pSS, RA, and HID). Fisher's exact test was used to analyse the differences between R:S ratios in the CDRs for the whole panels of RF from pSS, RA and HID. Differences were considered significant at two-sided P values < 0.05.
RESULTS
Specificity of monoclonal RF autoantibodies
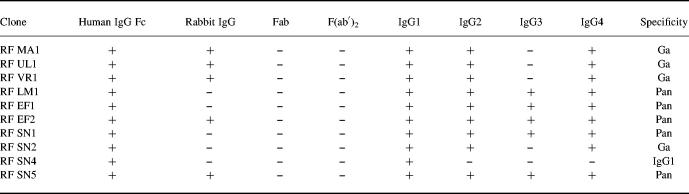
We have derived 10 IgM RF clones monospecific for the Fc region of human IgG from six different patients with pSS (Table 1). Five of the clones (50%) were also found to be reactive with rabbit IgG (RF MA1, RF UL1, RF VR1, RF EF2, RF SN5). Five clones (RF EF1, RF EF2, RF SN1, RF SN5, RF LM1) showed pan specificity (reactive to all human IgG subclasses), while four clones (RF MA1, RF UL1, RF SN2, RF VR1) showed the classical Ga specificity (reactive with IgG1, IgG2 and IgG4 but not IgG3). One clone (RF SN4) was found only reactive with IgG1. None of the 10 clones was reactive with Fab, F(ab′)2, tetanus toxoid, or ssDNA.
Table 1.
Specificities of rheumatoid factor (RF) monoclonal autoantibodies from patients with pSS
None of the clones showed any reactivity with tetanus toxoid or ssDNA.
V gene sequence analysis
The sequences of the heavy chains (Table 2 and Fig. 1) revealed that four of the clones (40%) were encoded by VH4 family genes (RF SN2, RF SN4, RF VR1, RF EF1), four (40%) used VH1 family genes (RF MA1, RF UL1, RF EF1, RF SN1), one used a VH3 gene (RF LM1) and one clone used a VH2 gene (RF SN5). All of the VH genes used have been described before in RF from either RA patients or HID [21–23]. Of the VH1 family clones, one of them (RF MA1) was closest to DP-10 GL gene, RF UL1 was highly homologous to DP-88 GL gene, RF EF2 used DP-14 GL and RF SN2 used DP-7. Interestingly, three of the four VH4 clones were closest to the GL gene DP-79 and all three rearranged to the JH4b segment. Two of these were from one patient (RF SN2 and RF SN4) and one from a different patient (RF VR1). The fourth VH4 clone (RF EF1) used DP-65 GL gene. The VH3 clone (RF LM1) had closest homology to the DP-46 GL gene and the VH2 clone was closest to the DP-26 GL gene. All of the clones were rearranged to different D-segments (Fig. 2) except two pairs. RF SN4 and RF EF1 used the D3–22 segment in two different reading frames and RF SN2, RF SN5 used the D1–26 segment also in two different reading frames. Analysis of the J segments showed that JH6 was preferentially used, four clones rearranged to the JH6b segment (RF UL1, RF EF2, RF SN1, RF LM1), while the fifth clone (RF MA1) rearranged to the JH6c segment (RF MA1). Four clones rearranged to JH4b (RF SN5, RF SN2, RF SN4, RF VR1), and only one clone used the JH5b segment (RF EF1).
Table 2.
Monoclonal IgM rheumatoid factor (RF) from pSS patients are shown with family gene, the closest identified VH, D and JH germ-line (GL) gene segment, and the length (L) of CDRH3 amino acid sequence
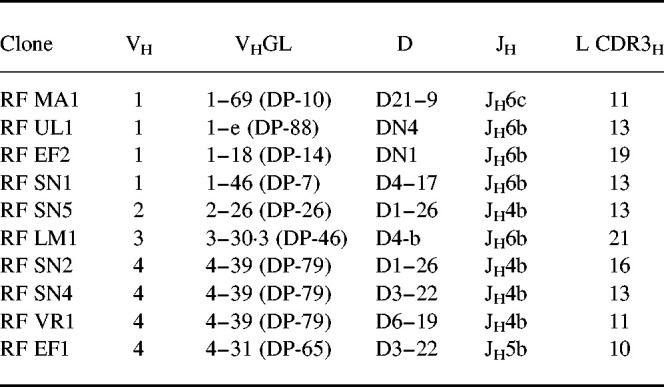
Fig. 1.
Amino acid sequences of the VH gene region of IgM rheumatoid factor (RF) from pSS. Closest identified germ-line genes in the V BASE and DNAPLOT are shown. Dashes denote homology, uppercase letters denote replacement mutation, whereas lower case letters denote silent mutation. The codons are numbered according to the definition of Kabat [51].
Fig. 2.
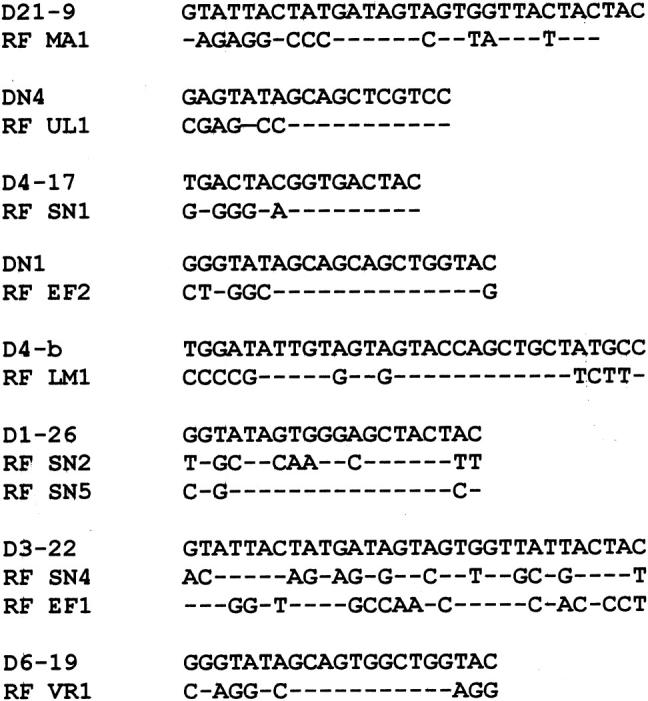
Nucleotide sequences of D segment of pSS rheumatoid factors (RF) compared with the closet germ-line (GL) D segment. Upper case denotes mismatches with the GL.
Analysis of the mutational patterns
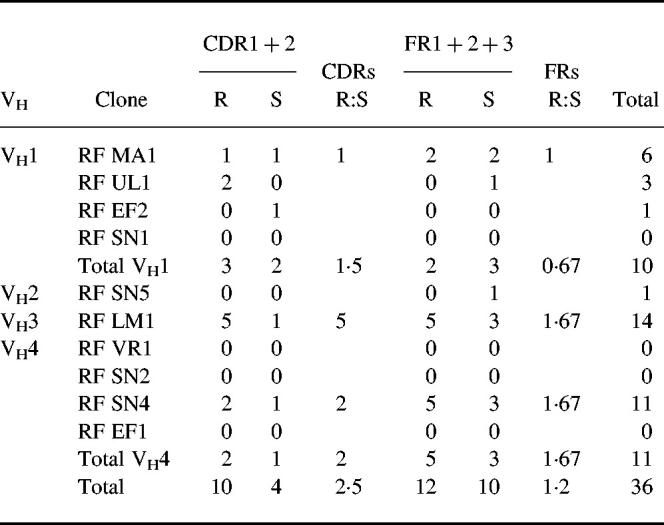
In contrast to what has been found in RF from RA and HID, most of the present clones were found to be highly homologous to their closest GL gene. Surprisingly, four of the clones were found to be unmutated copies of their closest GL genes at the nucleotide level. Three of them used VH4 family genes (RF VR1, RF SN2 and RF EF1), while the fourth used a VH1 family gene (RF SG1) (Table 3). Two other clones had only one silent mutation (RF EF2 and RF SN5). Thus six clones were 100% homologous to the GL product at the amino acid level. One clone had three mutations (RF UL1); two replacement mutations in the CDRH1 and one silent mutation in framework two. Only three clones were moderately to highly mutated comparable to that seen in RF of RA and HID (RF MA1, RF SN4, RF LM1 had six, 11, and 14 mutations per VH gene, respectively). All three had from two to five replacement mutations in the CDR regions. The mean number of mutations was 3.6 per VH gene. This is statistically significantly lower than the mean numbers for RA RF (mean 11 and P = 0.0028, Mann–Whitney U-test) and HID RF (mean 10 and P = 0.0051) [11,22]. The R:S ratio for CDRH1 + 2 for RF in pSS was 2.5 (Table 3), which is not statistically different from the ratios found in RA or HID RF.
Table 3.
Total number of mutations, R:S ratios of CDRH1 + 2 and FRH1 + 2 + 3 of the VH segment of IgM rheumatoid factor (RF) of pSS in different VHfamilies and the overall panel of IgM pSS RF
DISCUSSION
RF are autoantibodies against antigenic determinants on the Fc region of human IgG, but many RF are also reactive to IgG of other species, e.g. rabbit IgG [4,17]. Our pSS RF satisfy these criteria. In addition, the majority showed the pan and Ga classical specificity similar to RA RF [17,31].
In humans, VH3 family genes are the most frequent family used by antibodies (50–60%) [32–34]. This is also true for RF from RA, where VH3 has been found to be the most frequent family (60–80%) followed by VH1 and VH4 [35–37]. However, if RF only derived from blood of RA are considered, VH1 is the predominant family (50%), followed by VH4 (25%) and VH3 (25%) [38–40]. This is similar to RF derived from the peripheral blood of HID (53.4% used VH1, 37.9% used VH3, and 5.2% used VH4) [12,14]. In pSS RF only one clone used VH3, whereas VH1 and VH4 were equally represented (40% each). This looks closer to RF derived from the blood of both RA patients and normals, but clearly different from the distribution found in the synovial tissues of RA patients. This finding is supported by idiotype studies done previously, where elevated levels of VH1-associated cross-reactive idiotype in the sera from patients with pSS were found [36,41,42]. In addition, our panel also showed a high degree of heterogeneity with eight different GL genes encoding 10 RF clones. This is comparable to RF from RA and HID, which also showed a large degree of heterogeneity. All GL genes utilized by pSS RF were seen previously in RA and/or HID RF. Two GL genes each, used once by our clones (VH1 DP-10 and VH2 DP-26) were seen before in both RA and HID RF; DP-10 was used by several clones from HID, RA, and MC RF [33,43], while DP-26 was used before by two clones from HID and one RF clone from RA synovium [12].
The most frequent GL used by our clones was DP-79 (30%) and this was found to be used by several RF clones from both synovium and peripheral blood of RA but not of HID [22,23]. Interestingly, all our DP-79 clones rearranged to the JH4 segment which was similar to most DP-79 RF clones from RA which also used the JH4 segment. This suggests that a combination of VH DP-79 and JH4 might be important in RF specificity. The other GL genes used by our clones, and also seen before in RA RF, were DP-46, which were similarly used by two clones from RA synovium [22]. DP-14 and DP-7 were each used by one RF clone from RA synovium [22,23]. DP-88 and DP-65 GL genes were used by RF from HID and pSS but not from RA [12,22]. Although the frequency of VH family use was different, in general the GL gene usage of pSS RF seemed more similar to RA RF than HID RF.
A striking finding is the preferential use of the JH6 segment (50%). Generally JH4 is the most frequent J segment used (45–65%), followed by JH6 (about 20–25%) [44,45], and this was also true for RA and HID where JH4 is the most frequently used by RF [22]. This might be due to selective activation of B cells clones. D-segment use was diverse.
It has been postulated that the generation of autoantibodies can occur mainly through either an antigen-driven response [35], as in RA and HID RF, or through other mechanisms such as unspecific polyclonal or monoclonal activation. In the former mechanism the B cell undergoes a germinal centre reaction where the immunoglobulin V regions undergo a process of somatic hypermutation, affinity maturation and selection [35]. Clonal expansion and class switch have been seen in RF from RA. Following polyclonal and monoclonal activation the antibodies generated have low affinity and they are very close or directly encoded by the GL, as in MC RF.
The majority of pSS RF were 100% homologous to a GL gene product (six out of 10); to our knowledge only one RF clone out of 55 from RA synovium and only one out of 34 from HID were encoded directly by the GL [12,22,23,25]. Three of our pSS RF clones had a substantial number of mutations [11,29]. In the first one (RF LM1) there was evidence of targeting the replacement mutation in the CDR5 regions (R:S = 1 + 2); and this indicates that this RF had been selected during the germinal centre reaction. The second clone (RF SN4) had 11 mutations, but most of those mutations were in FR regions (eight mutations), while the third clone (RF MA1) had only one replacement and one silent mutation in CDRH1 and the rest in FRH3. This indicates that there was no pressure in the CDR region of these two clones but they had still undergone a germinal centre process.
Our data suggest that there is little or no role for somatic mutation in the generation of most RF in pSS. This has been suggested indirectly by previous studies with idiotype markers [34,36,37,41–43,46–48]. One explanation for this is that there may be intrinsic B cell defects resulting in failure to diversify the GL by somatic mutations [46]. The possible mechanism of generation of those antibodies is through unspecific polyclonal activation and expansion of B cells without an antigen-driven process and T cell help. This could be part of the immunological and pathological processes acting in SS, as indicated by the considerable number of SS patients who develop hypergammaglobulinaemia and lymphoma and monoclonal gammopathy due to monoclonal B cell activation and production of MC RF.
However, Stott et al. and Bahler & Swerdlow studied rearranged variable region genes without defined antibody specificities within the labial salivary glands from patients with SS and showed evidence of antigen-driven germinal centre response [49,50]. These findings suggest that our RF from peripheral blood of pSS might not be involved in the local pathology of the disease.
In conclusion, our data suggest that there is very little role for somatic mutation in the generation of RF from peripheral blood in pSS.
Acknowledgments
This study was supported by European Biomed concerted action BMH4-CT96-0595, the Research Council of Norway, Medinnova Foundation. We gratefully acknowledge the excellent technical help of Eilen Strand, Ingvild Vatan, and Lill Næss. We sincerely thank Professor Ove Mellby for provision of ssDNA, Professor Terje Michalsen for provision of IgG subclasses and Dr Eva Källberg for valuable discussion and advice.
REFERENCES
- 1.Tzioufas AG, Talal N, Moutsopoulos HM. Sjögren's syndrome: from polyclonal B cell activation to monoclonal B cell proliferation. In: Panayi GS, Whaley K, editors. Immunology of connective tissue diseases. Dordrecht: Kluwer Academic; 1994. pp. 335–54. [Google Scholar]
- 2.Lane HC, Fauci AS. In: Sjögren's syndrome. Harrison's principles of internal medicine. Wilson JD, Braunwald E, Isselbacher KJ, et al., editors. New York: McGraw-Hill; 1991. pp. 1449–50. [Google Scholar]
- 3.Tzioufas AG, Moutsopoulos HM. Sjögren's syndrome. In: Klippel JH, Dieppe PA, editors. Rheumatology. London: Mosby; 1998. pp. 7.32.1–12. [Google Scholar]
- 4.Børretzen M, Mellbye OJ, Thompson KM, Natvig JB. Rheumatoid factors. In: Peter JB, Shoenfeld Y, editors. Autoantibodies. Amsterdam: Elsiver Science B.V; 1996. pp. 706–15. [Google Scholar]
- 5.Markusse HM, Otten HG, Vroom TM, Smeets TJ, Fokkens N, Breedveld FC. Rheumatoid factor isotypes in serum and salivary fluid of patients with primary Sjögren's syndrome. Clin Immunol Immunopathol. 1993;66:26–32. doi: 10.1006/clin.1993.1004. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson JC, Fox PC, Travis WD, et al. IgA rheumatoid factor and IgA containing immune complexes in primary Sjögren's syndrome. J Rheumatol. 1989;16:1205–10. [PubMed] [Google Scholar]
- 7.Zvaifler NJ. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16:265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- 8.Fehr K, Zelvart M, Rauber M, Knopef M, Baici A, Salagam P, Boni A. Production of agglutinators and rheumatoid factors in plasma cells of rheumatoid and non-rheumatoid synovial tissues. Arthritis Rheum. 1981;24:510–9. doi: 10.1002/art.1780240310. [DOI] [PubMed] [Google Scholar]
- 9.Kalsi J, Isenberg D. Rheumatoid factor: primary or secondary event in the pathogenesis of RA? Int Arch Allergy Immunol. 1993;102:209–15. doi: 10.1159/000236528. [DOI] [PubMed] [Google Scholar]
- 10.Heyman B. Fc-dependent IgG mediated suppression of the antibody response: fact or artefact? Scand J Immunol. 1990;31:601–7. doi: 10.1111/j.1365-3083.1990.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 11.Posnett DN, Edinger J. When do microbes stimulate rheumatoid factor? J Exp Med. 1997;185:1721–3. doi: 10.1084/jem.185.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Børrezten M, Natvig JB, Thompson KM. Hetrogenous RF structures between and within healthy individuals are not related to HLA-DRB1*0401. Mol Immmunol. 1997;34:929–38. doi: 10.1016/s0161-5890(97)00087-4. [DOI] [PubMed] [Google Scholar]
- 13.Djavad N, Bas S, Shi X, Schwager J, Jeannet M, Vischer T, Roosnek E. Comparison of rheumatoid factors of rheumatoid arthritis patients, of individual with mycobacterial infections and of normal controls: evidence for maturation in the absence of an autoimmune response. Eur J Immunol. 1996;26:2480–6. doi: 10.1002/eji.1830261031. [DOI] [PubMed] [Google Scholar]
- 14.Thompson KM, Randen I, Børretzen M, Førre Ø, Natvig JB. Variable region gene usage of human monoclonal rheumatoid factors derived from healthy donors following immunization. Eur J Immunol. 1994;24:1771–8. doi: 10.1002/eji.1830240808. [DOI] [PubMed] [Google Scholar]
- 15.Randen I, Brown D, Thompson KM, et al. Clonally related IgM rheumatoid factors undergo affinity maturation in the synovial tissue. J Immunol. 1992;148:3296–301. [PubMed] [Google Scholar]
- 16.Randen I, Thompson KM, Thorpe S, Førre Ø, Natvig JB. Human monoclonal IgG rheumatoid factors from the synovial tissue of patients with rheumatoid arthritis. Scand J Immunol. 1993;37:668–72. doi: 10.1111/j.1365-3083.1993.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 17.Randen I, Thompson KM, Natvig JB, Førre Ø, Waalen K. Human monoclonal rheumatoid factors derived from the polyclonal repertoire of rheumatoid synovial tissue: production and characterization. Clin Exp Immunol. 1989;78:13–18. [PMC free article] [PubMed] [Google Scholar]
- 18.Børretzen M, Randen I, Zdarsky E, Førre Ø, Natvig JB, Thompson KM. Control of antibody affinity by selection against amino acid replacements in the complementary determining regions. Proc Natl Acad Sci USA. 1994;91:12917–21. doi: 10.1073/pnas.91.26.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual V, Randen I, Thompson K, Sioud M, Førre Ø, Natvig J, Capra JD. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. Further evidence that some autoantibodies are unmutated copies of germline genes. J Clin Invest. 1990;86:1320–8. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual V, Victor K, Randen I, et al. Nucleotide sequence analysis of rheumatoid factors and polyreactive antibodies derived from patients with rheumatoid arthritis reveals diverse use of VH and VL gene segments and extensive variability in CDR-3. Scand J Immunol. 1992;36:349–62. doi: 10.1111/j.1365-3083.1992.tb03108.x. [DOI] [PubMed] [Google Scholar]
- 21.Børrezten M, Randen I, Natvig JB, Thompson KM. Structural restriction in the heavy chain CDR3 of human rheumatoid factors. J Immunol. 1995;155:3630–7. [PubMed] [Google Scholar]
- 22.Børrezten M, Chapman C, Natvig JB, Thompson KM. Differences in mutational patterns between rheumatoid factors in health and disease are related to variable heavy chain family and germline gene usage. Eur J Immunol. 1997;27:735–41. doi: 10.1002/eji.1830270323. [DOI] [PubMed] [Google Scholar]
- 23.Ermel RW, Kenny TP, Wong A, Chen PP, Malyj W, Robbins DL. Analysis of the molecular basis of synovial rheumatoid factors in rheumatoid arthritis. Clin Immunol Immunopathol. 1997;84:307–17. doi: 10.1006/clin.1997.4399. [DOI] [PubMed] [Google Scholar]
- 24.Thompson KM, Randen I, Natvig JB, Mageed RA, Jefferis R, Carson DA, Tighe H, Førre Ø. Human monoclonal rheumatoid factors derived from the polyclonal repertoire of rheumatoid synovial tissue: incidence of cross-reactive idiotopes and expression of VH and V kappa subgroups. Eur J Immunol. 1990;20:863–8. doi: 10.1002/eji.1830200422. [DOI] [PubMed] [Google Scholar]
- 25.Jain RI, Fais F, Kaplan S, et al. Ig H and L chain variable region gene sequence analysis of twelve synovial tissue-derived B cell lines producing IgA, IgG, and IgM rheumatoid factors structure/function comparisons of antigenic specificity, V gene sequence, and IG isotype. Autoimmunity. 1995;22:229–43. doi: 10.3109/08916939508995321. [DOI] [PubMed] [Google Scholar]
- 26.Stüber F, Lee SK, Bridges SL, Jr, Koopman WJ, Schroeder HW, Jr, Gaskin F, Fu SM. A rheumatoid factor from a normal individual encoded by VH2 and VκII gene segments. Arthritis Rheum. 1992;35:900–4. doi: 10.1002/art.1780350808. [DOI] [PubMed] [Google Scholar]
- 27.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of an EEC prospective concerted action. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 28.Kjeldsen-Kragh J, Mellbye OJ, Haugen M, Mollnes TE, Hammer HB, Sioud M, Førre Ø. Changes in laboratory variables in rheumatoid arthritis patients during a trial of fasting and one-year vegetarian diet. Scand J Rheumatol. 1995;24:85–93. doi: 10.3109/03009749509099290. [DOI] [PubMed] [Google Scholar]
- 29.Thompson KM, Hough DW, Maddison PJ, Melamed MD, Hughes JN. The efficient production of stable human monoclonal antibody secreting hybridomas from EBV transformed lymphocytes using the mouse myeloma X63-Ag8.653 as fusion partner. J Immunol Methods. 1986;94:7–12. doi: 10.1016/0022-1759(86)90208-5. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Gaarder PI, Natvig JB. Hidden rheumatoid factors reacting with ‘Non a’ and other antigens of native autologus IgG. J Immunol. 1970;105:928–37. [PubMed] [Google Scholar]
- 32.Guigou V, Cuisinier AM, Tonnelle C, Moinier D, Fougereau M, Fumoux F. Human immunoglobulin VH and VK repertoire revealed by in situ hybridization. Mol Immunol. 1990;27:935–40. doi: 10.1016/0161-5890(90)90161-r. [DOI] [PubMed] [Google Scholar]
- 33.Zouali M, Theze J. Probing VH gene-family utilization in human peripheral B cells by in situ hybridization. J Immunol. 1991;146:2855–64. [PubMed] [Google Scholar]
- 34.Shokri F, Mageed RA, Maziak BR, Jefferis R. Expression of VHIII-associated cross-reactive idiotypes on human B-lymphocytes: association with staphylococcal protein A (SPA) binding and staphylococcus aureus Cowan I (SAC) stimulation. J Immunol. 1991;146:936–40. [PubMed] [Google Scholar]
- 35.Randen I, Thompson KM, Natvig JB, et al. Rheumatoid factor V genes from patients with rheumatoid arthritis are diverse and show evidence of antigen driven response. Immunol Rev. 1992;128:49–71. doi: 10.1111/j.1600-065x.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 36.Shokri F, Mageed RA, Kitas GD, Katsikis P, Moutsopoulos HM, Jefferis R. Quantification of cross-reactive idiotype-positive rheumatoid factor produced in autoimmune rheumatic diseases. An indicator of clonality and B cell proliferative mechanisms. Clin Exp Immunol. 1991;85:20–27. doi: 10.1111/j.1365-2249.1991.tb05676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melero J, Aguilera I, Mageed RA, Jefferis R, Tarragó D, Núòez-Roldán A, Sánchez B. The frequent expansion of a subpopulation of B cells that express RF-associated cross reactive idiotypes: evidence from analysis of a panel of autoreactive monoclonal antibodies. Scand J Immunol. 1998;48:152–8. doi: 10.1046/j.1365-3083.1998.00373.x. [DOI] [PubMed] [Google Scholar]
- 38.Harindranath N, Goldfarb IS, Ikematsu H, Burastero SE, Wilder RL, Notkins AL, Casali P. Complete sequence of the genes encoding the VH and VL regions of low and high affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int Immunol. 1991;3:1188–99. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youngblood K, Fruchter L, Ding G, Lopez J, Bonagura V, Davidson A. Rheumatoid factors from the peripheral blood of two patients with rheumatoid arthritis are genetically heterogeneous and somatically mutated. J Clin Invest. 1994;93:852–61. doi: 10.1172/JCI117040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani L, Wilder RL, Casali P. Human rheumatoid B-1a (CD5+ B) cells make hypermutated high affinity IgM rheumatoid factors. J Immunol. 1993;151:473–88. [PMC free article] [PubMed] [Google Scholar]
- 41.Shokri F, Mageed RA, Maziak BR, Talal N, Amos N, William BD, Jefferis R. Lymphoproliferation in primary Sjögren's syndrome—evidence of selective expansion of a B cell subset characterized by the expression of cross reactive idiotypes. Arthritis Rheum. 1993;36:1128–36. doi: 10.1002/art.1780360814. [DOI] [PubMed] [Google Scholar]
- 42.Shokri F, Mageed RA, Kitas GD, Moutsopoulos HM, Jefferis R. The level of rheumatoid factor associated cross-reactive idiotypes in Sjögren's syndrome, rheumatoid arthritis, SLE and healthy individuals (abstract) Br J Rheumatol. 1989;28(Suppl. 1):40. [Google Scholar]
- 43.Shimizu S, Sugai S, Imaoka T, Sawada M, Konda S. Monoclonal anti-idiotypic antibodies against monoclonal rheumatoid factors derived from patients with Sjögren's syndrome. Scand J Rheumatol. 1986;(Suppl. 61):106–10. [PubMed] [Google Scholar]
- 44.Sanz I. Multiple mechanism participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991;147:1720–9. [PubMed] [Google Scholar]
- 45.Yamada M, Wasserman R, Reichard BA, Shane S, Caton AJ, Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991;173:395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox RI, Chen P, Carson DA, Fong S. Expression of cross-reactive idiotype on rheumatoid factor in patients with Sjögren's syndrome. J Immunol. 1986;136:477–83. [PubMed] [Google Scholar]
- 47.Sugai S, Saito I, Masaki Y, Takeshita S, Shimizu S, Tachibana J, Miyasaka N. Rearrangement of the rheumatoid factor related germline gene V g and bcl-2 expression in lymphoproliferative disorders in patients with Sjögren's syndrome. Clin Immunol Immunopathol. 1994;72:181–6. doi: 10.1006/clin.1994.1127. [DOI] [PubMed] [Google Scholar]
- 48.Deacon EM, Mathews JB, Potts AJ, Hamburger J, Mageed RA. Expression of rheumatoid factor associated cross reactive idiotopes by glandular B cells in Sjögren's syndrome. Clin Exp Immunol. 1991;83:280–5. doi: 10.1111/j.1365-2249.1991.tb05628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stott DI, Hlepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren's syndrome. J Clin Invest. 1998;102:938–46. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahler DW, Swerdlow SH. Clonal salivary gland infiltrates associated with myoepithelial sialadenitis (Sjögren's syndrome) begin as nonmalignant antigen-selected expansions. Blood. 1998;91:1864–72. [PubMed] [Google Scholar]
- 51.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. Bethesda: NIH Publication; 1991. pp. 91–3243. [Google Scholar]