Abstract
We have previously shown an increased susceptibility of T cell subsets to anti-Fas-induced apoptosis in human ageing [1]. In this study, we have examined the role of downstream mediators, including caspases, in Fas-mediated apoptosis in lymphocytes from ageing humans. The cleavage activity of caspase-8 and caspase-3 was compared between ageing and young subjects at different times following anti-Fas treatment, using colorimetric detection analysis. The expression of Fas-associated death domain (FADD), caspase-8, and caspase-3 in lymphocytes was compared at the protein level using Western blotting, and at the mRNA level by Northern blot analysis. In lymphocytes from ageing subjects, there was an early increase in the cleavage activity of caspase-8 and caspase-3 compared with young controls. Furthermore, increased protein expression of FADD, caspase-8 and caspase-3 at the basal level was observed in lymphocytes from ageing humans. Our results suggest that the altered expression and activity of molecules in the Fas/FasL signalling pathway may play a role in increased Fas-induced apoptosis and T cell deficiency in ageing humans.
Keywords: anti-Fas, caspase-3, caspase-8, ageing
INTRODUCTION
Apoptosis plays an important role in cellular homeostasis. In the immune system, especially in lymphocytes, apoptosis plays an important role in maintaining the T cell repertoire and in deleting autoreactive lymphocytes, thus regulating the immune response [2–4]. Cross-linking of surface monomeric Fas molecules by Fas ligand or agonistic anti-Fas antibody activates the apoptotic death programme in vitro and in vivo[5,6]. Mutational analyses have shown that a 70aa cytoplasmic death domain plays a role in signalling through this receptor [7,8]. Upon cross-linking of Fas antigen, Fas-associated death domain (FADD/MORT1), a death domain-containing protein, is recruited to the cell membrane. FADD/MORT1 then recruits caspase-8 (FLICE/MACH/Mch5) to the death-inducing signalling complex (DISC), which subsequently leads to activation of a downstream protease cascade. The identities of all the caspases involved in Fas-induced apoptosis are still unknown; however, caspase-3 (CPP32/Yama/apopain) has been shown to play an important role [9,10]. Activation of caspase-3 leads to cleavage of self and its nuclear substrates, including poly (ADP-ribose) polymerase (PARP) [11–13], resulting in sequential degradation of cell assembly.
Ageing is characterized by a progressive decline in immune functions (reviewed in [14–16]). Recently, we [1] and others [17–19] have shown increased T cell apoptosis in ageing humans. This increase is associated with up-regulation of Fas and Fas ligand (FasL) expression and increased susceptibility to Fas-mediated apoptosis [1]. However, downstream signalling in the anti-Fas-induced pathway in ageing subjects has not been explored. Therefore, in the present study we have analysed the expression and activity of FADD and caspases (caspase-8 and caspase-3) in lymphocytes from ageing humans following anti-Fas treatment, and compared them with lymphocytes from young controls. To the best of our knowledge, this is the first report of caspase activation in human ageing.
Our data show that there are increased constitutive levels of FADD, caspase-8, and caspase-3 in lymphocytes from ageing, compared with young subjects. Furthermore, following anti-Fas antibody treatment, there is an early activation of caspase-8 and caspase-3 in the lymphocytes from ageing subjects, compared with that observed in the young controls.
SUBJECTS AND METHODS
Subjects
Peripheral blood was obtained from healthy young (18–22 years old) and ageing volunteers (65–92 years old) following written consent. The study protocol was approved by the Institutional Review Board of the University of California, (Irvine, CA).
Antibodies
MoAbs against FADD, caspase-3 and horseradish peroxidase (HRP)-conjugated mouse IgG were purchased from Transduction Laboratories (Lexington, KY). FADD antibody was raised in the mouse against a 24-kD protein fragment corresponding to the death domain of FADD. It is an IgG1 isotype, clone 1, and is specific for human, dog, and mouse FADD protein. Caspase-3 antibody was raised in the mouse against a 24.7-kD protein fragment corresponding to amino acids 1–219 of human CPP32. It is an IgG2a isotype, clone 19, and is specific for human and chick caspase-3 protein. Polyclonal antibody against caspase-8 (Mch5) and HRP-conjugated goat antibody were purchased from Santa Cruz Technology (Palo Alto, CA). Anti-caspase-8 antibody was raised in goat against a peptide corresponding to amino acids 354–373 (carboxy terminus of human caspase-8) and is reactive to caspase-8 of human origin by Western blotting and immunohistochemistry. MoAb against PARP (clone C2-10) was obtained from PARP Laboratories (Quebec, Canada). Anti-Fas MoAb was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). It was raised in the mouse using human diploid fibroblast cell line (FS-7) as an immunogen. It is an IgM isotype, clone CH-11, and specifically recognizes Fas expressed in various cell lines. It does not cross-react with either tumour necrosis factor (TNF) or mouse Fas.
Probes
Plasmids pcDNA/FADD U1, pcDNA/Yama and pcDNA/FLICE were a gift from Dr V. Dixit (Ann Arbor, MI), now at Genentech (South San Francisco, CA). Plasmids were grown in Escherichia coli HB101 and plasmid DNA was purified on tip 100 Qiagen columns (Qiagen, Chatsworth, CA). Purified DNA preparations were digested with (i) Kpn/BamH1 to give approx. 700-bp FADD insert, (ii) Kpn/BamH1 to give approx. 1.5-kb FLICE (caspase-8) insert, and (iii) EcoRI/XbaI to give approx. 850-bp Yama (caspase-3) insert.
Cell culture
Mononuclear cells (MNC) were separated from freshly isolated peripheral blood using Ficoll–Hypaque gradient. Cells were > 95% lymphocytes and < 5% monocytes. Cells (2 × 106 cells/ml) were activated with anti-CD3 MoAb (25 ng/ml) for 48 h. Following activation, the cells were washed and incubated in the presence of recombinant IL-2 (10 ng/ml) for a duration of another 4 days. The cells were washed, and viable cells were counted by trypan blue dye exclusion. Ten million viable cells (1 × 106 cells/ml) were treated with anti-Fas MoAb (1 μg) or its isotype-matched control IgM antibody (1 μg) for different time periods.
FADD expression
FADD expression was determined at the protein level using Western blotting (described below) and at the mRNA level using Northern blotting (described below).
Caspase activity
Cell extracts were prepared from the previously activated lymphocytes (as described in Cell culture) treated with anti-Fas MoAb for 0, 4, 8 and 16 h.
Caspase-8 activity was determined using FLICE/Caspase-8 colorimetric assay kit (BioVision Research Products, Palo Alto, CA). The cleavage of synthetic caspase-8 substrate IETD-pNA was detected spectrophotometrically by the formation of pNA.
Caspase-3 activity was determined using the following criteria: (i) its ability to cleave self; (ii) its ability to cleave its nuclear substrate PARP; and (iii) its ability to cleave a synthetic peptide substrate DEVD-pNA. The cleavage of caspase-3 and PARP was monitored by the appearance of their cleaved forms (p20 and p10 for caspase-3 and p85 for PARP, respectively), using Western blotting. The cleavage activity of DEVD-pNA was determined colorimetrically by the formation of cleaved substrate (pNA) using ApoAlert Assay kit (Clontech, Palo Alto, CA).
Western blotting
Cellular extracts from lymphocytes were prepared by lysing the cells in a buffer containing 142.5 mm KCl, 5 mm MgCl2, 10 mm HEPES pH 7.2, 1 mm EGTA, 0.2% NP40, 0.2 mm phenylmethylsulfonyl fluoride, 0.2 trypsin inhibitory units/ml aproteinin, 0.7 μg/ml pepstatin, and 1 μg/ml leupeptin. Cells were homogenized and centrifuged at 1000 rev/min for 8 min to precipitate cell debris. The supernatants were centrifuged at 30 000 g for 45 min to precipitate membrane fractions. The protein was estimated and equal amounts of protein were loaded in each lane. Cell lysates containing 25 μg protein were loaded onto 4–20% SDS–PAGE gels and electrophoresed. The proteins were transferred to nitrocellulose membrane for 4 h/overnight in cold. The blots were blocked with PBS containing 3% non-fat dry milk and 0.1% Tween-20 (PBS–M–T) for 1 h at 37°C. The membranes were incubated with the primary antibody diluted in PBS–M–T overnight at 4°C and washed three times with PBS–M–T. The blots were then incubated with HRP-conjugated secondary antibody for 30 min at 37°C and washed with PBS–M–T. After several washes the blots were developed using enhanced chemiluminescence detection method (Amersham Inc., Arlington Heights, IL). The blots were scanned using ImageQuant Software (Molecular Devices, Sunnyvale, CA) and data expressed in optical density (OD).
Colorimetric assay
Caspase-8 and caspase-3 activities were quantified by spectrophotometric detection of chromophore (pNA) following its cleavage from labelled substrates IETD-pNA and DEVD-pNA, respectively. Lymphocytes were cultured as described before and cells were treated with anti-Fas antibody (1 μg/ml) or its isotype-matched control for different time periods. Following incubation, the cells were lysed and incubated as follows: (i) caspase-8 substrate IETD-pNA; (ii) CPP32 substrate DEVD-pNA; and (iii) CPP32 substrate in the presence of specific peptide inhibitor (DEVD-CHO) for 1 h according to the instructions provided by the manufacturers (the inhibitor for caspase-8 substrate was not available). All reactions were performed in triplicate. The samples were read by spectrophotometer at 405 nm. The results are expressed as relative caspase activity [20], which is the ratio between the caspase activity of the sample and that measured in control (without treatment). In the case of caspase-3 activity, background remaining after inhibition by DEVD-CHO was subtracted from all the samples.
Northern blotting
Expression at mRNA level was determined using Northern blot analysis. The amount of RNA was determined using spectrophotometer and equal amounts of RNA were loaded in each lane. Total cellular RNA (20 μg) was separated by electrophoresis in a denaturing agarose gel containing 6.5% formaldehyde, transferred onto a charged nylon membrane (Schleicher & Schuell Inc., Keene, NH) by capillary blotting in 20× sodium chloride and sodium citrate buffer (SSC) for 20 h, and cross-linked to the membrane by UV irradiation. The blots were prehybridized at 42°C for 6 h in a solution of 5× SSC, 5× Denhart's solution (0.5 g Ficoll, 0.5 g polyvinylpyrrolidone, 0.5 g bovine serum albumin (BSA)), 50 mm EDTA, 10 μg/ml poly(A), 100 μg/ml yeast RNA and 200 μg/ml heat-denatured salmon sperm DNA. Hybridizations were done for 20 h at 42°C in the same solution containing 10% dextran sulfate and α-32P-labelled probe (2 × 106 ct/min per ml), labelled using a random priming DNA labelling kit (Amersham). The blots were washed under stringent conditions at 0.2× SSC/ 0.1% SDS at 65°C for 1 h, and exposed to X-OMAT AR film (Eastman Kodak, Rochester, NY) at −80°C for 16 h with an intensifying screen before development. The blots were scanned using ImageQuant Software (Molecular Devices) and data expressed in OD.
TUNEL assay
The DNA fragmentation was determined using TUNEL assay (terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling). Cells were harvested and washed with Dulbecco's phosphate buffered saline (DPBS) and the assay was carried out as described before [1]. Briefly, cells (1 × 106 cells/ml) were fixed in 4% paraformaldehyde for 30 min at room temperature followed by permeabilization with sodium citrate buffer containing 1% Triton X-100 for 2 min on ice. After washing, cells were incubated with FITC-labelled dUTP in the presence of terminal deoxynucleotidyl transferase enzyme solution for 1 h at 37°C using cell death detection kit (Boehringer Mannheim Corp., Indianapolis, IN). Five thousand cells were acquired and analysed using FACScan and Consort 30 software (Becton Dickinson, San Jose, CA).
RESULTS
FADD expression
Following lymphocyte treatment with an anti-Fas antibody, the Fas receptor is cross-linked resulting in the recruitment, to Fas, of a protein with a death domain, FADD, which in turn, associates with downstream members of the caspase family through its interaction with the death-effector domain (DED). Therefore, we first examined if there were any differences in constitutive FADD expression in lymphocytes from ageing and young humans. Expression at the protein level was measured by Western blotting using samples from six ageing and six young subjects. The expression at the mRNA level was measured using Northern blot analysis on samples from two ageing and two young subjects. One ageing and one young subject were analysed simultaneously. Blots were scanned and data represented as OD. The OD values were normalized for background variation and gel to gel variations. Comparing data, there was found to be increased basal expression (P < 0.001) of FADD protein in ageing (OD mean ± s.d. 4.9 ± 0.5) compared with young controls (OD 1.8 ± 0.6). Figure 1a shows a representative Western blot from two ageing and two young subjects. In contrast, the FADD mRNA expression was comparable (P > 0.5) between ageing and young subjects (Fig. 1b) (ageing: OD 6.8 ± 1.4; young: OD 5.7 ± 1.7). No further changes in the expression of FADD were observed following treatment with anti-CD3 or anti-Fas antibody in either young or ageing subjects (data not shown).
Fig. 1.
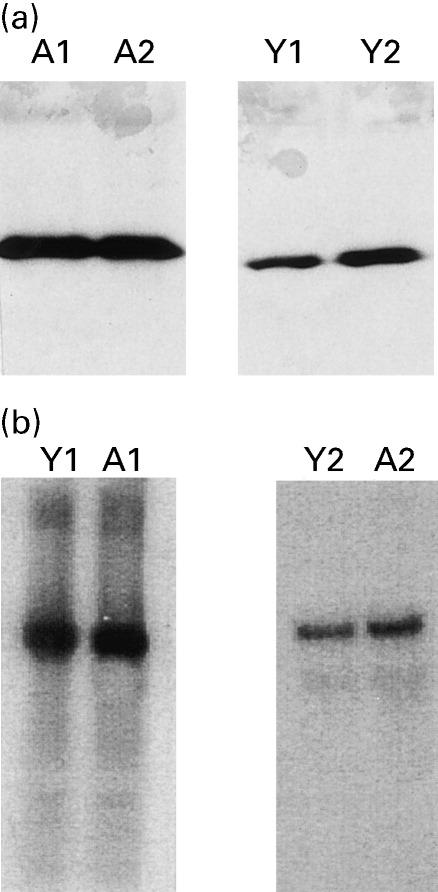
(a) Fas-associated death domain (FADD) protein expression. Increased FADD expression at basal levels in lymphocytes from ageing compared with young subjects as detected by Western blotting. Representative gels from two subjects in each group are shown. A, Ageing; Y, young. (b) FADD mRNA expression. Comparable FADD mRNA expression at basal levels in lymphocytes from ageing and young subjects as detected by Northern blotting. Blots from two ageing and two young subjects are shown.
Caspase-8 activity and expression
Caspase-8 is the most proximal member of the caspase family to be recruited to the cytoplasmic domain of the Fas receptor following its trimerization. Over-expression of caspase-8 is shown to induce apoptosis [21]. Therefore, we compared the activity and expression of caspase-8 in lymphocytes from ageing and young subjects. The activity of caspase-8 was assayed by the cleavage of a synthetic peptide substrate IETD-pNA into pNA, which was measured spectrophotometrically. The increase in caspase-8 activity at 4, 8, and 16 h following anti-Fas treatment was 2.5-, 2.9- and 5.7-fold in ageing subjects compared with 1.1-, 1.5- and three-fold in the young controls (Fig. 2).
Fig. 2.
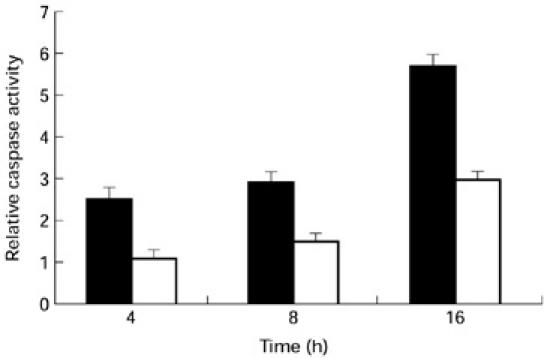
Caspase-8 activity. Increased caspase-8 activity is seen in samples from ageing humans at 0, 4, 8 and 16 h following anti-Fas treatment. Mean (± s.d.) data from 10 ageing (▪) and 10 young subjects (□) are shown.
Next, we compared the constitutive expression of caspase-8 in lymphocytes from six ageing and six young subjects. An increase (P < 0.001) in caspase-8 expression was observed in lymphocytes from ageing (OD 4.9 ± 0.2) compared with young (OD 1.1 ± 0.1) subjects. One ageing and one young subject were analysed simultaneously. A representative gel photograph from three ageing and three young subjects is shown in Fig. 3a. At the mRNA level, there was an increase (P < 0.05) in caspase-8 expression in two ageing subjects (OD 10.2 ± 0.5) compared with the young controls (OD 4.1 ± 1.0). A representative gel photograph from one ageing and one young subject is shown in Fig. 3b. No further change in caspase-8 expression was observed following treatment with anti-CD3 or anti-Fas antibody in either the ageing or young group (data not shown).
Fig. 3.
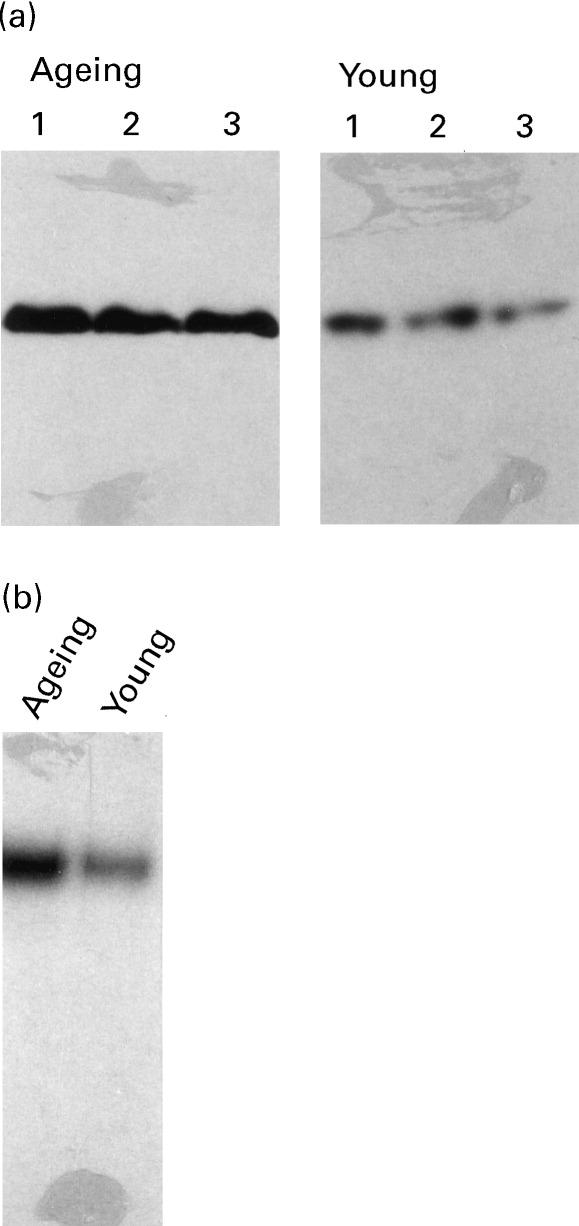
(a) Caspase-8 protein expression. Increased caspase-8 expression at basal levels in lymphocytes from ageing compared with young controls as detected by Western blotting. Representative gels from three subjects in each group are shown. (b) Caspase-8 mRNA expression. Increased caspase-8 mRNA expression at basal levels in lymphocytes from the ageing as compared to the young as detected by Northern blotting. A representative gel from one ageing and one young subject is shown.
Caspase-3 activity and expression
Next, we compared the expression and activity of caspase-3. The specific substrates for caspase-3 are known; therefore, caspase-3 activity was analysed using the following criteria: (i) cleavage of caspase-3; (ii) cleavage of a specific nuclear substrate, PARP; and (iii) cleavage of a synthetic peptide substrate, DEVD-pNA. In lymphocytes from ageing subjects, caspase-3 cleavage fragments (p20 and p10) were detected within 4 h of anti-Fas antibody treatment, whereas in the young the cleaved forms of caspase-3 were detected only after 16 h of anti-Fas antibody treatment (Fig. 4).
Fig. 4.
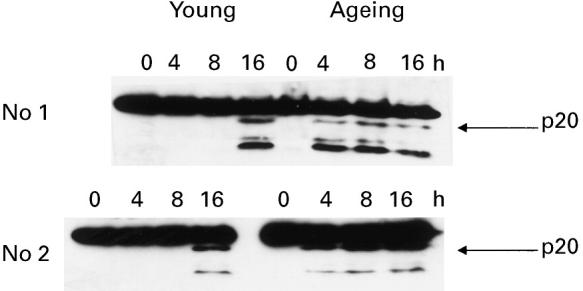
Cleavage of caspase-3. Activated lymphocytes (as described in Cell culture section) from young and ageing subjects were treated with anti-Fas antibody for 0, 4, 8 or 16 h. Following incubation, the proteins were extracted and caspase-3 cleavage examined using Western blotting. Representative gels from two ageing and two young subjects are shown. Cleaved forms of caspase-3 (p20 and p10) were seen at 4 h in ageing subjects compared with 16 h in the young.
Next, we compared the cleavage of PARP, a nuclear substrate of caspase-3, in lymphocytes from ageing and young humans following anti-Fas antibody treatment. In ageing subjects, the appearance of a signature 85-kD fragment was seen within 4 h of anti-Fas antibody treatment. However, in young subjects the cleavage of the 116-kD pro-form was not seen until 16 h of anti-Fas treatment (Fig. 5). The treatment of lymphocytes with isotype-matched IgM did not show any cleavage in either the ageing or young group (data not shown).
Fig. 5.
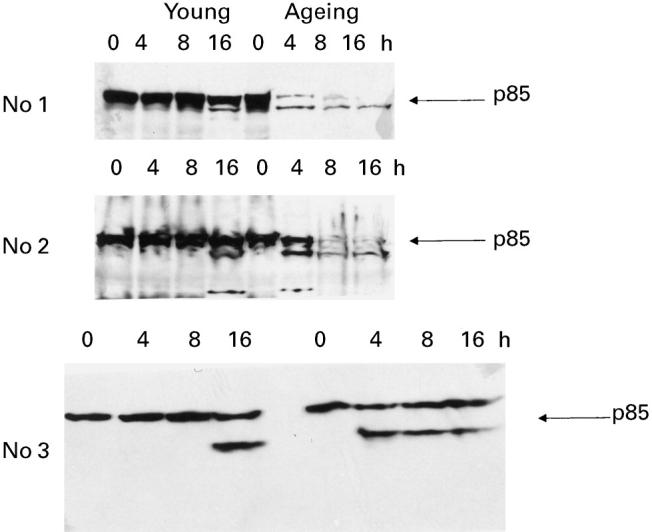
Cleavage of poly ADP-ribose polymerase (PARP) Activated lymphocytes (as described in Cell culture section) from young and ageing subjects were treated with anti-Fas antibody for 0, 4, 8 or 16 h. Following incubation, the protein was extracted and PARP cleavage examined by Western blotting. The cleaved form of PARP (p85) was seen at 4 h in the ageing compared with 16 h in the young.
The activity of caspase-3 was also assayed by the cleavage of a synthetic peptide substrate, DEVD-pNA, and analysed using a colorometric detection method. The caspase-3 activity was determined on samples from 10 ageing and 10 young subjects, analysed simultaneously. The results are shown as relative caspase activity (described above). In samples from ageing subjects, the increase in caspase-3 activity following anti-Fas treatment at 4, 8 and 16 h was 6.7-, 8.7- and 17.3-fold, respectively, whereas in young subjects the increase was two-, three- and 12-fold (Fig. 6).
Fig. 6.
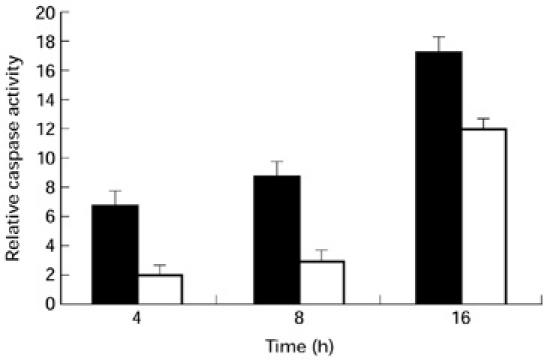
Caspase-3 activity. Increased caspase-3 activity is seen in samples from ageing subjects at 0, 4, 8 and 16 h following anti-Fas treatment. Data (mean ± s.d.) from 10 ageing (▪) and 10 young subjects (□) are shown.
Finally, we compared the constitutive expression of caspase-3 in lymphocytes from ageing and young subjects. Caspase-3 protein expression was measured in lymphocytes from six ageing and six young subjects. There was increased (P < 0.05) protein expression from ageing (OD 5.9 ± 1.0) compared with young (OD 2.1 ± 0.6) subjects. A representative gel photograph from three ageing and three young subjects is shown in Fig. 7a. No further increase in caspase-3 expression was observed following treatment with anti-CD3 or anti-Fas antibody in either the ageing or young group (data not shown). Caspase-3 mRNA levels were measured in three ageing and three young subjects. At the mRNA level, there was an increase (P < 0.002) in caspase-3 expression in ageing (OD 4.2 ± 0.4) compared with young (OD 1.4 ± 0.5) subjects. A gel photograph for caspase-3 mRNA expression is shown in Fig. 7b.
Fig. 7.
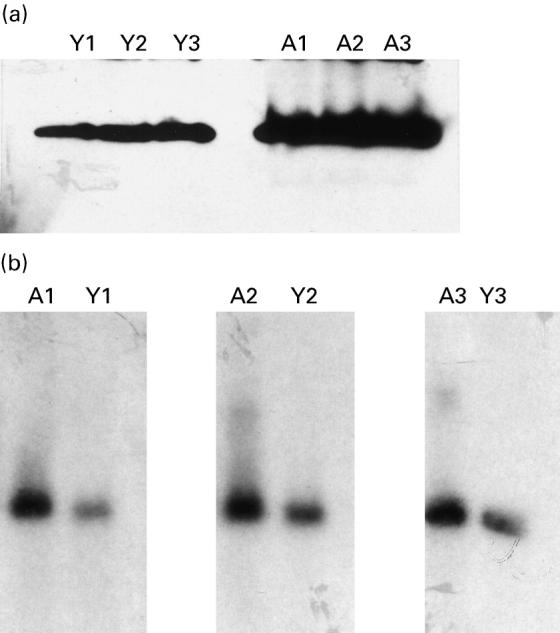
(a) Caspase-3 protein expression. Increased caspase-3 expression at basal levels in lymphocytes from ageing compared with young subjects, as detected by Western blotting. Representative gels from three ageing and three young subjects are shown. (b) Caspase-3 mRNA expression. An increased caspase-3 mRNA expression at basal levels in lymphocytes from ageing compared with young as detected by Northern blotting. Representative gels from three ageing and three young subjects are shown. A, Ageing; Y, young.
DNA fragmentation
Next, we compared the DNA fragmentation of lymphocytes at different time periods following anti-Fas antibody treatment of MNC from three ageing and three young subjects using TUNEL assay. A significantly increased (P < 0.001) proportion of TUNEL+ cells was observed in ageing subjects (% TUNEL+ cells 61 ± 7%) at 16 h following treatment with anti-Fas antibody compared with young controls (% TUNEL+ cells 22 ± 4%). Representative histograms from one ageing and one young subject are shown in Fig. 8.
Fig. 8.
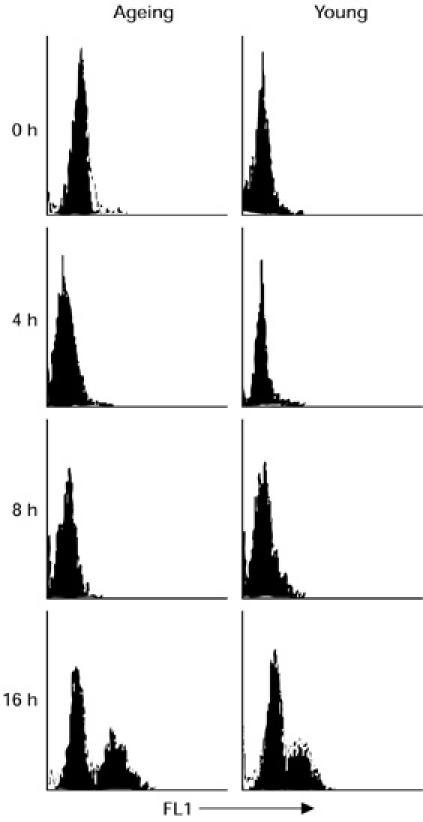
DNA fragmentation in lymphocytes. Activated lymphocytes were treated with anti-Fas antibody for 0, 4, 8 or 16 h. Following incubation, cells were harvested and percent apoptotic cells determined using TUNEL assay. Representative histograms from one ageing and one young subject are shown. Significantly increased TUNEL+ cells are seen in lymphocytes from ageing subjects following 16 h of anti-Fas treatment compared with young controls.
DISCUSSION
The Fas/FasL pathway has been shown to play a central role in maintaining T cell homeostasis. We have recently shown that there is a higher susceptibility of CD4+ and CD8+ T cell subsets from ageing humans to undergo Fas-mediated apoptosis that is associated with an increased expression of Fas/FasL on T cells from ageing humans compared with the young [1]. In the present study, we have observed increased levels of constitutive expression of FADD, caspase-8 and caspase-3 in lymphocytes from ageing compared with young subjects. Furthermore, following anti-Fas antibody treatment, an increased activation of caspase-8 and caspase-3 was observed in the ageing compared with young controls.
In the present study, we observed increased constitutive expression of FADD in ageing subjects at the protein, but not at the mRNA level, suggesting a post-translational modification of FADD in ageing humans. We did not observe any further increase in FADD expression in lymphocytes from either ageing or young subjects (data not shown) following treatment with anti-CD3 or anti-Fas antibody. There are no published reports to suggest that activation up-regulates FADD expression. The importance of the increased constitutive expression of FADD protein in ageing is unclear. Over-expression of FADD [7] in MCF7 cells (a breast carcinoma cell line that expresses Fas and is sensitive to Fas-induced killing) and in BJAB1 cells (a B cell lymphoma cell line which is sensitive to anti-Fas-induced apoptosis) has been shown to initiate apoptosis, which is inhibited by crmA, an inhibitor of caspase-3. However, we did not observe any increased apoptosis in freshly isolated MNC in ageing compared with young controls (data not shown). It remains to be determined whether the over-expression of constitutive levels of FADD in lymphocytes from ageing humans leads to its early recruitment to the Fas receptor, upon receptor activation, resulting in an early activation of caspases and increased apoptosis (as observed in the ageing).
Caspases are present in zymogen form and are activated to release their enzymatically activated forms. Following trimerization of the Fas receptor, pro-caspase-8, one of the most proximal members of caspases, is recruited to the Fas receptor–FADD complex and is physically associated with FADD via a DED homology interaction [8]. This results in the activation of procaspase-8 to activated caspase-8 and finally to the activation of caspase-3. In this study, we observed increased levels of constitutive expression of both caspase-8 and caspase-3 and an increased activation of both caspases in MNC from ageing subjects. Similar to over-expression of FADD, the role of increased constitutive levels of caspases in ageing is not clear. Transfection of MCF7 cells and 293-EBNA cells (embryonic kidney cells) with an expression vector encoding caspase-8 has been shown to result in massive cell death in these cells [22]. Kumar et al. [23] observed decreased expression of caspases, including caspase-3, in STAT-1 null fibroblasts that were resistant to TNF-α-induced apoptosis. Therefore, it is possible that the constitutive levels of caspases may play a role in the kinetics of caspase activation. However, these investigators did not examine anti-Fas-induced apoptosis in these cell lines. In the present study, we observed an early and increased activation of both caspase-8 and caspase-3 in ageing subjects following anti-Fas antibody treatment. The possibility that increased caspase activity in lymphocytes from ageing humans is due to an increased number of cells undergoing apoptosis cannot be excluded. However, the early activation of caspases (at 4 h) in samples from ageing subjects following anti-Fas treatment when no apoptosis is observed by TUNEL assay (apoptosis is observed at 16 h) may suggest that the increased caspase-8 and caspase-3 activities are due, at least in part, to the increased activation of caspases and perhaps the early recruitment of caspases to DISC.
In conclusion, increased apoptosis in lymphocytes from ageing humans is associated with increased constitutive expression and activity of certain adaptor molecules and caspases involved in Fas/FasL pathway-mediated apoptosis.
Acknowledgments
We wish to thank Dr V. M. Dixit (Genentech, South San Francisco) for providing plasmids for FADD, caspase-8 and caspase-3.
REFERENCES
- 1.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), FasL, Bcl-2 and Bax. J Immunol. 1998;160:1627–37. [PubMed] [Google Scholar]
- 2.Green DR, Scott DW. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;6:476–87. doi: 10.1016/0952-7915(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 3.Osborne BA. Apoptosis and the maintenance of homeostasis in the immune system. Curr Opin Immunol. 1996;8:245–54. doi: 10.1016/s0952-7915(96)80063-x. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S. Apoptosis mediated by the Fas system. Prog Mol Subcell Biol. 1996;16:87–103. doi: 10.1007/978-3-642-79850-4_6. [DOI] [PubMed] [Google Scholar]
- 5.Dhein J, Daniel PT, Trauth BC, Oehm A, Moller P, Krammer PH. Induction of apoptosis by monoclonal antibody anti-Apo-1 class switch variants is dependent on cross-linking of Apo-1 cell surface antigens. J Immunol. 1992;149:3166–73. [PubMed] [Google Scholar]
- 6.Nagata S. Fas-mediated apoptosis. Adv Exp Med Biol. 1996;406:119–24. doi: 10.1007/978-1-4899-0274-0_12. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan AM, O'rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan AM, Dixit VM. Portrait of an executioner: the molecular mechanism of FAS/APO-1-induced apoptosis. Semin Immunol. 1997;9:69–76. doi: 10.1006/smim.1996.0055. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta converting enzyme. J Biol Chem. 1992;269:30761–4. [PubMed] [Google Scholar]
- 10.Orth K, O'rourke K, Salvesen GS, Dixit VM. Molecular ordering of mammalian CED-3/ICE like proteases. J Biol Chem. 1996;271:20977–80. doi: 10.1074/jbc.271.35.20977. [DOI] [PubMed] [Google Scholar]
- 11.Tewari M, Quan L, O'rourke K, et al. Yama/CPP32 beta, a mammalian homolog of CED-3 is a crmA inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase. Cell. 1995;81:801–9. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 12.Talanian RV, Quinlan C, Trautz S, et al. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–82. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 13.Villa P, Kaufmann SH, Earnshaw WC. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–93. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- 14.Miller RA. The aging immune system. Primers Prospectus Sci. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 15.Saltzman RL, Peterson PK. Immunodeficiency of the elderly. Rev Infect Dis. 1987;9:1127–39. doi: 10.1093/clinids/9.6.1127. [DOI] [PubMed] [Google Scholar]
- 16.Wick G, Grubeck-Loebenstein B. The aging immune system: primary and secondary alterations of immune reactivity in the elderly. Exp Gerontol. 1997;32:401–13. doi: 10.1016/s0531-5565(96)00152-0. [DOI] [PubMed] [Google Scholar]
- 17.Herndon FJ, Hsu HC, Mountz JD. Increased apoptosis of CD45RO- T cells with aging. Mech Ageing Dev. 1997;94:123–34. doi: 10.1016/s0047-6374(97)01882-4. [DOI] [PubMed] [Google Scholar]
- 18.Phelouzat MA, Arbogast A, Laforge T, Quadri RA, Proust JJ. Excessive apoptosis of mature T lymphocytes is a characteristic feature of human immune senescence. Mech Ageing Dev. 1996;88:25–38. doi: 10.1016/0047-6374(96)01714-9. [DOI] [PubMed] [Google Scholar]
- 19.Phelouzat MA, Laforge T, Arbogast A, Quadri RA, Boutet S, Proust JJ. Susceptibility to apoptosis of T lymphocytes from elderly humans is associated with increased in vivo expression of functional Fas receptors. Mech Ageing Dev. 1997;96:35–46. doi: 10.1016/s0047-6374(97)01883-6. [DOI] [PubMed] [Google Scholar]
- 20.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;295:801–4. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 21.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell. 1996;85:817–27. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 22.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/Apo-1- and TNF receptor-induced cell death. Cell. 1996;85:803–15. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–2. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]


