Abstract
The pathogenesis of minimal-change nephrotic syndrome (MCNS) is obscure. It has been postulated that this glomerular disease may be a systemic disorder of cell-mediated immunity. IL-12 is a heterodimeric cytokine that is produced primarily by antigen-presenting cells and plays a primary role in the induction of cell-mediated immunity. To gain insight into the involvement of this cytokine, in vitro IL-12 levels were assessed in patients with MCNS and IgA nephropathy (IgAN) who were in either a stable or active clinical condition. The levels were compared with values in healthy controls. Significantly increased spontaneous and lipopolysaccharide (LPS)-stimulated release of IL-12 was detected in peripheral blood monocyte (PBM) cultures of MCNS and IgAN patients with the nephrotic syndrome (NS) compared with those of normal controls. Moreover, when individual MCNS patients were followed through their clinical illness, IL-12 levels were increased during the active phase and normalized as the patients went into remission. The amounts of IL-12 are significantly correlated with the levels of vascular permeability factor (VPF) in MCNS patients. This study describes a correlation between in vitro IL-12 release by PBM from two types of nephrotic patients and their disease activity, as well as a correlation with release of VPF by the same cultures. The study contributes to the understanding of this class of diseases and the observations may have a prognostic/diagnostic value.
Keywords: minimal-change nephrotic syndrome, IgA nephropathy, IL-12, vascular permeability factor
INTRODUCTION
Minimal-change nephrotic syndrome (MCNS) is a kidney disease of unknown aetiology characterized by nephrotic syndrome (NS). Although the precise mechanism of NS remains to be solved, several cytokines have been implicated as important inducers of vascular permeability factor (VPF) in MCNS [1,2]. For example, IL-12 induces MCNS cells to produce VPF [3].
IL-12 is a cytokine that has important and novel effects on cellular immunity [4]. It is produced by a variety of antigen-presenting cells including macrophages and dendritic cells, and was originally demonstrated to bind to receptors on activated T cells and natural killer (NK) cells [5]. The ability of IL-12 to control immunity is believed to reside in its interrelationship with other cytokines [6]. In previous studies we have shown that IL-12 and IL-15 act additively to produce VPF in MCNS [7].
To examine the possibility that IL-12 participates in the pathophysiology of the acute phase of NS, we determined here in vitro levels of IL-12 in patients with renal diseases. We also investigated the relationship between IL-12 levels and VPF activity. Ours is the first demonstration that shows that IL-12 levels relate to clinical and laboratory parameters in MCNS patients.
PATIENTS AND METHODS
Patients
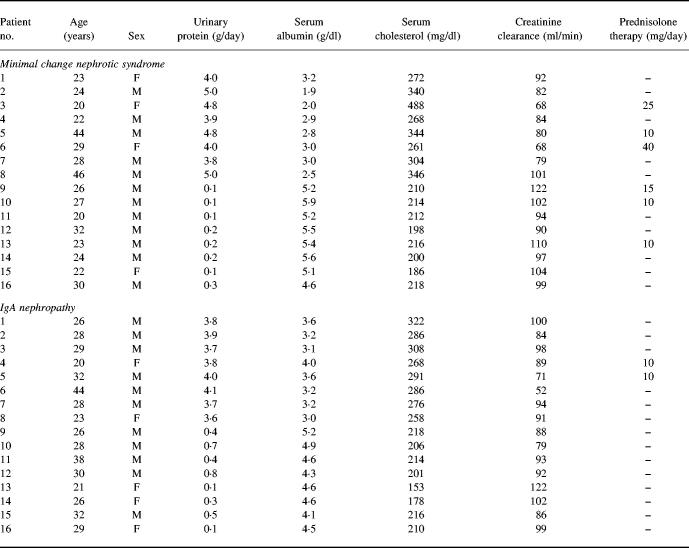
Two groups of patients from whom informed consent had been obtained were studied: 16 had MCNS and 16 IgA nephropathy (IgAN). The mean age of the patients with MCNS (12 males, four females) was 28 years; eight had NS, eight were in remission. Renal biopsy was performed in all MCNS patients, and the diagnosis was made by light and immunofluorescence microscopy. On light microscopy, the glomeruli appeared normal or showed only minor changes. Immunofluorescence revealed lack of deposition of immunoglobulins and complement in the glomeruli. Diagnostic criteria for NS were massive proteinuria (> 3.5 g/day) and hypoalbuminaemia (< 4.0 g/100 ml) with or without oedema. The dose of prednisolone was < 15 mg/day in four patients, while patients 3 and 6 were receiving 25 mg/day and 40 mg/day, respectively. None of these patients was treated with other medications that might affect our interpretation of the data, such as angiotensin-converting enzyme inhibitor, non-steroidal anti-inflammatory drugs, cyclosporin, or cyclophosphamide. The mean age of the IgAN patients (11 males, five females) was 29 years. The criteria described by Schena [8] were used to characterize the IgAN: haematuria and mesangial deposits of IgA on renal biopsy. Systemic lupus erythematosus, Henoch–Schönlein purpura, and hepatic diseases were excluded on the basis of clinical history, the results of examination, and laboratory data. Two IgAN patients were receiving prednisolone alone. Sixteen healthy subjects (10 males, six females, age 28 years) served as controls. Table 1 summarizes the clinical characteristics of the study group.
Table 1.
Clinical characteristics of patients with renal disease
One point of concern was the prednisolone treatment received by a minority of patients. This treatment might have interfered with the IL-12 release by peripheral blood monocytes (PBM) of NS patients. This work on the effects of steroids is included.
Production of monocyte supernatant
Monocytes were enriched from EDTA (0.002%) blood. The cells were isolated by Nycodenz (density 1.006 g/ml) (Nyegaard A/S, Oslo, Norway) gradient centrifugation [9]. Over 90% of the cells in the preparation were CD14+ cells. Cell viability was assessed by the trypan blue dye exclusion test.
The monocytes (1 × 105 cells/ml) were then incubated in RPMI 1640 tissue culture medium (Gibco Ltd, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin in the presence or absence of lipopolysaccharide (LPS; 100 ng/ml, Escherichia coli 055:B5; Difco Labs, Detroit, MI). The cell-free supernatants were collected after 24 h incubation at 37°C in 5% CO2 and stored at −20°C until use.
Measurement of IL-12 release
The amount of IL-12 present in culture supernatants was quantified by commercially available ELISA kits (Cat. no. Q1200; R&D Systems, Minneapolis, MN). This kit detects the bioactive p70 form of IL-12. Results are expressed as pg/ml. The detection limit for IL-12 was 0.7 pg/ml. There was no cross-reactivity in this ELISA with other cytokines and growth factors including IL-1, IL-2, IL-3, IL-4, IL-6, tumour necrosis factor-alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF).
Assay for VPF
A lymphokine, VPF, which increases vascular permeability in the guinea pig skin, has been characterized in MCNS [1,2,3,7]. VPF activity was measured using the method of Lagrue et al. [10], with minor modifications. Briefly, 0.1 ml of the lymphocyte culture supernatant was injected intradermally into the freshly shaved abdominal skin of guinea pigs, avoiding the median line. Hartley strain guinea pigs, weighing 300 ± 50 g, were used in all experiments. We estimated the protein contents of the culture supernatants using a sandwich ELISA assay [11] and normalized them in order to be able to compare the results. A total of 10 μg/ml of protein was injected into each animal. The culture medium served as negative control. A solution of Evans blue (1% in 0.15 m NaCl) was injected intravenously 5 min later; the total dosage administered was 0.5 ml/100 g body weight. The animals were killed 30 min later under ether anaesthesia. Each supernatant was assayed in triplicate. The diameter of the blue area surrounding each injection site was measured and the blue area calculated.
Statistical analysis
Statistical significance was analysed using the Mann–Whitney U-test. Spearman's test was used for correlation analysis. P < 0.05 was considered significant.
RESULTS
Induction of IL-12 by LPS: dose and kinetics
To determine the concentration of LPS that would be optimal in our culture system for the secretion of IL-12 protein, supernatants were removed from PBM cultures of healthy donors 24 h after the addition of various doses of LPS and assayed for IL-12. As shown in Fig. 1a,b, by 3 h after the addition of LPS, PBM responded to LPS concentrations of > 1 ng/ml by producing more IL-12 than unstimulated PBM. Maximal IL-12 release was induced by 100 ng/ml LPS. High concentrations of LPS (> 500 ng/ml) resulted in a continued decline in IL-12 release. Therefore, in all subsequent experiments for studying IL-12 release we used LPS at an optimum dose of 100 ng/ml for the activation of NS PBM.
Fig. 1.
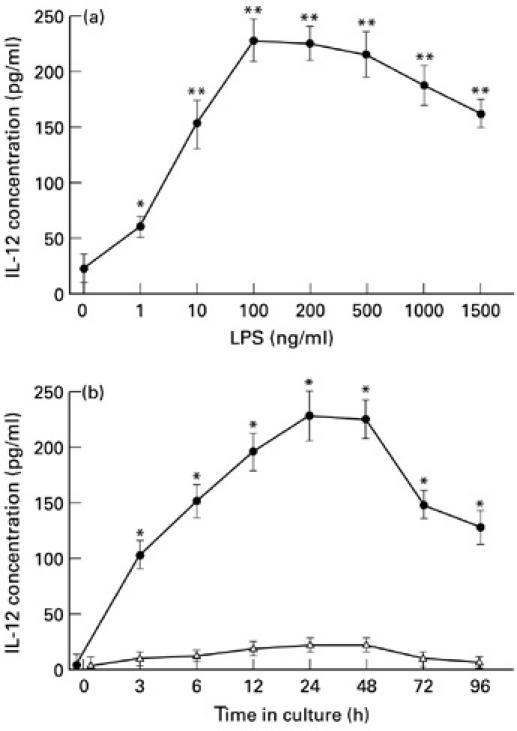
(a) Dose-dependent IL-12 release after stimulation with lipopolysaccharide (LPS). The supernatants were collected at the time point of 24 h. The release of IL-12 into the supernatant of normal peripheral blood monocyte (PBM) cultures shows linear dose dependency in the presence of LPS in dose from 1 to 100 ng/ml: **P < 0.01; *P < 0.05 versus controls which did not contain LPS. High concentrations of LPS (> 500 ng/ml) resulted in a continued decline in IL-12 release. (b) Kinetics of IL-12 release in normal cell cultures. PBM (1 × 105) were cultured alone (▵) or with 100 ng/ml of LPS (•) in 24-well plates in a total 1.0-ml volume for 3–96 h. Culture supernatants were harvested and tested for release of IL-12 by ELISA. The increase in IL-12 was statistically significant when compared with the base line value (0 h): *P < 0.01. Each point represents the mean ± s.d. of triplicate cultures in 16 experiments.
As shown in Fig. 1b, the time course of the release of IL-12 in PBM cultures stimulated with LPS in 16 healthy controls revealed a peak at 24–48 h, with a rapid decline subsequently. Therefore, subsequent studies were performed by culturing 1 × 105 cells/ml for 24 h.
IL-12 levels in patients with MCNS and IgAN
IL-12 content in supernatant of PBM cultured for 24 h was measured by ELISA in 32 patients with renal disease and 16 normal controls. The spontaneous and LPS-induced IL-12 levels of the MCNS and IgAN patients with NS were significantly higher than in both the MCNS and IgAN groups without NS and 16 normal subjects, whereas the mean values of the MCNS and IgAN groups without NS were not significantly different from normal controls (Fig. 2). These in vitro findings suggest that IL-12 may be an indicator of disease activity in patients with NS.
Fig. 2.
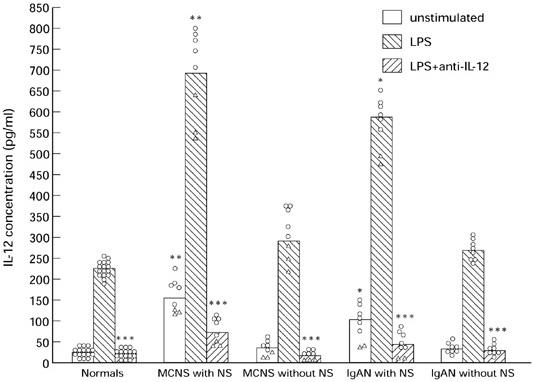
IL-12 release by peripheral blood monocytes (PBM) (1 × 105 cells/ml) of nephrotic syndrome (NS) patients or healthy controls with (100 ng/ml) or without lipopolysaccharide (LPS) stimulation after a 24-h incubation period. The release of IL-12 was enhanced significantly (P < 0.01) after treatment of the cells with LPS. IL-12 release of NS PBM was significantly increased compared with that of normal controls: **P < 0.01; *P < 0.02. The release of IL-12 was further confirmed by experiments using 5 μg/ml of anti-human IL-12 MoAb. The antibody neutralized the IL-12 activity observed (***P < 0.01). Minimal-change nephrotic syndrome (MCNS) and IgA nephropathy (IgAN) patients were divided into untreated (○), and treated with prednisolone (▵). Each data point represents data from patient or control. Mean values are shown by vertical bars.
Six additional patients with non-glomerular disease (three with polycystic kidney, one with horseshoe kidney and two with nephrolithiasis) were studied as a disease control. Each of these patients had normal IL-12 release, and the mean levels of IL-12 with or without LPS for all these patients were 238.2 ± 26.9 pg/ml and 30.2 ± 5.9 pg/ml, respectively, which did not differ from the normal subjects. Cells from six patients with chronic bronchitis and other non-renal inflammatory disease (i.e. chronic sinusitis and chronic tonsillitis) released approximately equal amounts of IL-12 (241.6 ± 28.8 pg/ml) and more than that from cells of healthy subjects, but the differences were not statistically significant.
We investigated the effect of steroid therapy on IL-12 release. MCNS and IgAN patients were divided into untreated, and treated with prednisolone. Figure 2 shows that PBM of the steroid-treated patients released lower levels of IL-12 than the untreated patients.
Neutralization of IL-12 activity
The release of IL-12 by PBM was further confirmed by neutralization experiments using 5 μg/ml of anti-human IL-12 MoAb (R&D Systems) in the ELISA assay. The activity could be neutralized by a 3-h preincubation with the anti-IL-12, but not by control mouse IgG (Fig. 2). Thus, the PBM culture supernatants used in the study appeared to contain predominantly IL-12 activity.
Relationship between IL-12 release and in vitro levels of VPF in MCNS patients
A positive correlation was found between the in vitro levels of IL-12 and in vitro production of VPF in 16 patients with MCNS (Fig. 3). Therefore the IL-12 induced by LPS parallels in vitro production of VPF in patients with MCNS.
Fig. 3.
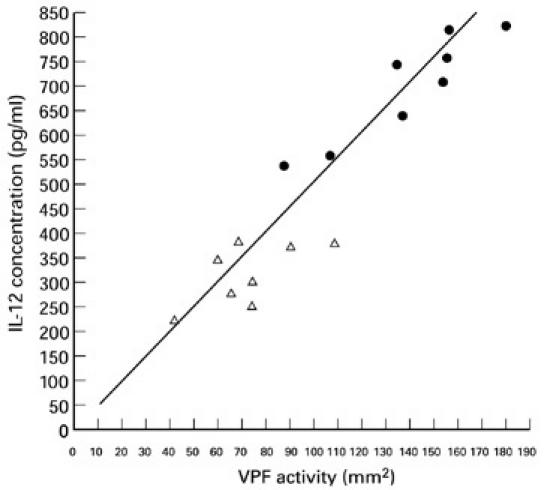
There is correlation (r = 0.701, P < 0.01) between IL-12 and vascular permeability factor (VPF) content in supernatant of lymphocytes cultured for 24 h in the presence of 5 μg/ml concanavalin A. •, Minimal-change nephrotic syndrome (MCNS) with nephrotic syndrome (NS); ▵, MCNS without NS.
Follow-up studies of MCNS patients
Compared with random determinations, serial studies of IL-12 in individual patients were much more informative. Two patients are shown in Figs 4 and 5. The first patient (case 1) was a 44-year-old man who had a relapse of MCNS with heavy proteinuria and hypoalbuminaemia. He had high levels of IL-12, particularly in the beginning of acute exacerbations, and decreased to relatively normal levels with the onset of clinical remission.
Fig. 4.
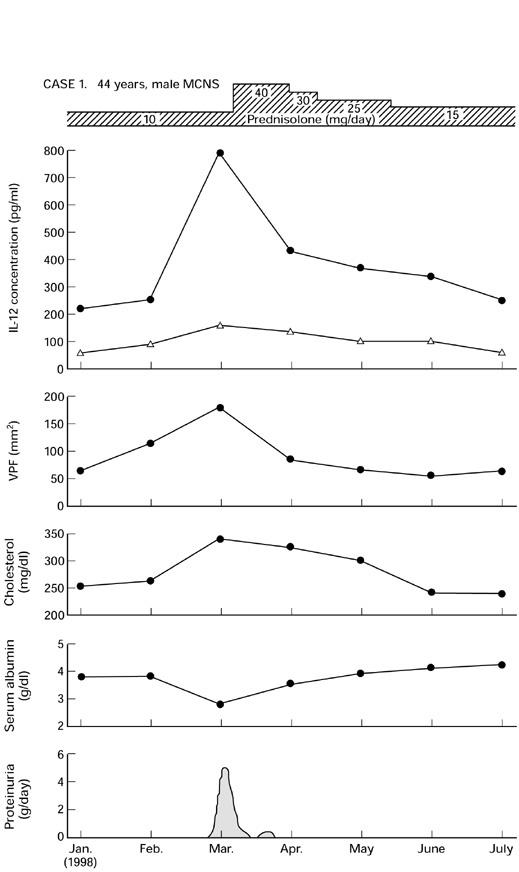
Serial study of IL-12 release by peripheral blood monocytes (PBM) from a patient with minimal-change nephrotic syndrome (MCNS) (no. 5, Table 1). VPF, Vascular permeability factor. •, LPS; ▵, unstimulated.
Fig. 5.
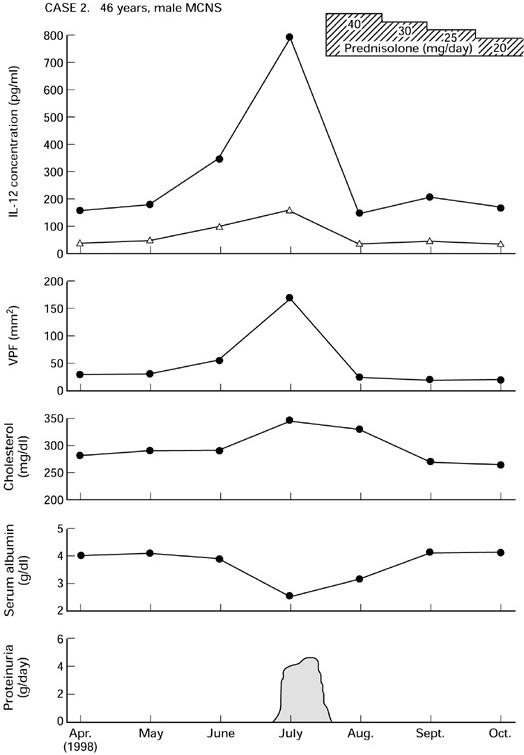
Serial measurement of IL-12 release in a 46-year-old man with minimal-change nephrotic syndrome (MCNS) (no. 8, Table 1). VPF, Vascular permeability factor. •, LPS; ▵, unstimulated.
The second patient (case 2) was a 46-year-old man with steroid-responsive MCNS who presented with relapse. In this patient, the IL-12 levels tended to be very high in the relapse, decreased sharply after the onset of relapse, and then returned to relatively normal levels with the improvement of his clinical and laboratory data.
DISCUSSION
Using a sensitive ELISA method, we found that in vitro concentrations of IL-12 were elevated in nephrotic patients during acute exacerbations. From the results presented in Figs 4 and 5, it is evident that levels of IL-12 were markedly increased when disease activity was at its highest. To our knowledge this is the first report demonstrating that in vitro IL-12 and VPF levels are also significantly correlated.
Recently, IL-12 was shown to promote the production of VPF [3], supporting the idea that IL-12 may play an important role in the process of VPF production. The precise mode of action of IL-12 remains to be determined in light of its stimulatory effect on Th1 cells. IL-12 has been shown to induce interferon-gamma (IFN-γ) and IL-2 production from Th1 clones [12]. It is intriguing to assume that stimulation with IL-12 induces Th1 cell development and IL-2 production, which shares a lot of biological activities with VPF [13].
Previous studies have shown that the concentration of serum IL-8 may be correlated with clinical disease activity in NS [14]. Indeed, the usefulness of the measurement of serum levels of cytokines was confirmed in a study by Daniel et al. [15]. Our study of the release of IL-12 by PBM and its relationship to VPF release is clearly described. As with all studies of this nature it is unclear whether we are dealing with cause or effect with relationship to the nephrotic state.
We should be cautious when translating in vitro findings into potential in vivo function. Certainly, in the present study the PBM were taken out of their natural environment and could not interact with other cells such as vascular endothelial cells. In addition, in the culture dish they lacked exposure to a multitude of other cytokines and regulatory molecules.
Glucocorticoids are widely used for treatment of NS and it could be assumed that their effect is partly mediated by the inhibition of cytokine production of NS PBM [1,2]. As shown in Fig. 2, NS patients treated with steroid had significantly lower levels of IL-12 than were seen in untreated patients. The MCNS patients shown in Figs 4 and 5 were given prednisolone to control the exacerbation. The issue of steroid treatment on IL-12 release in these patients and the impact on data interpretation deserves more extensive discussion. After therapy with prednisolone, IL-12 levels declined and returned towards normal within 10 days. This treatment decreased the ability of their monocytes to secrete IL-12. It is possible that the beneficial effect of steroids in the studied renal diseases is due to steroid down-regulation of cytokine production.
The positive correlations between in vitro IL-12 and VPF release, as well as in vitro IL-12 release and disease status, are well demonstrated. One point to which we need to pay careful attention is the interpretation of a causal relationship between IL-12 and disease pathogenesis. While there is a strong correlation between in vitro IL-12 release and disease activity, which may be proven to be of diagnostic or prognostic value, a causal relationship has not been proven and should not be inferred without proper qualification. Further functional studies are needed to determine whether IL-12 is involved in the pathogenesis of MCNS.
REFERENCES
- 1.Matsumoto K. Interleukin-10 and interleukin-13 synergize to inhibit vascular permeability factor release by peripheral blood mononuclear cells from patients with lipoid nephrosis. Nephron. 1997;77:212–8. doi: 10.1159/000190275. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto K, Ohi H, Kanmatsuse K. Interleukin-4 cooperates with interleukin-10 to inhibit vascular permeability factor release by peripheral blood mononuclear cells from patients with minimal-change nephrotic syndrome. Am J Nephrol. 1999;19:21–27. doi: 10.1159/000013420. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Ohi H, Kanmatsuse K. Interleukin-12 upregulates the release of vascular permeability factor by peripheral blood mononuclear cells from patients with lipoid nephrosis. Nephron. 1998;78:403–9. doi: 10.1159/000044968. [DOI] [PubMed] [Google Scholar]
- 4.Locksley RM. Interleukin-12 in host defense against microbial pathogens. Proc Natl Acad Sci USA. 1993;90:5879–80. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai BB, Quinn PM, Wolitzky AG, Mongini PKA, Chizzonite R, Gately MK. IL-12 receptor.II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–32. [PubMed] [Google Scholar]
- 6.Yoshimoto T, Takeda K, Takeda T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–7. [PubMed] [Google Scholar]
- 7.Matsumoto K, Kanmatsuse K. Interleukin-15 and interleukin-12 have an additive effect on the release of vascular permeability factor by peripheral blood mononuclear cells in normals and in patients with nephrotic syndrome. Clin Nephrol. in press. [PubMed] [Google Scholar]
- 8.Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy world-wide. Am J Med. 1990;89:209–15. doi: 10.1016/0002-9343(90)90300-3. [DOI] [PubMed] [Google Scholar]
- 9.Yi Q, Holm G, Lefvert AK. Idiotype-induced T cell stimulation requires antigen presentation in association with HLA-DR molecules. Clin Exp Immunol. 1996;104:359–65. doi: 10.1046/j.1365-2249.1996.27735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagrue G, Xheneumont S, Branellec A, Hirbec G, Weil B. Vascular permeability factor elaborated from lymphocytes. I. Demonstration in patients with nephrotic syndrome. Biomedicine. 1975;23:37–40. [PubMed] [Google Scholar]
- 11.Danielpour D, Roberts AB. Specific and sensitive quantitation of transforming-growth factor β3 by sandwich enzyme linked immunosorbent assay. J Immunol Methods. 1995;180:265–72. doi: 10.1016/0022-1759(94)00322-n. [DOI] [PubMed] [Google Scholar]
- 12.Manetti R, Parronchi P, Giudizi MG, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of interleukin-4 producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heslan J-MJ, Branellec A, Pilatte Y, Lang P, Lagurue G. Differentiation between vascular permeability factor and IL-2 in lymphocyte supernatants from patients with minimal-change nephrotic syndrome. Clin Exp Immunol. 1991;86:157–62. doi: 10.1111/j.1365-2249.1991.tb05789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garin EH, Blanchard K, Matsushima K, Djeu JY. IL-8 production by peripheral blood mononuclear cells in nephrotic patients. Kidney Int. 1994;40:913–6. doi: 10.1038/ki.1994.171. [DOI] [PubMed] [Google Scholar]
- 15.Daniel V, Trautmann Y, Konrad M, Nayir A, Scharer K. T-lymphocyte populations, cytokines and growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997;47:289–97. [PubMed] [Google Scholar]