Abstract
Chlamydia psittaci is an obligate intracellular pathogen that causes abortion in both sheep and humans. The disease in sheep (but not humans) is characterized by a long-term persistent phase that appears to be under the control of interferon-gamma. However, nothing is known about cytokine induction that precedes the persistent phase in sheep. Primary alveolar lavage cells recovered from normal adult sheep were used to study cytokine production in the first 72 h of infection with C. psittaci. These cells were phenotypically characteristic of macrophages, being adherent, phagocytic, CD14+ and staining positive for non-specific esterase. In vitro infection of the macrophages with C. psittaci resulted in the release of IL-1β, IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) as measured by ovine-specific ELISAs. Heat-treated chlamydiae (1 h at 65°C) did not induce the release of IL-1β, but the release of IL-8 was similar to that induced by untreated organisms. The cells from different sheep varied most notably in their patterns of GM-CSF release in response to heat-treated and untreated organisms.
Keywords: innate immunity, cytokines, Chlamydia psittaci, ovine
INTRODUCTION
Cytokine induction by bacteria is a complex phenomenon that depends on several factors. These include the phenotype of the target host cell, bacterial components such as lipopolysaccharide (LPS) and bacterial processes such as adhesion and invasion [1,2]. Recent interest has focused on the induction of proinflammatory cytokines and chemokines by bacteria, especially IL-1β, IL-6, IL-12, tumour necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-8 [2]. These immune mediators have the potential to recruit and activate effector cells to the site of infection. IL-8 production by epithelial cells appears to be a common feature of the host response to many bacteria that infect mucosal surfaces, including Salmonella typhimurium, Escherichia coli, Helicobacter pylori and Chlamydia trachomatis [3–6].
The role of innate immunity in orchestrating the development of the acquired immune response is widely recognized and is especially important in situations where an infectious agent can establish a chronic infection in the host. From this viewpoint, chlamydial infections are particularly interesting since they are often characterized by recurrence, persistence and latency [7]. The genus Chlamydia encompasses four species of obligate intracellular Gram-negative bacteria that have a wide host range and are characterized by diverse pathological manifestations [8]. Some of these organisms will cross species barriers, notably the avian and ovine strains of C. psittaci that are zoonotic and cause ornithosis/psittacosis and abortion, respectively, in humans. Sheep are natural hosts of C. psittaci and therefore offer an ideal experimental system to address the fundamental questions of persistence and development of immunity. An important step in this process is to identify the immunological events that occur early in acute infection that may lead to persistence.
The route of natural infection in sheep with abortifacient C. psittaci appears to be oral/nasopharyngeal. Collective data from experimental infections in sheep suggest that the organism disseminates from this site and possibly resides in lymphoid tissues during the persistent phase [9]. The immune events that occur early in infection, and in particular the role of mononuclear phagocytes, are as yet undefined. Therefore, we have examined the release of cytokines by ovine alveolar macrophages in the first 72 h following experimental infection in vitro with C. psittaci.
MATERIALS AND METHODS
Cell culture
All cells were grown or maintained in Iscove's modified Dulbecco's medium (IMDM; Gibco BRL, Paisley, UK) supplemented with appropriate concentrations of fetal bovine serum (FBS; Sigma, Poole, UK). No antibiotics or other supplements were used unless stated otherwise. The ovine ST-6 cell line was routinely grown in 225-cm2 vented plastic flasks (Costar, High Wycombe, UK) in IMDM supplemented with 5% FBS as previously described [10].
Chlamydiae
The S26/3 ovine abortion strain of C. psittaci was used throughout. The growth and titration of C. psittaci in ST-6 cells and the maintenance of stocks have been described in detail elsewhere [11]. Heat-treated organisms were incubated at 65°C in a water bath for 1 h.
Alveolar lavage
Six-month-old sheep with no history of infection with C. psittaci or abortion were euthanized by i.v. injection of sodium pentobarbitol (Euthatal; Rhone Merieux, Harlow, UK). At post mortem, the trachea was clamped before removal of the lungs to avoid contamination with blood. Alveolar lavage was performed according to the previously published technique [12]. Briefly, the lungs were filled with approximately 1 l of sterile PBS, massaged gently and the lavage fluid recovered by pouring through sterile gauze. Lavage fluids that showed signs of discoloration with blood were discarded. Cells were pelletted by centrifugation at 400 g for 10 min then washed three times in Hanks' balanced salt solution (HBSS) supplemented with 2% FBS, 10 U/ml preservative-free heparin (Sigma), 4 μg/ml amphotericin B (Sigma), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco) and 50 μg/ml gentamycin sulphate (Gibco). The cells were counted, adjusted to 1 × 107/ml and cryopreserved in 1-ml vials (Gibco) in a mixture of IMDM containing 50% FBS and 10% dimethyl sulphoxide (Sigma).
Infection of cells with C. psittaci
Lavage cells were thawed in a 37°C water bath and washed once with IMDM containing 5% FBS and 50 μg/ml gentamycin sulphate. They were then adjusted to 1 × 106/ml in the same medium and 100 μl/well seeded into 96-well flat-bottomed microtitre plates (Costar) and cultured overnight at 37°C in a 5% CO2/humid atmosphere. The plates were then washed with warm PBS and the adherent cells were exposed to live C. psittaci at either 1 × 104 or 1 × 103 inclusion-forming units (IFU) per well in 100 μl IMDM containing 2% FBS, the equivalent number of heat-treated organisms, or medium alone. All cultures were performed in quadruplicate. After a 3-h incubation to allow the chlamydiae to infect the cells, a further 100 μl of medium were added to the wells. Supernatants were harvested from the plates 24, 48 and 72 h after infection (180 μl/well), transferred to Falcon Microtest flexible assay plates (Becton Dickinson, Oxnard, CA) and stored at −70°C until analysis for cytokines. After the supernatants were harvested, the plates were fixed for 10 min in methanol and the cells stained with Giemsa (BDH, Poole, UK).
Characterization of lavage cells
The alveolar cell suspensions were phenotypically analysed prior to adherence on the plates with a panel of MoAbs specific for ovine cell surface molecules. These were 36F (CD2) [13], VPM65 (CD14) [14], CC21 (CD21) [15], SBU LCA1.28 (CD45) [16], 86D (γδ TCR) [17] and the control antibody VPM49 (specific for an antigen of ruminant pestiviruses) [18]. Prior to staining, the cells were incubated for 15 min with heat-inactivated normal rabbit serum diluted 1:50 in HBSS containing 2% FBS and 0.01% sodium azide. Thereafter, the washing and staining was performed as previously described [19]. A rabbit anti-mouse polyclonal antiserum conjugated to FITC (Dako Ltd, High Wycombe, UK) was used as second-stage reagent. The cells were analysed by flow cytometry using a Becton Dickinson FACScan (Mountain View, CA) with linear amplification for forward scatter and side scatter and logarithmic amplification for FITC (green) fluorescence.
The adherent cells were stained for non-specific esterase using a commercial kit (Sigma). The cells were allowed to adhere to glass Lab-Tek II Chamber Slides (Nalge Nunc Int., Napierville, IL) by overnight culture, then washed with PBS as described in the previous section. The cells were fixed and stained according to the manufacturer's instructions. The phagocytic capacity of the cells was also measured after adherence. Cells were seeded into 96-well plates, allowed to adhere, then washed as before. Latex beads (Sigma) of 3 μm diameter were washed by centrifugation at 2000 g in IMDM supplemented with 2% FBS. A 50-μl suspension of a 1:500 dilution of the manufacturer's stock was added to each well and the plates incubated overnight to allow cells to phagocytose the beads. The plates were then washed, fixed and stained as described above. The percentage of cells that had phagocytosed particles was evaluated microscopically.
Cytokine analysis by enzyme-linked immunoassay
The protocols for the ELISAs to measure ovine IL-1β [20], IL-8 [21] and GM-CSF [22] have been described in detail elsewhere. The specificities and sensitivities of these ELISAs have also been described [23]. Test supernatants were thawed and assayed by all three cytokine ELISAs on the same day (50 μl/well). Supernatants were assayed on a well-for-well basis from the original 96-well plate cultures, therefore the quadruplicates for each treatment measured in the ELISA represented four separate culture wells. Each ELISA plate was standardized with a titration of the appropriate recombinant ovine cytokine, thereby allowing both intraplate and interplate comparisons to be drawn. Tetramethylbenzidine substrate (Kirkegaard & Perry Labs, Gaithersburg, MD) was used throughout. The optical density (OD) was measured using a Flow Titertek Multiskan ELISA reader equipped with a 450-nm filter. Cytokine concentrations were extrapolated from a standard curve that was calculated by quadratic regression with tails using Dynatech PC Software (Dynatech, Billingshurst, UK).
Statistical analysis
Results were analysed using the unpaired two-tailed Student's t-test assuming equal variance.
RESULTS
Phenotypic characterization of alveolar lavage cells
FACS analyses of the cells recovered from the four sheep used in all subsequent cytokine experiments are shown in Fig. 1. These represent the cell populations following resuscitation from liquid nitrogen and prior to culture. All populations predominantly consisted of CD14+ mononuclear cells. Although the staining for CD14 was duller than that for CD45, an increase in the median fluorescence for CD14 staining was observed compared with the control MoAb for all four sheep (sheep 1 (Fig. 1a): 178 versus 36; sheep 2 (Fig. 1b): 142 versus 79; sheep 3 (Fig. 1c): 124 versus 42; sheep 4 (Fig. 1d): 138 versus 35). There was no positive staining for CD2, γδ TCR and CD21 (not shown).
Fig. 1.
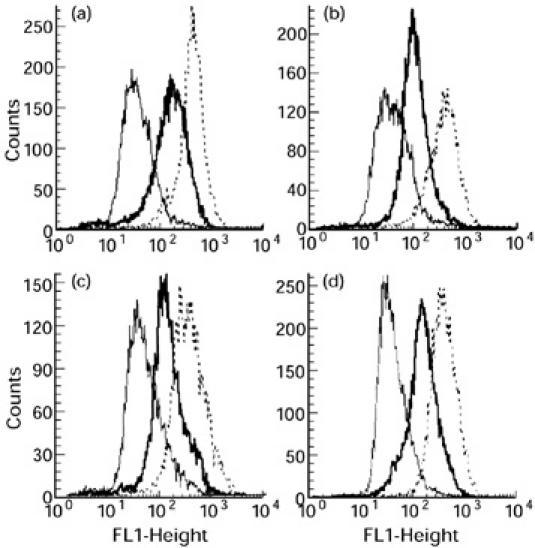
FACS profiles of alveolar lavage cells. Cells were phenotyped with mouse MoAbs recognizing sheep CD14 (——), sheep CD45 (- - - - -) and a control MoAb (——), then stained with a rabbit anti-mouse FITC conjugate. Cell number (ordinate) is plotted against log fluorescence intensity (abscissa). The cells from the individual sheep numbered 1–4 correspond to profiles a–d, respectively.
Following adherence, the percentages of cells staining positive for non-specific esterase were 99.5%, 98.5%, 99.0% and 90.6% for sheep numbers 1, 2, 3, and 4, respectively. Functional studies showed that the adherent cells were phagocytic, the percentages being 96.0%, 94.5%, 92.6% and 93.0% for sheep 1, 2, 3 and 4, respectively. These combined data were taken as evidence that the majority of the cells adhered to the plates were of the monocyte/macrophage lineage.
Release of cytokines following infection with C. psittaci
The release of IL-1β by macrophages from each sheep following exposure to C. psittaci is shown in Fig. 2. Peak levels were found in supernatants harvested 24 h after infection of cells from all four sheep with 104 IFU of C. psittaci, all significantly greater than untreated controls (P ≤ 0.02). The amount of IL-1β detected then decreased over 48 h and 72 h. Although much lower amounts of IL-1β were released by cells exposed to 103 IFU, there was a gradual upward trend over 72 h of culture. The release of IL-1β was dependent on multiplication of the organism, since no significant IL-1β was detected in the supernatants of cells exposed to heat-treated organisms. The cells from sheep numbers 1, 2 and 3 released very similar amounts of IL-1β in response to the various treatments, whereas the amounts released by cells from sheep number 4 were around two-to-three-fold higher.
Fig. 2.
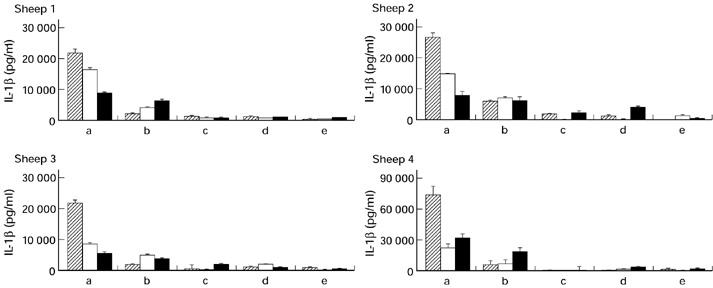
IL-1β release by alveolar macrophages from four different sheep after exposure to Chlamydia psittaci. Cells (1 × 105/well) were seeded into 96-well plates and exposed to: (a) 1 × 104 inclusion-forming units (IFU) C. psittaci; (b) 1 × 103 IFU C. psittaci; (c) 1 × 104 IFU heat-treated C. psittaci; (d) 1 × 103 IFU heat-treated C. psittaci; or (e) medium alone. Heat treatment was performed at 65°C for 1 h. All cultures were performed in quadruplicate. Supernatants were harvested after 24 h (hatched), 48 h (□) and 72 h (▪) and individually assayed for the presence of ovine IL-1β by ELISA. Note that the ordinate values vary between sheep.
Figure 3 shows the release of IL-8 by cells from the individual sheep. For sheep 1, 2 and 3 both the untreated and heat-treated organisms induced the release of IL-8 over the first 48 h of culture compared with cells cultured in medium alone (P ≤ 0.05). For those cells, heat treatment had no marked effect on the release of IL-8. Supernatants of cells from sheep 1 and 2 exposed to untreated or heat-treated organisms also contained significantly more IL-8 at 72 h than the untreated controls (P < 0.02). For cells from sheep 4, IL-8 release was only significantly enhanced in the first 24 h after infection with 104 IFU untreated C. psittaci(P < 0.05). The amounts of IL-8 released varied between the sheep, with the highest amounts released by cells from sheep 4.
Fig. 3.
IL-8 release by alveolar macrophages from four different sheep after exposure to Chlamydia psittaci. Cells (1 × 105/well) (macrophages) or 5 × 103 cells/well (ST-6) were seeded into 96-well plates and exposed to: (a) 1 × 104 inclusion-forming units (IFU) C. psittaci; (b) 1 × 103 IFU C. psittaci; (c) 1 × 104 IFU heat-treated C. psittaci; (d) 1 × 103 IFU heat-treated C. psittaci; or (e) medium alone. Heat treatment was performed at 65°C for 1 h. All cultures were performed in quadruplicate. Supernatants were harvested after 24 h (hatched), 48 h (□) and 72 h (▪) and individually assayed for the presence of ovine IL-8 by ELISA. Note that the ordinate values vary between sheep.
The pattern of GM-CSF release in response to the various treatments was not consistent between sheep (Fig. 4). Cells from sheep 1 released GM-CSF in response to 104 IFU C. psittaci and the amount did not vary over 72 h. Cells exposed to 104 heat-treated organisms at 24 h also released GM-CSF, but this was not detected at 48 h or 72 h. The cells from sheep 2 released GM-CSF in response to both live and dead organisms at both concentrations, notably at 72 h (P < 0.05). Significant amounts relative to untreated controls were detected in cells infected with 104 IFU live C. psittaci at both 48 h and 72 h (P < 0.03). Only 104 IFU live C. psittaci induced the release of GM-CSF from the macrophages derived from sheep 3, the peak occurring at 72 h (P < 0.01). The maximum amount of GM-CSF released by cells from sheep 4 occurred at 24 h and 48 h after infection with 104 IFU live organisms, but these corresponded to only a two-fold increase relative to untreated controls. The untreated cells from sheep 4 released more GM-CSF than the untreated cells from the other three sheep.
Fig. 4.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) release by alveolar macrophages from four different sheep after exposure to Chlamydia psittaci. Cells (1 × 105/well) were seeded into 96-well plates and exposed to: (a) 1 × 104 inclusion-forming units (IFU) C. psittaci; (b) 1 × 103 IFU C. psittaci; (c) 1 × 104 IFU heat-treated C. psittaci; (d) 1 × 103 IFU heat-treated C. psittaci; or (e) medium alone. Heat treatment was performed at 65°C for 1 h. All cultures were performed in quadruplicate. Supernatants were harvested after 24 h (hatched), 48 h (□) and 72 h (▪) and individually assayed for the presence of ovine GM-CSF by ELISA.
The data from all four sheep were pooled and the amount of cytokine induced by each treatment relative to the medium control was calculated for each time point. The results are shown in Table 1. The greatest increase (60-fold) for any of the cytokines studied was the release of IL-1β in the first 24 h after infection with 104 IFU C. psittaci. This decreased over the following 48 h. The same treatment also induced the greatest release of IL-8 (a maximum six-fold increase at 24 h) and GM-CSF (a maximum 20-fold increase at 72 h).
Table 1.
Cytokine release by ovine macrophages following exposure to Chlamydia psittaci
Data were pooled from the macrophages derived from four different sheep and the amounts of each cytokine released were calculated relative to cells cultured in medium alone.
Effects of Chlamydia infection on the cells
Infection with 104 IFU C. psittaci resulted in widespread cellular destruction within 24–48 h. Cell lysis was less marked in the cells infected with 103 IFU. Inclusion bodies were observed within the macrophages indicating multiplication of the chlamydiae. Exposure to 104 heat-treated organisms resulted in the appearance of large, vacuolated cells. There was no visible evidence of cell death. Heat-treated organisms (103) had a reduced effect. Cells cultured in medium alone remained intact and smaller than their treated counterparts. Heat-treated organisms failed to form inclusions in the ovine ST-6 cell line that was used as a marker of infectivity. In contrast, chlamydial inclusions were easily identifiable in ST-6 cells that had been infected with 104 or 103 IFU, although unlike the macrophages, very little lysis was observed within the time course of these experiments (data not shown).
DISCUSSION
Identification of the early release of cytokines by cells in response to infectious agents is crucial to our understanding of disease pathogenesis and the development of immunity. The applications of this knowledge can be seen most clearly by the increasing use of cytokines as therapeutic agents to modulate immune responses in a variety of situations, including neoplasia, autoimmunity and prophylactic vaccination [24]. We have found that primary ovine macrophages will release proinflammatory cytokines/chemokines (IL-1β, GM-CSF and IL-8) within the first 72 h of infection with C. psittaci. The kinetics of cytokine release varied for the different cytokines (Table 1). The cytokine patterns and concentrations released also varied between macrophages derived from different animals (Fig. 2–4). This is not surprising, since the primary cells were derived from an outbred population of sheep.
The greatest increase relative to background for any of the cytokines measured was displayed by IL-1β in the first 24 h after infection with 104 IFU C. psittaci(Table 1). Given the experimental protocol, it was surprising that the concentrations of IL-1β in the supernatants dropped over the following 48 h. One would expect the cytokine concentrations to be cumulative over the 72-h culture period. Indeed, this has been observed for IL-1β released into the supernatants of the Mono Mac 6 cell line infected with C. pneumoniae over a 96-h culture period [25]. It is possible that the IL-1β was either being utilized by the cells, blocked by free receptors or degraded by proteolytic enzymes released by the dying macrophages. The effect appears to be specific for IL-1β, since concentrations of IL-8 in the supernatants did not decrease over the same time period (Fig. 3). Certain bacteria can produce proteases that degrade IL-1β, although this has not been described for C. psittaci [26]. The release of IL-1β may be linked to the death of the macrophages, since human monocytes have been shown to apoptose and release IL-1β within 24 h of infection with the guinea pig inclusion conjunctivitis serovar of C. psittaci [27].
The interpretation of the data for IL-8 induction by C. psittaci was complicated by the spontaneous release of high concentrations of IL-8 by the macrophages from all four sheep (Fig. 3). The production of IL-8 appears to be a common feature of chlamydial infection in a number of human epithelial and monocytic cell lines. However, those lines did not spontaneously release IL-8 [6,28]. It has been noted that primary human endocervical cells spontaneously produce IL-8 in the first 72 h of culture. Moreover, the concentrations of IL-8 released by those human cells are similar to those observed for the ovine macrophages [6]. The spontaneous release of IL-8 may be a reaction by primary cells to the foreign environment of tissue culture. Indeed, it has been reported that pulmonary ovine macrophages do not spontaneously produce IL-8 in situ in normal sheep lungs [29]. It is possible that the in vitro environment lacks the down-regulatory elements (such as IL-10) that can suppress IL-8 production. IL-10 can inhibit IL-1β-induced IL-8 production by mononuclear cells and can also inhibit spontaneous IL-8 production by human monocytes [30]. The relationships between the production of these cytokines following chlamydial infection remains to be investigated. The role of LPS cannot be ruled out in activating pulmonary macrophages in vivo prior to isolation and therefore contributing to the apparent spontaneous release of IL-8. However, there was no enhancement of IL-8 production by ovine macrophages incubated with S. minnesota Re 595 LPS at concentrations of up to 10 μg/ml compared with uninfected or otherwise unstimulated controls (data not shown).
GM-CSF is a particularly interesting cytokine to study with regard to alveolar macrophages. It is known to activate macrophages and up-regulate CD14 and MHC class II expression [31]. As a result, macrophages present antigen more efficiently, which has clear implications for the subsequent development of protective immunity. In further experiments it would be interesting to correlate the ability of cells from individual sheep to produce GM-CSF in response to chlamydial infection with T cell reactivity and protection from abortion. GM-CSF is one of the cytokines that are important for successful pregnancy, and its production in the reproductive tract can be down-regulated by interferon-gamma (IFN-γ). Infectious organisms that elicit a Th1-type response (e.g. Leishmania) can result in pregnancy failure, which has been attributed at least in part to IFN-γ production [32]. IFN-γ is a key cytokine in the ovine response to C. psittaci infection. It is produced in vivo in response to experimental challenge and also exerts chlamydiostatic effects on infected cells [11]. The induction of GM-CSF and IFN-γ by different cell types may prove to be one of the key elements in the persistence of C. psittaci and subsequent abortion of the fetus.
GM-CSF was the least abundant of the cytokines measured but, like IL-8, was induced by both live and heat-treated organisms. In other bacterial infections, the release of IL-8 and GM-CSF by epithelial cells is associated with bacterial invasion, whereas bacterial adhesion is a less potent stimulus [2]. The regime of heat treatment described here (65°C for 1 h) was sufficient to inhibit totally inclusion formation in fibroblastic ST-6 cells. However, the phenotype of the host cell has an important bearing on the uptake of heat-treated organisms. Multiple means of chlamydial attachment to host cells exist, and heat-treated C. psittaci can enter murine macrophages and subsequently form vacuoles that differ from those induced by untreated, infectious organisms [33]. Therefore, although inclusions were not observed by conventional staining in the ovine macrophages or ovine ST-6 cells exposed to heat-treated organisms, it is possible that the chlamydiae were present in a morphologically altered form [34]. In support of these host cell phenotypic differences, heat-treated C. trachomatis serovar L2 (75°C, 10 min) fails to induce IL-8 secretion by the SW620 human epithelial cell line [6]. However, the same organism has been reported to induce IL-8 secretion by the U-937 human monocytic cell line after even after more stringent heat treatment (100°C, 1 h) [27].
The ability of heat-treated chlamydiae to stimulate cytokine production and initiate immune responses has important implications for rational design of safe vaccines to control chlamydial infections. The formulation of chlamydial vaccines incorporating heat-treated organisms has not been a favoured approach in the past, since they are not effective in inducing protective immunity. However, it appears that the route of delivery of the organisms is critical, since it has recently been shown that dendritic cells pulsed with heat-treated C. trachomatis can induce protective immunity to genital infection after adoptive transfer to naive mice [35]. The protection was comparable to that induced by live organisms. Although IL-1β, IL-8 and GM-CSF were not measured in those experiments, the pulsed dendritic cells produced IL-6 and IL-12, two cytokines that will be investigated in ovine cells in the future as reagents become available. As more information becomes available on the cytokines induced in the acute stages of chlamydial infection, their role in disease pathogenesis can be elucidated, thereby allowing control strategies to be formulated.
Acknowledgments
This work was funded by the Scottish Office Agriculture, Environment and Fisheries Department (G.E., R.W., J.B.) and the Co-operative Research Centre for Vaccine Technology, Australia (P.M.W., J.-P.S., P.R.W.). The authors thank Helen Rowe and Sarah Lund (Moredun Research Institute) for help in harvesting alveolar lavages, Andrew Sanderson (Edinburgh University) for support with the FACS analysis and Ian McKendrick (BioSS) for expert statistical evaluation of the results.
REFERENCES
- 1.Henderson B, Wilson M. Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine. 1996;8:269–82. doi: 10.1006/cyto.1996.0036. [DOI] [PubMed] [Google Scholar]
- 2.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–9. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agace W, Hedges S, Andersson U, Anderson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–9. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsey N, Primrose JN, Lindley IJ. Interleukin-8 expression in Helicobacter pylori infected, normal and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen SJ, Eckmann L, Quayle AJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levitt D, Barol J. The immunobiology of Chlamydia. Immunol Today. 1987;8:246–51. doi: 10.1016/0167-5699(87)90175-7. [DOI] [PubMed] [Google Scholar]
- 8.Herring AJ. Typing Chlamydia psittaci—a review of methods and recent findings. Br Vet J. 1993;149:455–75. doi: 10.1016/S0007-1935(05)80111-3. [DOI] [PubMed] [Google Scholar]
- 9.Rodolakis A, Salinas J, Papp J. Recent advances on ovine chlamydial abortion. Vet Res. 1998;29:275–88. [PubMed] [Google Scholar]
- 10.Entrican G, Haig DM, Norval M. Identification of ovine interferons: differential activities derived from fibroblast and lymphoid cells. Vet Immunol Immunopathol. 1989;21:187–95. doi: 10.1016/0165-2427(89)90066-4. [DOI] [PubMed] [Google Scholar]
- 11.Graham SP, Jones GE, Maclean M, Livingstone M, Entrican G. Recombinant ovine interferon-γ inhibits the multiplication of Chlamydia psittaci in ovine cells. J Comp Pathol. 1995;112:185–95. doi: 10.1016/s0021-9975(05)80060-x. [DOI] [PubMed] [Google Scholar]
- 12.Burrells C. Cellular and humoral elements of the lower respiratory tract of sheep. Immunological examination of cells and fluid obtained by bronchoalveolar lavage of normal lungs. Vet Immunol Immunopathol. 1985;10:225–43. doi: 10.1016/0165-2427(85)90049-2. [DOI] [PubMed] [Google Scholar]
- 13.Mackay CR, Hein WR, Brown MH. Unusual expression of CD2 in sheep: implications for T cell interaction. Eur J Immunol. 1988;18:1681–8. doi: 10.1002/eji.1830181105. [DOI] [PubMed] [Google Scholar]
- 14.Gupta VK, McConnell I, Dalziel RG, Hopkins J. Identification of the sheep homologue of the monocyte cell surface molecule—CD14. Vet Immunol Immunopathol. 1996;51:89–99. doi: 10.1016/0165-2427(95)05512-6. [DOI] [PubMed] [Google Scholar]
- 15.Naessens J, Howard CJ. Monoclonal antibodies reacting with bovine B cells (BoWC3, BoWC4, BoWC5) Vet Immunol Immunopathol. 1991;27:77–85. doi: 10.1016/0165-2427(91)90083-o. [DOI] [PubMed] [Google Scholar]
- 16.Maddox JF, Mackay CR, Brandon MR. The sheep analogue of leukocyte common antigen (LCA) Immunology. 1985;55:347–53. [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay CR, Beya M-F, Matzinger P. γ/δ T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989;19:1477–83. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- 18.Dutia BM, Entrican G, Nettleton PF. Cytopathic and non-cytopathic biotypes of border disease virus induce polypeptides of different molecular weight with common antigenic determinants. J Gen Virol. 1990;71:1227–32. doi: 10.1099/0022-1317-71-5-1227. [DOI] [PubMed] [Google Scholar]
- 19.Entrican G, Hopkins J, Maclean M, McConnell I, Nettleton PF. Cell phenotypes in the efferent lymph of sheep persistently infected with border disease virus. Clin Exp Immunol. 1992;87:393–8. doi: 10.1111/j.1365-2249.1992.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothel JS, Hurst L, Pepin M, Berton P, Corner LA, Wood PR. Analysis of ovine IL-1β production in vivo and in vitro by enzyme immunoassay and immunohistochemistry. Vet Immunol Immunopathol. 1997;57:267–78. doi: 10.1016/s0165-2427(96)05754-6. [DOI] [PubMed] [Google Scholar]
- 21.Haig D, Deane D, Percival A, et al. The cytokine response of afferent lymph following orf virus reinfection of sheep. Vet Dermatol. 1996;7:11–20. doi: 10.1111/j.1365-3164.1996.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Entrican G, Deane D, Maclean M, Inglis L, Thomson J, McInnes C, Haig DM. Development of a sandwich ELISA for ovine granulocyte/macrophage colony-stimulating factor. Vet Immunol Immunopathol. 1996;50:105–15. doi: 10.1016/0165-2427(95)05468-5. [DOI] [PubMed] [Google Scholar]
- 23.Scheerlinck J-PY, Chaplin PJ, Wood PR. Ovine cytokines and their role in the immune response. Vet Res. 1998;29:369–83. [PubMed] [Google Scholar]
- 24.Kelso A. Cytokines: principles and prospects. Immunol Cell Biol. 1998;76:300–17. doi: 10.1046/j.1440-1711.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 25.Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydiae pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–5. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher J, Nair S, Poole S, Henderson B, Wilson M. Cytokine degradation by biofilms of Porphyromonas gingivalis. Curr Microbiol. 1998;36:216–9. doi: 10.1007/s002849900297. [DOI] [PubMed] [Google Scholar]
- 27.Ojcius DM, Souque P, Perfettini J-L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–6. [PubMed] [Google Scholar]
- 28.Bianchi A, Dosquet C, Henry S, Couderc M-C, Ferchal F, Scieux C. Chlamydia trachomatis growth stimulates interleukin 8 production by human monocytic U-937 cells. Infect Immun. 1997;65:2434–6. doi: 10.1128/iai.65.6.2434-2436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legastelios I, Cottin V, Mornex J-F, Cordier G. Alveolar macrophages from sheep naturally infected by visna-maedi virus contribute to IL-8 production in the lung. Vet Immunol Immunopathol. 1997;59:131–9. doi: 10.1016/s0165-2427(97)00055-x. [DOI] [PubMed] [Google Scholar]
- 30.Gesser B, Leffers H, Jinquan T, et al. Identification of functional domains on human IL-10. Proc Natl Acad Sci USA. 1997;94:14620–5. doi: 10.1073/pnas.94.26.14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caulfield JJ, Hawrylowicz CM, Kemeny DM, Lee TH. GM-CSF increases the ability of cultured macrophages to support autologous CD4+ T-cell proliferation in response to Dermatophagoides pteronyssinus and PPD antigen. Immunology. 1997;92:123–30. doi: 10.1046/j.1365-2567.1997.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. J Immunol. 1996;156:653–62. [PubMed] [Google Scholar]
- 33.Escalante-Ochoa C, Ducatelle R, Haesebrouck F. The intracellular life of Chlamydia psittaci: how do the bacteria interact with the host cell? FEMS Microbiol Rev. 1998;22:65–78. doi: 10.1111/j.1574-6976.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 34.Beatty WL, Morrison RP, Byrne GI. Persistent Chlamydiae: from cell culture to a paradigm for Chlamydial pathogenesis. Microbiol Rev. 1994;58:686–99. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–18. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]


