Abstract
There are three classes of RNA polymerase enzyme (RNAPs I, II and III). In systemic sclerosis (SSc), three main groups of anti-RNAP sera have been characterized by radioimmunoprecipitation techniques: anti-RNAP I/III sera, anti-RNAP I/II/III sera, and a group precipitating both RNAP II and topoisomerase I (topo I). Some sera in this third group precipitate the phosphorylated (IIO) form of RNAP II in the absence of the unphosphorylated (IIA) form. Certain other antinuclear antibodies (ANA) have also been detected in anti-RNAP IIO/IIA/topo I and anti-RNAP IIO/topo I sera. In the present study of 155 SSc patients, clinical features of individuals from each of these antibody groups were assessed and compared with those of patients from other autoantibody-defined groups. The anti-RNAP I/II/III antibody specificity was closely associated with the presence of diffuse cutaneous SSc (dc-SSc) (77.8%; cf. remaining group, 12.4%; P < 0.001; relative risk (RR) 6.3). Patients with anti-RNAP I/III antibodies also had an increased incidence of dc-SSc, but this was not significant (42.9%; cf. remainder, 15.7%). Anti-RNAP+ patients had a significantly increased incidence of renal involvement (29.0%, cf. remainder, 11.3%; P < 0.05; RR 2.6), with 40% of anti-RNAP I/II/III patients having renal disease. Meanwhile, the presence of anti-centromere antibodies (ACA) was associated with limited cutaneous SSc (lc-SSc) (100.0%; cf. remainder, 75.3%; P < 0.005), together with reduced incidences of both renal disease (2.4%, cf. remainder, 22.1%: P < 0.01) and pulmonary fibrosis (21.4%, cf. remainder, 52.3%; P < 0.005; RR 1.9). Anti-topo I antibodies were associated with the presence of pulmonary fibrosis (69.7%; cf. remainder, 32.6%; P < 0.001; RR 2.1). A majority of anti-topo I sera were from lc-SSc patients, regardless of whether anti-topo I antibodies occurred alone (75.0%) or together with anti-RNAP IIO + IIA antibodies (75.0%), and this was similar to the remainder (86.5%; NS). However, when anti-topo I+ patients were compared with the ACA group, and then with all anti-RNAP I+ patients (37.5% lc-SSc), significant differences were found in the occurrence of dc- versus lc-SSc (P < 0.005 and P < 0.05, respectively). In conclusion, these results confirm that there are three main groups of SSc sera, each characterized by the presence of a mutually exclusive SSc-specific autoantibody (ACA, anti-topo I or anti-RNAP I), and distinguished by patterns of cutaneous involvement and specific clinical features. It appears that, in each of the three groups of SSc patients, distinct pathological processes are occurring, which are responsible for the characteristic symptoms, for the modification of particular autoantigens and, consequently, for the production of particular autoantibodies. Based on these data, together with our previous results, it is further hypothesized that anti-RNAP II antibodies may be produced in the context of two different immune response pathways.
Keywords: RNA polymerase, autoantibody, systemic sclerosis
INTRODUCTION
Three main groups of anti-RNA polymerase (anti-RNAP) sera have been described in systemic sclerosis (SSc) [1–5]. The first group precipitates RNAPs I and III. The second group precipitates RNAPs I, II and III (i.e. both phosphorylated (IIO) RNAP and unphosphorylated (IIA) RNAP are precipitated by this group of sera). The third group precipitates RNAP IIO together with topoisomerase I (topo I), and sometimes also RNAP IIA [6–8].
The three multi-subunit RNAPs (I, II and III) share a number of sequence homologies, and some of the smaller subunits are common to two or all three enzymes [9–11]. Unlike the other two RNAPs, the largest subunit of RNAP II contains a carboxy terminal repeat domain (CTD) [12], which is highly phosphorylated in the transcriptionally active (IIO) form of the enzyme [13,14]. Consequently, the dephosphorylated (IIA) form of RNAP II is conformationally distinct [15,16].
The presence of anti-topo I antibodies in approx. 25% of SSc sera [17–20] has been associated with diffuse cutaneous SSc (dc-SSc) [19–23], and with the occurrence of pulmonary interstitial fibrosis [17,19,24,25]. However, most studies linking anti-topo I antibodies with dc-SSc were carried out before the characterization of anti-RNAP antibodies. Therefore, in these studies, anti-topo I+ patients could only be considered in relation to anti-centromere antibody (ACA)-positive patients, or, alternatively, compared with anti-topo I− groups: the presence of ACA has been closely linked with a particular subtype of SSc found in about 25% of patients and characterized by very limited skin involvement, often confined to sclerodactyly [17,18,26,27].
Since then, anti-RNAP I and anti-RNAP III antibodies have each been associated with an increased risk of developing dc-SSc [5,28], and with a higher incidence of renal disease [5]. For the purposes of clinical comparisons, some studies have tended to classify all sera containing anti-RNAP antibodies into a single group, often due to the small numbers involved: however, similar associations with dc-SSc [1–4] and with renal involvement [1,4] have been observed. In addition, some groups have reported an association of anti-RNAP antibodies with cardiac involvement [3,4]. Different clinical features in patients with anti-RNAP antibodies compared with those with anti-topo I antibodies have also been noted by some of these authors [1,4,5], including an increased incidence of diffuse disease and renal involvement in the anti-RNAP group [1,5], and a higher rate of pulmonary involvement in patients with anti-topo I [1,5]. Clinical associations were also considered in the study of Satoh et al. [8], who found that patients with both anti-RNAP IIO and anti-topo I antibodies had a significantly higher incidence of dc-SSc than did patients who had anti-topo I antibodies alone. However, only a small number of groups has so far studied the clinical associations of ACA, anti-topo I antibodies and anti-RNAP antibodies in the context of a single study [1,4]. Based on their own results, together with others [5], one of these studies concluded that there are three main, mutually exclusive groups of SSc sera: those with ACA, those with both anti-RNAP I and anti-RNAP III antibodies, and those with anti-topo I antibodies [1].
During our earlier studies concerning the co-precipitation of topo I and RNAP IIO (+ IIA) by a subset of SSc sera [6], a number of patients were found to have sera which precipitated topo I together with Jo-1 or Ro (+ La) autoantigens, some of which also contained anti-RNAP IIO (+ IIA) antibodies. These sera were of particular interest, since Satoh et al. had reported on the co-precipitation of RNAP IIO and topo I together with either U1 RNP, Ku or Ro autoantigens [8]. Bunn et al. have also noted that these particular anti-nuclear antibodies (ANA) did not occur in any of their anti-RNAP III, anti-RNAP I/III or anti-RNAP I/II/III sera [1]. Consequently, our findings concerning multiple ANA specificities in SSc [6] are reported here in more detail, along with their clinical associations.
PATIENTS AND METHODS
Patients and sera
A total of 155 patients was studied, all fulfilling criteria for SSc as described by Masi et al. [29]. Most patients had ostensibly idiopathic SSc, and were consecutively recruited via the Royal National Hospital for Rheumatic Diseases (Bath, UK). However, sera and clinical data were available for nine male patients with silica-associated SSc (Si-SSc) (kindly provided by Dr U. Haustein, University of Leipzig, Germany [30]), and these patients were also included: previous studies have shown that Si-SSc is clinically, serologically and immunologically indistinguishable from idiopathic SSc [30,31]. Blood samples were taken from the remaining patients, from which sera were prepared, all sera being stored at −20°C before serological analysis.
Disease subtype was classified as either limited cutaneous SSc (lc-SSc) or dc-SSc [32]. Limited cutaneous SSc was defined as cutaneous sclerosis not extending proximal to the elbow, knee or neck, while dc-SSc was defined as cutaneous sclerosis which did extend proximal to the elbow, knee or neck.
Detailed clinical notes were available from most of these patients, enabling any visceral complications to be noted. Pulmonary involvement was assessed by chest x-ray and standard carbon monoxide diffusion capacity (DLCO) tests. If the DLCO value was < 80% of that predicted, and/or significant shadowing of the lungs characteristic of bibasilar pulmonary fibrosis was apparent by x-ray examination, pulmonary involvement was recorded [33]. One or more of the following features recorded on more than one occasion (unless attributable to unrelated disease) was taken to imply renal involvement: (i) creatinine clearance rate < 60 ml/min (or serum creatinine > 1.3 mg/dl); (ii) active urinary sediment; (iii) proteinuria (> 0.5 g/day); and (iv) accelerated hypertension (diastolic B.P. > 105 mmHg) [33].
Indirect immunofluorescence
As described previously [34], HEp-2-cell slides (Biodiagnostics Ltd, Upton-Upon-Severn, UK) were incubated with serum diluted 1:40 with PBS for 1 h. Washed cells were incubated with FITC-conjugated goat anti-human polyvalent immunoglobulins (1:1000) (Sigma Chemical Co., Poole, UK). After a second wash, the slides were mounted in glycerol/PBS containing 2.5% DABCO (1,4-diazobicyclo-[2,2,2]-octane), and viewed under a fluorescent microscope.
Immunodiffusion
Ouchterlony double immunodiffusion was carried out on all sera, as previously described [35], excluding those sera already known to contain ACA. Anti-centromere antibodies are not detected by this method, and sera containing ACA are generally considered to form a mutually exclusive group (see below). The following extracts were used as antigen sources: rabbit thymus extract (Bradshaw Biologicals, Market Harborough, UK), Ro/La (SS-A/SS-B) extract (Biodiagnostics Ltd) and Scl-70 (topo I) extract (Biodiagnostics Ltd).
Radioimmunoprecipitation
On account of previous routine indirect immunofluorescence (IF) tests or immunoblot (IB) studies, 42 sera were already known to contain ACA. Anti-centromere antibodies are not well detected by immunoprecipitation (IP) assays [36], and, in our previous studies [34,37] no ACA had been detected in conjunction with anti-topo I, anti-RNAP antibodies or any other ANA. Consequently, only a proportion of ACA+ sera was tested by IP in the present study, 14 (33%) being excluded.
The remaining 141 sera were subject to IP assays using 35S-methionine-labelled K562 cell extracts for the detection of antibodies precipitating RNAP, topo I, Jo-1, U1 RNP (+ Sm), Ku, PL-7, Ro and/or La antigens, as previously described [34].
Statistical analysis
All comparisons were performed by χ2 analysis of 2 × 2 tables, with Yates' correction where appropriate (level of significance: P < 0.05).
RESULTS
Immunodiffusion
A total of 32 sera had anti-topo I antibodies by immunodiffusion, while antibodies recognizing Jo-1, U1 RNP + Sm, Ro and/or La antigens were detected in seven, four, six and three sera, respectively. No anti-Ku antibodies were detected by this method.
Radioimmunoprecipitation
IP profiles produced by ANA+ SSc sera are shown in Fig. 1. In the present study, all ANA specificities identified by immunodiffusion were confirmed on IP gels by comparison with prototype sera of known autoantibody specificity. Since radioimmunoprecipitation is a more sensitive technique than immunodiffusion, additional sera containing antibodies to RNAP II, RNAP III, topo I, Jo-1, U1 RNP + Sm, Ro and/or La were identified by IP techniques (Table 1). Several of these samples had shown a weak positive unidentified result by immunodiffusion. A number of sera were found to precipitate more than one of these nuclear antigens. One anti-PL-7 serum was detected (specificity kindly confirmed by Dr I. Targoff, Department of Medicine, Oklahoma University Sciences Centre, OK, USA).
Fig. 1.
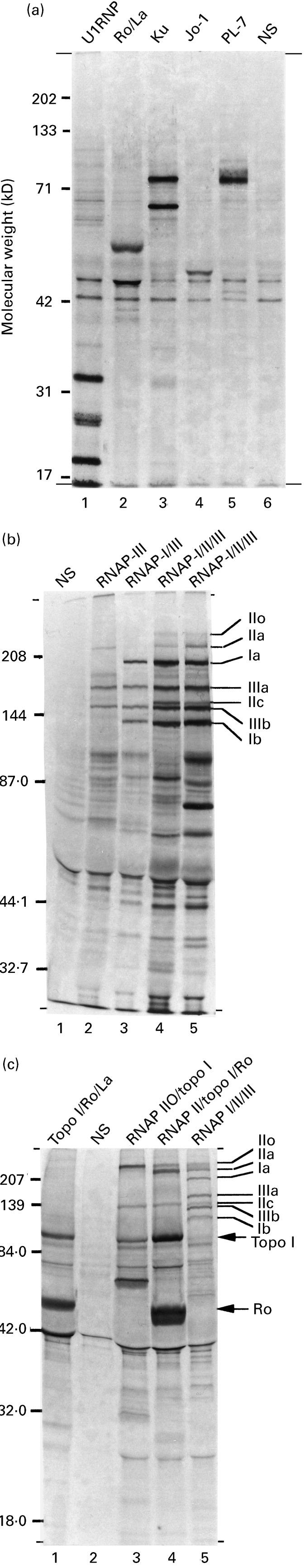
Radioimmunoprecipitation profiles produced by systemic sclerosis (SSc) sera. Autoradiographs of SDS–PAGE-separated 35S-methionine-labelled proteins precipitated by Protein-A Sepharose beads coated with patient antibodies. (a) Anti-nuclear antigens precipitated by standard SSc sera containing anti-U1 RNP antibodies (lane 1), anti-Ro/La antibodies (lane 2), anti-Ku antibodies (lane 3), anti-Jo-1 antibodies (lane 4) and anti-PL-7 antibodies (lane 5). A normal serum (NS) is shown in lane 6 for comparison. (b) In the present study three main groups of anti-RNA polymerase (RNAP) sera were detected. The first group precipitated RNAPs I and III (lane 3): the largest two subunits of each enzyme are indicated (subunits Ia and Ib, and IIIa and IIIb, respectively). The second group of sera also precipitated RNAP II, in both its phosphorylated (IIO) and dephosphorylated (IIA) form (lane 4): the largest subunits of RNAP IIO and RNAP IIA are indicated (subunits IIo, and IIa, respectively, with the second largest subunit (IIc) being common to both forms of RNAP II). Lane 1, NS; lane 2, anti-RNAP III standard; lane 5, anti-RNAP I/II/III standard. (c) The third group of anti-RNAP sera in SSc precipitates both topoisomerase I (topo I) and RNAP IIO (lanes 3 and 4). Some sera in the third group also precipitate RNAP IIA (lane 4). A proportion of anti-topo I sera also contain anti-Ro antibodies (c, lanes 1 and 4), some of which also precipitate RNAP IIO (+IIA) (c, lane 4). Lane 2, NS; lane 5, anti-RNAP I/II/III serum.
Table 1.
Autoantibodies detected in the sera of systemic sclerosis (SSc) patients by radioimmunoprecipitation assays, and their clinical associations
Figures in parentheses are percentages.*1P < 0.05; *2P < 0.01; *3P < 0.005; *4P < 0.001 when compared with the respective remaining group; *5P < 0.05 when compared with each other; *6P < 0.025 when compared with each other; *7P < 0.025 when compared with each other; *8P < 0.025 when compared with each other; *9P < 0.005 when compared with each other; *10P < 0.005 when compared with each other; *11P < 0.001 when compared with each other (all comparisons by χ2 test with Yates' correction where appropriate).
† For details, see text.
‡ For organ involvement definitions, see text.
§ Total numbers of patients on whom this category of clinical information was available.
¶ Some sera had multiple antibody specificities.
** Determined by immunoblotting and/or immunofluorescence assays.
*** Th RNP, U3 RNP, Ku, Pm-Scl and/or PL-7.
RNAP, RNA polymerase; topo I, topoisomerase I; CENPs, centromere proteins.
The most common IP profiles produced by anti-RNAP+ SSc sera are shown in Fig. 1b,c. In the present study, 34 anti-RNAP sera were detected (21.9%) and, as previously reported [6–8], three main groups were identified: anti-RNAP I/III sera (n = 7; 4.5%) (Table 1), anti-RNAP I/II/III sera (n = 11; 7.1%) (Table 1), and sera precipitating the phosphorylated (IIO) form of RNAP II in the absence of RNAPs I and III (n = 15; 9.7%). All sera in the third group also precipitated topo I, and six of them also precipitated the unphosphorylated (IIA) form of RNAP II. As reported previously [2,6,8], all anti-RNAP I/II/III sera precipitated both the IIO and the IIA form of RNAP II (Table 1). In addition, one serum precipitated RNAP III in the absence of RNAPs I and II.
As shown in Fig. 1, a number of SSc sera contained other multiple ANA specificities. A total of 18 sera containing anti-Ro antibodies was detected (11.6%) (Table 1), of which six also contained anti-La antibodies (3.9%). Only one anti-La serum failed to precipitate the Ro antigen (an anti-Pm-Scl serum) (Table 1). Of the 36 sera (23.2%) containing anti-topo I antibodies (Table 1), eight (5.2%) also precipitated the Ro antigen (Table 1), including two anti-RNAP II/topo I sera and two anti-RNAP IIO/topo I sera. Immunoprecipitation profiles produced by SSc sera containing both anti-topo I and anti-Ro antibodies are shown in Fig. 1c. Notably, the Ro, La and Jo-1 antigens were only precipitated by those anti-RNAP sera which also contained anti-topo I antibodies. Despite a fairly high prevalence of sera containing anti-Jo-1 and/or anti-U1 RNP antibodies (each was detected in 10 sera (6.5%); Table 1), most were monospecific, and only one was found to co-precipitate an RNAP (one anti-RNAP IIO/topo I/Jo-1 serum). However, a small number of sera containing other multiple ANA combinations was detected, including one anti-Ro/U1 RNP serum, one anti-Ro/Jo-1, and one anti-Jo-1/U1 RNP/Sm serum. Despite reports on the co-precipitation of RNAP IIO and the Ku antigen [8], no serum was found to contain anti-Ku antibodies. All nine Si-SSc sera were found to contain anti-topo I antibodies, including one anti-RNAP IIO/IIA/topo I/Ro serum. This would be consistent with previous observations suggesting that ACA are extremely rare in male idiopathic SSc patients [30].
Meanwhile, sera containing ACA (n = 42; 27.1%) appeared to form a separate group: of the 28 ACA sera tested by IP, none was found to precipitate any of the other autoantibodies which we have previously detected. Furthermore, anti-RNAP I+ sera were not found to co-precipitate topo I. Together, these results confirm the existence of three mutually exclusive groups of SSc sera, each characterized by the presence of an SSc-specific autoantibody (ACA, anti-topo I and anti-RNAP I).
Clinical associations of anti-RNA polymerase autoantibodies in SSc
Clinical data from patients whose sera contained a particular individual antibody were compared with clinical data from patients in the corresponding remaining group (i.e. patients whose sera did not contain that particular antibody) (Table 1). Comparisons were also made between groups of sera with different antibody profiles (Table 1). Patients whose sera were already known to contain other defined specificities (mainly other anti-nucleolar antibody (ANoA)-positive patients) were classified together (Table 1), and patients in whose sera no defined autoantibody specificities have definitely been characterized to date are also indicated (Table 1).
Disease subtype and cutaneous involvement
The occurrence of lc- versus dc-SSc in the different antibody groups can be compared in Table 1. The presence of ACA was associated with lc-SSc (100.0%; cf. remaining group, 75.3%; P < 0.005). The individual antibody which showed the closest and most significant association with dc-SSc was anti-RNAP I (62.5%; cf. remaining group, 10.4%; P < 0.001; relative risk (RR) 6.0), and anti-RNAPs IIO, IIA and III were also each associated with the development of diffuse disease (RNAP IIO, 50.0%, cf. remainder, 10.0%; P < 0.001, RR 5.0; RNAP IIA, 61.5%; cf. remainder, 11.9%; P < 0.001, RR 5.2; RNAP III, 58.8%; cf. remainder, 10.5%; P < 0.001, RR 5.6). Furthermore, patients with anti-RNAP I/II/III antibodies had the highest calculated incidence of diffuse skin involvement (77.8%; cf. remaining group 12.4%; P < 0.001, RR 6.3). Although the anti-RNAP I/III group also showed an increased incidence of dc-SSc (42.9%), this was not significantly different from the remaining group (15.7%).
A majority of anti-topo I sera (72.7%) came from patients with lc-SSc, but this was not significantly different from the remaining group (86.5%). The same proportion of anti-topo I+ patients had lc-SSc regardless of whether anti-topo I antibodies occurred alone (75.0%) or together with anti-RNAP IIO + IIA antibodies (75.0%) (Table 1). Anti-topo I+ patients appeared to represent a group with an intermediate frequency of diffuse cutaneous involvement. Thus, when anti-topo I+ patients were compared with the ACA group, the anti-topo I group had a significantly higher frequency of diffuse disease (P < 0.005) (Table 1). Meanwhile, comparison of the anti-topo I group with the anti-RNAP I/II/III group revealed a significantly lower frequency of dc-SSc in the anti-topo I+ patients (P < 0.025) (Table 1).
Together, these results indicate that the three main groups of SSc patients (characterized by the presence of anti-centromere, anti-topo I and anti-RNAP I antibodies, respectively) are each associated with a different risk of diffuse cutaneous involvement.
All the remaining ANA (anti-Ro, anti-La, anti-Jo-1 and anti-U1 RNP) had a higher than average occurrence of lc-SSc (Table 1), although none was significant when compared with the respective remaining group.
Renal involvement
The incidence of renal involvement in each of the different antibody groups is shown in Table 1. The previously reported association of anti-RNAP antibodies with renal disease was confirmed (29.0%, cf. remaining group, 11.3%; P < 0.05; RR 2.6). Each of the individual anti-RNAP antibody groups had a higher than average incidence of kidney involvement (Table 1), but none of these results reached significance. The anti-RNAP+ antibody profile which had the highest rate of renal involvement was the anti-RNAP I/II/III group (40.0% with renal involvement): however, this was not significant when compared with the remaining group (13.6%), possibly due to the small numbers involved. The anti-RNAP I/III group actually had a lower than average incidence of renal involvement (14.3%).
The highest rate of kidney involvement (60.0%) occurred in patients with anti-La antibodies, and, despite the low numbers involved, this was found to be a significant association (cf. remaining group, 13.8%; P < 0.05; RR 4.3). Furthermore, the anti-Ro+ group was also significantly associated with renal involvement (41.7%, cf. remaining group, 12.9%; P < 0.05; RR 3.2). However, four of these sera also contained anti-RNAP antibodies.
The ACA+ group had a reduced incidence of renal involvement (2.4%, cf. remainder, 22.1%; P < 0.01), similar to previous reports [25]. Renal involvement in the anti-topo I group was higher than average (24.2%, cf. remaining group, 12.6%), but this was not significant.
The incidence of renal involvement in patients with anti-RNAP I/II/III antibodies was found to be significantly increased compared with the ACA group (P < 0.005). Furthermore, the anti-topo I group also had a significantly higher incidence of renal involvement than the ACA+ group (P < 0.025).
Pulmonary involvement
The frequency of pulmonary involvement in each of the different antibody groups is shown in Table 1. As reported previously, lung involvement was closely associated with anti-topo I antibodies (69.7%; cf. remaining group, 32.6%; P < 0.001; RR 2.1). The frequency of pulmonary involvement was even higher in patients with anti-Ro antibodies, and this was also significant (75.0%, cf. remaining group, 38.8%; P < 0.05; RR 1.9). Furthermore, a higher incidence of pulmonary involvement occurred in those anti-Ro+ patients whose sera did not precipitate topo I (80.0%) than in anti-topo I+ patients who did not also have anti-Ro antibodies (69.2%), although the difference between the two groups was not significant. When sera containing either anti-topo I and/or anti-Ro antibodies were considered, the incidence of pulmonary involvement was found to be highly significant (71.1%, cf. remainder, 30.0%; P < 0.001; RR 2.4). The ACA group had a reduced incidence of pulmonary involvement (21.4%, cf. remaining group, 52.3%; P < 0.005), confirming previous findings [25].
The anti-topo I+ group was shown to have a significantly increased incidence of lung involvement compared with the ACA group (21.4%; P < 0.001).
The nine anti-topo I+ Si-SSc patients all had pulmonary involvement.
When considering clinical associations, it was difficult to separate the influence of the individual anti-RNAP antibodies. However, by comparing anti-RNAP I/II/III patients with the anti-RNAP I/III group, the effect of the additional presence of anti-RNAP IIO + IIA antibodies could be assessed. Also, anti-RNAP IIO/IIA/topo I patients could be compared with patients precipitating topo I in the absence of any RNAPs. Thus, any clinical effects associated with the additional presence of anti-RNAP IIO and IIA antibodies could be calculated in two different contexts (Table 1). The prevalence of dc-SSc in anti-RNAP I/II/III sera was higher than in the anti-RNAP I/III group (77.8% versus 42.9%, respectively), but this did not reach significance, possibly due to the small numbers involved. Meanwhile, anti-topo I+ patients had the same low incidence of dc-SSc, regardless of the additional presence of anti-RNAP IIO and IIA antibodies (25.0%).
DISCUSSION
In the present study, a total of 18 sera from 155 SSc patients (12%) was found to precipitate both RNAP I and III. Thus, these results are in general agreement with previous findings in this area [1–5]. The frequencies of anti-topo I, anti-centromere, anti-Jo-1 and anti-U1 RNP antibodies were also compatible with previous data [1,17–20,25,36,38]. Meanwhile, the frequency of anti-Ro antibodies detected here (12%) was higher than recently found in SSc patients by Bunn et al. (5%) [1]. However, in addition to the method of immunodiffusion, we have included anti-Ro antibodies detected by the more sensitive technique of IP. Fujimoto et al. [39] have detected anti-Ro antibodies in 11% of SSc patients by immunodiffusion. Furthermore, using a sensitive ELISA method, anti-Ro antibodies have been reported to occur in 37% of SSc patients by Bell et al. [40].
Subgroups of anti-RNAP antibodies were similar to those which have been reported before [1–3,5], i.e. a very small group with anti-RNAP III antibodies alone (in the present study, 1%), a larger group with anti-RNAP I/III antibodies (5%), and another group capable of precipitating all three RNAPs (7%). In addition, sera with antibodies which precipitated both RNAP IIO and topo I in the absence of RNAPs I and III were detected in 10% of our sera, similar to the overall figure of 12% found by Satoh et al. [8]. Of these, roughly half also precipitated RNAP IIA, as stated in our original report [6]: the existence of such a subgroup of SSc sera has been confirmed in a separate publication by Satoh et al. [41]. It is of considerable interest that no sera have been reported that precipitate only RNAPs I, IIO and III, considering that anti-RNAP IIO/topo I sera are relatively frequent.
Of the 15 anti-RNAP IIO/topo I and anti-RNAP IIO/IIA/topo I sera studied here, four (27%) also contained anti-Ro antibodies. The incidence of sera which co-precipitated RNAP IIO and Ro was therefore very similar to that reported by Satoh et al. (cf. 33%) [8]. However, no other ANA were found in conjunction with anti-RNAP I, anti-RNAP III or ACA, a finding also supported by Bunn et al. [1]. In accordance with previous reports [5], none of the other SSc-specific antibodies, i.e. anti-Th RNP, -Pm-Scl or -U3 RNP antibodies, were found to coexist with any of the anti-RNAP antibodies detected here. Together, these results support the existence of three main, mutually exclusive groups of SSc sera, each characterized by the presence of a particular SSc-specific antibody (ACA, anti-topo I antibodies or anti-RNAP I antibodies), as suggested by Bunn et al. [1]. Anti-topo I antibodies are frequently accompanied by antibodies to Ro (+ La) and/or RNAP IIO (+ IIA). Meanwhile, anti-RNAP I antibodies are accompanied by antibodies recognizing RNAP III, and sometimes also by both anti-RNAP IIO and anti-RNAP IIA antibodies.
Results presented here confirm the very close association between the presence of ACA and limited disease, and the association between the presence of each individual anti-RNAP antibody and diffuse disease was also in support of previous data. Meanwhile, the association of anti-topo I antibodies with diffuse disease has not been confirmed. Several factors could have led to a majority of our anti-topo I+ patients having lc-SSc, including slightly different clinical interpretations of diffuse versus limited disease. However, the present results confirm that the anti-topo I group is associated with a significantly higher risk of dc-SSc compared with the ACA group, while the anti-RNAP I+ group is shown to have an even greater incidence of diffuse disease, significantly higher than either the anti-centromere- or the anti-topo I-positive groups. Similarly, Okano et al. [5] showed that, amongst dc-SSc patients, only 27% had anti-topo I antibodies, while 45% had anti-RNAP III antibodies.
As previously reported, anti-topo I antibodies were associated with pulmonary fibrosis. In addition, the association of renal involvement with anti-RNAP antibodies was confirmed in the present study, and we also found a significant association between renal involvement and the presence of anti-La and/or anti-Ro antibodies. However, some of these particular sera contained other ANA specificities, including anti-RNAP II, and the numbers involved were relatively small. Therefore, the existence of a link between anti-Ro (+ La) antibodies and renal involvement would require confirmation. In the present study, the anti-topo I group had a significantly increased rate of renal involvement compared with the ACA group.
Thus, it appears that the three main SSc-specific antibody groups are each distinguished by the frequency of diffuse cutaneous involvement and the frequency of specific clinical features: members of the ACA group usually have limited cutaneous involvement, and there is a low incidence of internal organ involvement; patients in the anti-RNAP I group frequently have the diffuse form of SSc, and this group has the highest incidence of renal disease; the anti-topo I group has the highest frequency of pulmonary fibrosis and these patients appear to have an intermediate risk of diffuse cutaneous manifestations. While this latter observation could reflect an intermediate degree of cutaneous spread in many anti-topo I+ patients, definitive conclusions were not possible in the present study, since patients had been assigned to either limited or diffuse subtypes [32], with no intermediate categories of cutaneous involvement.
Previous data regarding the autoreactive subunits of the RNAPs [2,5,6,42] suggest that, while anti-RNAP IIO/IIA/topo I sera and anti-RNAP IIO/topo I sera only recognize RNAP subunits which are specific to one or both forms of RNAP II, anti-RNAP I/III sera and anti-RNAP I/II/III sera also recognize subunits common to two or all three RNAPs, and also recognize RNAP III-specific subunits. Regarding patients with anti-RNAP I/II/III antibodies, the models suggested by both Hirakata et al. [2,43] and by Kuwana et al. [42] favour the initial production of anti-RNAP I and/or anti-RNAP III antibodies, followed by spreading of the antibody response to subunits shared by RNAPs I and III [42]. Then, due to recognition of a subunit shared by all three RNAPs, further spreading of the immune response to include the RNAP II enzyme [2], with eventual intramolecular epitope spreading within RNAP II which, according to the presence of appropriate HLA alleles, would often include antibodies recognizing the IIo subunit and/or the IIa subunit. Indeed, concerning the anti-RNAP III group, one possibility is that the anti-RNAP I/III group and the anti-RNAP I/II/III group are actually a single subgroup of patients at a different stage of their disease [42].
Meanwhile, our previous study [6] demonstrated that sera which precipitate only RNAP IIO recognize only subunit IIO-specific epitopes. Furthermore, it appears that the anti-RNAP IIO/topo I response is capable of spreading to RNAP IIA (specifically subunit IIa [6]) in certain patients, and, again, this may be due to the presence of an appropriate HLA background. To summarize, the anti-RNAP I/III antibody response appears to spread to include both RNAP IIO and RNAP IIA but not topo I, while the anti-RNAP IIO/IIA/topo I antibody response does not appear to spread to include RNAPs I and III. Such discretionary epitope spreading may be explained by the nature of antigen processing by B cells, which is known to be affected by the specificity of the particular B cell concerned: since the antigenic site bound by the immunoglobulin molecule is protected from proteolysis, different peptides are generated by B cells depending on their particular epitope specificity (for review see [44]). A consequence of the production of anti-RNAP II antibodies by two distinct pathways concerns autoantibody associations with particular HLA alleles, since different epitopes may be involved in overcoming tolerance to the same molecule.
Interestingly, our data suggest that the presence of anti-RNAP IIO + IIA antibodies contributes to the high incidence of dc-SSc in the anti-RNAP I/II/III group, but that a similar effect does not occur in the anti-RNAP IIO/IIA/topo I group (Table 1). Although the numbers involved were too small to allow definitive conclusions, it appears that the presence of anti-RNAP II antibodies per se is not associated with an increased incidence of dc-SSc.
Based on a detailed analysis of HLA associations of SSc-specific autoantibodies [45], together with their previous results [1], it has been suggested by Fanning et al. [45] that the three main serologically defined subgroups of SSc patients actually represent patients with three different diseases. The present study would support this hypothesis, but with the added point that the anti-topo I group is also associated with the occurrence of anti-Ro and/or anti-RNAP IIO (+ IIA) antibodies. It appears possible that, in the context of this particular subset of SSc patients, RNAP II, Ro and topo I are embroiled in a sequence of events which results in a breakdown of tolerance to one, two or all three molecules. Significantly, both RNAP II and topo I (but not RNAP I, RNAP III or centromere proteins) were among the SSc antigens found to be uniquely susceptible to metal ion-catalysed fragmentation under conditions of oxidative stress by Casciola-Rosen et al. [46]. Since metal ions co-localize with SSc antigens, such fragmentations may occur in vivo during ischaemic reperfusion [47]. Thus, the subset of patients with anti-RNAP II and/or topo I antibodies described here may correspond to the particular group of SSc patients proposed by Casciola-Rosen et al. [46] to have unique access to RNAP II and topo I antigens in a particular, novel fragmented form. In their paper, it was hypothesized that these modified proteins permitted the presentation of cryptic epitopes to T cells, resulting in a breakdown of tolerance to these antigens. Thus, the findings of Casciola-Rosen et al. could explain the mutual exclusivity of the anti-topo I+ group from the ACA group and the anti-RNAP III+ group. Other distinct pathogenic mechanisms may underlie the breakdown of tolerance to RNAP I and centromere proteins. Were this shown to be the case, the separate disease model of Fanning et al. [45] would be inherently supported.
In conclusion, these results suggest that anti-RNAP I, anti-topo I and ACA are markers of distinct pathological processes which are responsible for the characteristic symptoms, for the modification of particular autoantigens and, consequently, for the production of particular autoantibodies. Identification of the original inciting antigen recognized by SSc sera may be the key to the subgrouping of SSc patients, as implied by the work of Casciola-Rosen et al. [46,47].
Acknowledgments
This work was supported by grants from the Arthritis Research Campaign, UK. We are grateful to Professor U.-F. Haustein (University of Leipzig) for supplying some of the sera and clinical data included in the present study.
REFERENCES
- 1.Bunn CC, Denton CP, Shi-Wen X, Knight C, Black CM. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol. 1998;37:15–20. doi: 10.1093/rheumatology/37.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Hirakata M, Okano Y, Pati U, Suwa A, Medsger TA, Jr, Hardin JA, Craft JA. Identification of autoantibodies to RNA polymerase II. Occurrence in systemic sclerosis and association with autoantibodies to RNA polymerases I and III. J Clin Invest. 1993;91:2665–72. doi: 10.1172/JCI116505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwana M, Kaburaki J, Mimori T, Tojo T, Homma M. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest. 1993;91:1399–404. doi: 10.1172/JCI116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994;37:75–83. doi: 10.1002/art.1780370111. [DOI] [PubMed] [Google Scholar]
- 5.Okano Y, Steen VD, Medsger TA. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993;119:1005–13. doi: 10.7326/0003-4819-119-10-199311150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Harvey GR, Rands AL, McHugh NJ. Anti-RNA polymerase antibodies in systemic sclerosis (SSc): association with anti-topoisomerase I antibodies and identification of autoreactive subunits of RNA polymerase II. Clin Exp Immunol. 1997;105:468–74. doi: 10.1046/j.1365-2249.1996.d01-798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh M, Kuwana M, Ogasawara T, Ajmani AK, Langdon JJ, Kimpel D, Wang J. Association of autoantibodies to topoisomerase I and the phosphorylated (IIO) form of RNA polymerase in Japanese scleroderma patients. Arthritis Rheum. 1994;37(Suppl.):S261. (Abstr.) [PubMed] [Google Scholar]
- 8.Satoh M, Kuwana M, Ogasawara T, Ajmani AK, Langdon AJ, Kimpel D, Wang J, Reeves WH. Association of autoantibodies to topoisomerase I and the phosphorylated (IIO) form of RNA polymerase II in Japanese scleroderma patients. J Immunol. 1994;153:5838–48. [PubMed] [Google Scholar]
- 9.Buhler J-M, Huet J, Davies KE, Sentenac A, Fromageot P. Immunological studies of yeast nuclear RNA polymerases at the subunit level. J Biol Chem. 1980;255:9949–54. [PubMed] [Google Scholar]
- 10.Huet J, Sentenac A, Fromageot P. Spot-immunodetection of conserved determinants in eukaryotic RNA polymerases. J Biol Chem. 1982;257:2613–8. [PubMed] [Google Scholar]
- 11.Woychik NA, Laiao S, Kolodziej PA, Young RA. Subunits shared by eukaryotic nuclear RNA polymerases. Genes Dev. 1990;4:313–23. doi: 10.1101/gad.4.3.313. [DOI] [PubMed] [Google Scholar]
- 12.Corden JL, Cadena DL, Ahearn JM, Dahmus ME. A unique structure at the carboxyl terminal of the largest subunit of the eukaryotic RNA polymerase II. Proc Natl Acad Sci USA. 1985;82:7934–8. doi: 10.1073/pnas.82.23.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne JM, Laybourn PJ, Dahmus ME. The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIa. J Biol Chem. 1989;264:19621–9. [PubMed] [Google Scholar]
- 14.Zhang J, Corden JL. Identification of phosphorylation sites in the repetitive carboxyl-terminal domain of the mouse RNA polymerase II largest subunit. J Biol Chem. 1991;266:2290–6. [PubMed] [Google Scholar]
- 15.Cadena DL, Dahmus ME. Messenger RNA synthesis in mammalian cells is catalyzed by the phosphorylated form of RNA polymerase II. J Biol Chem. 1987;262:12468–74. [PubMed] [Google Scholar]
- 16.Zhang J, Corden JL. Phosphorylation causes a conformational change in the carboxyl-terminal domain of the mouse RNA polymerase II largest subunit. J Biol Chem. 1991;266:2297–302. [PubMed] [Google Scholar]
- 17.Catoggio LJ, Bernstein RM, Black CM, Hughes GRV, Maddison PJ. Serological markers in progressive systemic sclerosis: clinical correlations. Ann Rheum Dis. 1983;42:23–27. doi: 10.1136/ard.42.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catoggio LJ, Skinner RP, Maddison PJ. Frequency and clinical significance of anticentromere and anti Scl-70 antibodies in an English connective tissue disease population. Rheumatol Int. 1983;3:19–21. doi: 10.1007/BF00541227. [DOI] [PubMed] [Google Scholar]
- 19.Steen VD, Powell DL, Medsger TA. Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 20.Weiner ES, Earnshaw WC, Senecal J-L, Bordwell B, Johnson P, Rothfield N. Clinical associations of anticentromere antibodies and antibodies to topoisomerase I. A study of 355 patients. Arthritis Rheum. 1988;31:378–85. doi: 10.1002/art.1780310309. [DOI] [PubMed] [Google Scholar]
- 21.Ferri C, Bernini L, Cecchetti R, Latorraca A, Marotta G, Pasero G, Neri R, Bombardieri S. Cutaneous and serological subsets of systemic sclerosis. J Rheumatol. 1991;18:1826–32. [PubMed] [Google Scholar]
- 22.Giordano M, Valentini G, Migliaresis S, Picillo U, Vatti M. Different antibody patterns and different prognoses in patients with scleroderma with various extent of skin sclerosis. J Rheumatol. 1986;13:911–6. [PubMed] [Google Scholar]
- 23.van Venrooij WJ, Stapel SO, Houben H, Habets WJ, Kallenberg CGM, Penner E, van de Putte LB. Scl-86, a marker antigen for diffuse scleroderma. J Clin Invest. 1985;75:1053–60. doi: 10.1172/JCI111767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs DC, Vaughan RW, Welsh KI, Myers A, duBois RM, Black CM. Immunogenetic prediction of pulmonary fibrosis in systemic sclerosis. Lancet. 1991;338:661–2. doi: 10.1016/0140-6736(91)91235-m. [DOI] [PubMed] [Google Scholar]
- 25.Parodi A, Puiatti P, Rebora A. Serological profiles as prognostic clues for progressive systemic scleroderma: the Italian experience. Dermatologica. 1991;183:15–20. doi: 10.1159/000247625. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein RM, Steigerwald JC, Tan EM. Association of antinuclear and antinucleolar antibodies in progressive systemic sclerosis. Clin Exp Immunol. 1982;48:43–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Tan EM, Rodnan GP, Garcia I, Moroi Y, Fritzler MJ, Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis. Anticentromere antibody and its relationship to CREST syndrome. Arthritis Rheum. 1980;23:617–25. doi: 10.1002/art.1780230602. [DOI] [PubMed] [Google Scholar]
- 28.Reimer G, Rose KM, Scheer U, Tan EM. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987;79:65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masi AT, Rodnan GP, Medsger TA, Jr, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 30.McHugh NJ, Whyte J, Harvey G, Haustein UF. Anti-topoisomerase I antibodies in silica-associated systemic sclerosis. A model for autoimmunity. Arthritis Rheum. 1994;37:1198–205. doi: 10.1002/art.1780370814. [DOI] [PubMed] [Google Scholar]
- 31.Rustin MHA, Bull HA, Ziegler V, Mehlhorn J, Haustein U-F, Maddison PJ, James J, Dowd PM. Silica-associated systemic sclerosis is clinically, serologically and immunologically indistinguishable from idiopathic systemic sclerosis. Brit J Dermatol. 1990;123:725–34. doi: 10.1111/j.1365-2133.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 32.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 33.McHugh NJ, James IE, Maddison PJ. Differential isotype recognition of two centromere associated polypeptides by immunoblotting in connective tissue disease. Clin Exp Immunol. 1988;72:457–64. [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey GR, Black C, Maddison P, McHugh N. Characterization of antinucleolar antibody reactivity in patients with systemic sclerosis and their relatives. J Rheumatol. 1996;24:477–84. [PubMed] [Google Scholar]
- 35.Isenberg DA, Maddison PJ. Detection of antibodies to double stranded DNA and extractable nuclear antigen. Assoc Clin Pathol Broadsheet. 1987;117:1–8. doi: 10.1136/jcp.40.11.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kipnis RJ, Craft J, Hardin JA. The analysis of antinuclear and antinucleolar autoantibodies of scleroderma by radioimmunoprecipitation assays. Arthritis Rheum. 1990;33:1431–7. doi: 10.1002/art.1780330917. [DOI] [PubMed] [Google Scholar]
- 37.McHugh NJ, Whyte J, Artlett C, et al. Anti-centromere antibodies (ACA) in systemic sclerosis patients and their relatives: a serological and HLA study. Clin Exp Immunol. 1994;96:267–74. doi: 10.1111/j.1365-2249.1994.tb06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livingston JZ, Scott TE, Wigley FM, Anhalt GJ, Bias WB, McLean RH, Hochberg MC. Systemic sclerosis (scleroderma): clinical, genetic, and serological subsets. J Rheumatol. 1987;14:512–8. [PubMed] [Google Scholar]
- 39.Fujimoto M, Shimozuma M, Yazawa N, et al. Prevalence and clinical relevance of 52-kDa and 60-kDa Ro/SS-A autoantibodies in Japanese patients with systemic sclerosis. Ann Rheum Dis. 1997;56:667–70. doi: 10.1136/ard.56.11.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell S, Krieg T, Meurer M. Antibodies to Ro/SSA detected by ELISA. Correlation with clinical features in systemic scleroderma. Br J Dermatol. 1989;121:35–41. doi: 10.1111/j.1365-2133.1989.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 41.Satoh M, Ajmani AK, Ogasawara T, Langdon JJ, Hirakata M, Wang J. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. Specific recognition of the phosphorylated (IIO) form by a subset of human sera. J Clin Invest. 1994;94:1981–9. doi: 10.1172/JCI117550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuwana M, Okano Y, Kaburaki J, Medsger TA, Jr, Wright TM. Autoantibodies to RNA polymerases recognize multiple subunits and demonstrate cross-reactivity with RNA polymerase complexes. Arthritis Rheum. 1999;42:275–84. doi: 10.1002/1529-0131(199902)42:2<275::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Hirakata M, Kanungo J, Suwa A, Takeda Y, Craft J, Hardin JA. Autoimmunity to RNA polymerase II is focused at the carboxyl terminal domain of the large subunit. Arthritis Rheum. 1996;39:1886–91. doi: 10.1002/art.1780391115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mamula MJ, Janeway CA. Do B cells drive the diversification of immune responses? Immunol Today. 1993;14:151–2. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 45.Fanning GC, Welsh KI, Bunn C, Du Bois R, Black CM. HLA associations in three mutually exclusive autoantibody subgroups in UK systemic sclerosis patients. Br J Rheumatol. 1998;37:201–7. doi: 10.1093/rheumatology/37.2.201. [DOI] [PubMed] [Google Scholar]
- 46.Casciola-Rosen L, Wigley F, Rosen A. Scleroderma autoantigens are uniquely fragmented by metal-catalyzed oxidation reactions: implications for pathogenesis. J Exp Med. 1997;185:71–79. doi: 10.1084/jem.185.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen A, Casciola-Rosen L, Wigley F. Role of metal-catalyzed oxidation reactions in the early pathogenesis of scleroderma. Curr Opin Rheumatol. 1997;9:538–43. doi: 10.1097/00002281-199711000-00010. [DOI] [PubMed] [Google Scholar]



