Abstract
Cell accumulation and CC chemokine production were assessed in the peritoneal cavity of ovalbumin (OVA)-sensitized mice following antigen challenge. Intraperitoneal challenge with OVA induced a significant eosinophil influx from 6 h post-challenge with increased numbers persisting at 24 h. At 6 h there was also a marked presence of neutrophils. Messenger RNA expression and protein levels for the chemokines RANTES and MIP-1α were measured in the cell pellets and supernatants, respectively, from peritoneal washes following OVA challenge. RANTES mRNA was detected from 2 h to 4 h following OVA injection, whereas mRNA for MIP-1α was only detectable at 4 h. RANTES protein was first detected from 4 h after OVA injection and by 24 h the protein levels had increased further. Basal levels of MIP-1α were detected in peritoneal washes. These levels peaked at 2 h after OVA challenge and rapidly declined to basal levels by 6 h. A functional role for the chemokines was assessed using neutralizing polyclonal antibodies. Co-injection of OVA with anti-RANTES antibodies resulted in a significant inhibition of eosinophil infiltration into the cavity at 6 h and 24 h (63% and 52% inhibition, respectively) without significantly influencing the number of neutrophils present. In contrast, injection of anti-MIP-1α antibodies only inhibited neutrophil migration at the 6 h time point by 44% without significantly affecting the accumulation of eosinophils. These results demonstrate an important role for RANTES in mediating eosinophil influx in allergic inflammation and a contrasting role for MIP-1α in mediating neutrophil recruitment.
Keywords: eosinophils, neutrophils, chemokines, allergy
INTRODUCTION
Allergic inflammatory disorders are characterized by the presence of a large number of eosinophils at the site of inflammation. Eosinophils are able to release a number of inflammatory mediators which have been shown to be involved in mediating some of the symptoms associated with allergic diseases, and these cells have therefore been implicated in contributing to the pathogenesis of such disorders [1]. Migration of eosinophils from the systemic circulation to a specific tissue site involves a series of adhesive interactions which are controlled by inflammatory mediators such as chemokines, released into the surrounding milieu [2].
The chemokines are a family of over 35 small secreted proteins that mediate chemotaxis, and induce various functional changes, in subsets of leucocytes in vitro [3,4]. They are produced by a wide variety of cells, both of haematopoietic and non-haematopoietic origin, and are thought to play a critical role in the migration and activation of leucocytes in vivo. On the basis of a distinctive cysteine-containing amino acid motif present in the predicted primary amino acid sequence, the chemokines can be divided into two major subfamilies, C-X-C (α) and C-C (β), and two minor subfamilies, C (γ) and C-X3-C (δ). Members of the C-C subfamily, which presently contains the bulk of the known chemokines, all demonstrate juxtaposition of the first pair of cysteines within the cysteine signature, while thɛ C-X-C and C-X3-C subfamily members contain one or three intervening amino acids between these two cysteines, respectively. The sole member of the C subfamily is missing the first and third cysteines within the motif [3,4].
There is strong evidence which suggests that members of the C-C (or β) chemokine subfamily may be involved in allergic inflammatory conditions, including eotaxin, monocyte chemoattractant protein-3 (MCP-3), MCP-5, RANTES and MIP-1α [3]. For example, RANTES [5] and MIP-1α [6] induce human eosinophil chemotaxis in vitro and have no direct effect on neutrophils. Further, provocation with allergen has been shown to induce RANTES and MIP-1α protein production in the lungs of allergic individuals [7,8]. Several in vivo animal models have been used to investigate the importance of inflammatory mediators in controlling leucocyte trafficking in inflammation. In this respect, we have previously used the murine peritoneal cavity and air-pouch to understand the mechanisms involved in eosinophil [9,10] and neutrophil [11–15] migration in response to exogenously administered chemokines. The expression of either mRNA or protein for RANTES and MIP-1α has been demonstrated following antigen challenge in sensitized mice [16–18], but there is conflicting evidence for the involvement of these chemokines in mediating the eosinophil infiltration. Further, an indirect effect of MIP-1α on neutrophil infiltration in vivo has been demonstrated [14,15].
Due to the availability of appropriate neutralizing antisera, in the present study we have focused on the in vivo role of two C-C chemokines, namely RANTES and MIP-1α, in mediating eosinophil migration to antigen challenge in ovalbumin (OVA)-sensitized mice. Expression of both mRNA and protein was found for the chemokines following antigen challenge. However, when the functional role for the chemokines was investigated using neutralizing antisera, only neutralization of RANTES attenuated the eosinophil infiltration, while neutralization of MIP-1α inhibited neutrophil accumulation, but played no role in the recruitment of eosinophils.
MATERIALS AND METHODS
Animals
Female BALB/c mice (18–20 g in body weight; from Tuck, Raleigh, UK) were used for all experiments. Animals were housed in a 12-h light–dark cycle and allowed food and water ad libitum. On the day of experiment, the body weights had reached 22–24 g. All procedures were conducted in accordance with the Animal (Scientific Procedures) Act (1996).
OVA sensitization and challenge procedure
Mice were sensitized using a protocol previously described [19]. Briefly, animals were injected subcutaneously with 100 μg of OVA (Sigma Chemical Co, Poole, UK) adsorbed to 3.3 mg of aluminium hydroxide gel in sterile saline in a volume of 0.4 ml on days 1 and 8. On day 15, the mice were injected intraperitoneally with 0.4 ml of either vehicle (sterile saline) or 25 μg/ml OVA. At different time points post-OVA challenge, the animals were killed by CO2 asphyxiation. The peritoneal cavities were lavaged with 3 ml sterile PBS containing 10 mm EDTA (PBS–EDTA) and the lavages were spun (300 g, 10 min at 4°C). The supernatants were analysed for chemokine protein levels by ELISA (see below) and the cell pellets were used either for (i) total and differential cell counts (see below) or (ii) extraction of total RNA (see below).
RANTES injection into the peritoneal cavity
Either 0.4 ml vehicle (0.1% low endotoxin bovine serum albumin (BSA) in sterile PBS; Sigma) or 500 ng synthetic murine RANTES (generously provided by Dr I. Clark-Lewis, University of British Columbia, Vancouver, Canada [20]) were injected intraperitoneally into OVA-sensitized mice on day 15. The peritoneal cavities were lavaged at 6 h and total and differential cell counts performed (see below).
Treatment with anti-chemokine antibodies
The anti-RANTES and anti-MIP-1α polyclonal antibodies (IgG-purified) were raised in rabbits immunized with synthetic full-length or N-terminus peptides, respectively [14,15]. For in vivo administration, 200 μg of the antisera or control rabbit IgG (Sigma) were co-injected intraperitoneally with OVA into sensitized mice. Peritoneal cavities were lavaged 6 h or 24 h after injection, and total and differential cell counts performed as described below.
Quantification of peritoneal cavity cell infiltration
Total and differential leucocyte cell counts of the peritoneal cavity washes were performed as previously described [19]. Briefly, the peritoneal lavages were spun and the cell pellets were used for total cell counts (in a haemacytometer) and differential cell counts (on May–Grünwald–Giemsa-stained cytospin preparations).
Detection of chemokine mRNA by reverse transcriptase-polymerase chain reaction analysis
Messenger RNA for the chemokines RANTES and MIP-1α was analysed in peritoneal cavity cell pellets at different time points after OVA injection as previously described [21]. Total RNA was isolated using Trizol reagent (Gibco BRL, Paisley, UK) according to the manufacturer's instructions. The yield and purity of the RNA were estimated spectrophotometrically at 260 nm and 280 nm wavelength. Total RNA (3 μg) was used to generate cDNA and polymerase chain reaction (PCR) amplification reactions were performed on aliquots of the cDNA. The target primers were as follows: for murine RANTES (mRANTES): 5′-GCC-CAC-GTC-AAG-GAG-TAT-TTC-TAC-3′ and 5′-AGG-ACT-AGA-GCA-AGC-GAT-GAC-AGG-3′ (forward and reverse) which amplified a fragment of 205 base pairs in length; for murine MIP-1α (mMIP-1α): 5′-CCT-TGC-TGT-TCT-TCT-CTG-TAC-CAT-G-3′ and 5′-GCA-ATC-AGT-TCC-AGG-TCA-GTG-ATG-3′ (forward and reverse) which amplified a fragment of 255 base pairs; for murine GAPDH: 5′-ACC-ACA-GTC-CAT-GCC-ATC-AC-3′ and 5′-TCC-ACC-ACC-CTG-TTG-CTG-TA-3′ (forward and reverse) which amplified a product of 452 base pairs. All PCR reactions were performed in a final volume of 25 μl. For mRANTES, the PCR profile consisted of an initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C (45 s), annealing at 55°C (45 s) and extension at 72°C (30 s). Similar conditions were used for mMIP-1α, except the initial denaturation was followed by 35 cycles of denaturing at 94°C (45 s) and annealing at 56°C and for GAPDH the annealing was performed at 60°C (45 s). Amplification products were visualized by ethidium bromide fluorescence in agarose gels. Bands of expected sizes were obtained. Images were inverted using the Graphic Converter software (version 2.1) running on a Macintosh Performa 6200.
Quantification of chemokine protein levels by ELISA
The antibodies used for the RANTES ELISA assay were polyclonal antibodies (IgG-purified) raised in sheep (capture antibody) or rabbits (detection antibody). High-binding 96-well microtitre plates (Costar, Cambridge, MA) were coated with 100 μl of anti-RANTES capture antibody (diluted in 0.1 m NaHCO3, pH 8.3) and incubated at 4°C overnight. Plates were washed twice with PBS–Tween (0.2% polyoxyethylene-sorbitan monolaurate; Sigma) and blocked with 200 μl of PBS–3% BSA for 1 h at 37°C. Plates were washed twice as before, RANTES standard or sample added at 100 μl/well, and incubated for 90 min at 37°C. Plates were washed twice with PBS–Tween and incubated with the anti-RANTES detection antibody for 90 min at 37°C, then washed twice with PBS–Tween. Biotin-conjugated anti-rabbit F(ab)2 (Amersham, Aylesbury, UK; diluted 1:10 000 in PBS–3% BSA) was then added at 100 μl/well and incubated for 45 min at room temperature. Plates were washed twice in PBS–Tween, and 100 μl of streptavidin-horseradish peroxidase conjugate (1:3000 dilution in PBS–3% BSA) added per well. Plates were incubated for 30 min at room temperature, then washed four times in PBS–Tween. Peroxidase reactions were developed by the addition of 200 μl/well Fast-OPD substrate (Sigma), and the reaction terminated by the addition of 50 μl of 3 m HCl. Absorbance was determined at 485 nm on a Biolumin 96-well plate reader, using Xperiment software. The MIP-1α ELISA was performed as previously described [14,15].
Statistical analysis
All results are shown as the mean ± s.e.m. of n mice per group. Statistical differences were analysed using non-parametric tests on raw data. The Mann–Whitney U-test was used to analyse differences between two groups and the Kruskal–Wallis test was used if more than two groups were analysed.
RESULTS
Characterization of leucocyte influx into the peritoneal cavity after OVA challenge
Cell numbers from peritoneal washes of OVA-sensitized mice were quantified at 6 h and 24 h following i.p. injection of either vehicle or antigen. There were no significant differences observed in either the total number of cells (Fig. 1a) or mononuclear cells (Fig. 1b) at either time point. However, i.p. injection of OVA initiated a significant influx of granulocytes (neutrophils and eosinophils) into the cavity when compared with vehicle-injected mice. There was approximately a five-fold increase in granulocyte numbers in the peritoneal cavity when compared with saline-injected mice at 6 h and the numbers remained elevated at 24 h (Fig. 1c). Next, the time course of neutrophil and eosinophil infiltration was studied over a 24-h period (Fig. 2). Significant numbers of neutrophils and eosinophils were present in the peritoneal cavity from 4 h and 6 h post-challenge, respectively. The neutrophil numbers returned to baseline levels by 24 h but there was a further increase in eosinophils at this time point.
Fig. 1.
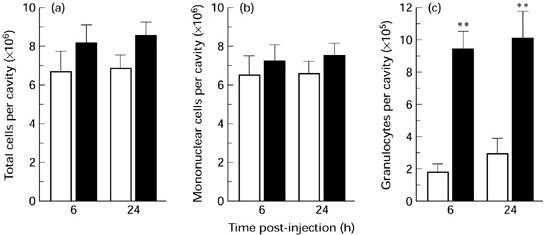
Leucocyte infiltration into the peritoneal cavity following challenge with ovalbumin (OVA). (a–c) The number of total cells, mononuclear cells and granulocytes, respectively, at 6 h and 24 h following i.p. injection of either saline (□; 0.4 ml) or OVA (▪; 10 μg) into OVA-sensitized mice. Data are expressed as the mean ± s.e.m. of six to eight mice. **P < 0.01 compared with saline controls.
Fig. 2.
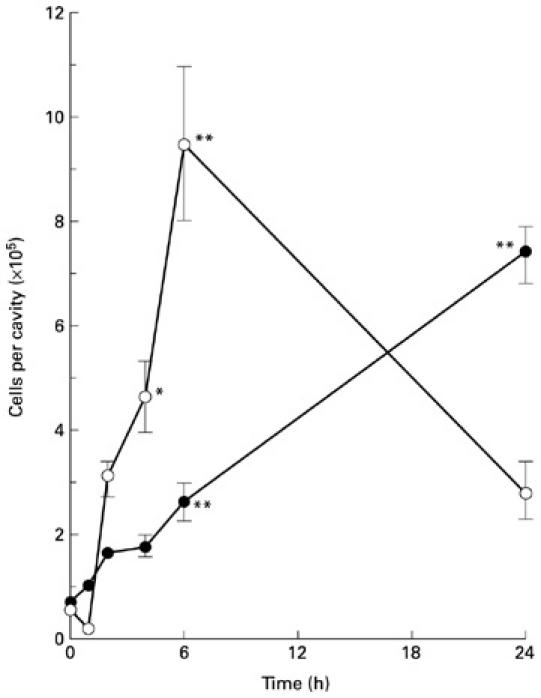
Time course of eosinophil and neutrophil accumulation into the peritoneal cavity following challenge with ovalbumin (OVA). Eosinophil (•) and neutrophil (○) numbers were quantified at different time points following i.p. challenge with OVA (0 h represents non-injected mice). Data are mean ± s.e.m. of 9–11 mice. *P < 0.05; **P < 0.01 compared with non-injected controls.
Time course of chemokine mRNA expression and protein secretion
Secreted protein levels and expression of mRNA in the supernatants and cell pellets, respectively, of peritoneal cavity lavages were quantified for RANTES and MIP-1α at different time points after antigen challenge. RANTES protein was not detected in the supernatants of peritoneal cavity lavages of control (non-injected), OVA-sensitized mice (Fig. 3a). Protein levels were significantly increased from 4 h after antigen injection, and levels continued to increase for up to 24 h (Fig. 3a). No mRNA expression for RANTES in the cell pellets was found under basal conditions (i.e. OVA-sensitized mice injected with vehicle; Fig. 3b). Increased levels of mRANTES mRNA were observed from as early as 2 h after OVA injection with levels persisting for up to 4 h. In contrast, MIP-1α immunoreactivity was present in the lavage fluid of non-injected mice (Fig. 4a). The level of MIP-1α protein increased rapidly after OVA injection, peaking at between 1 h and 2 h post-challenge, and returned to baseline levels within 6 h. In contrast, mRNA for mMIP-1α was only detected at the 4-h time point (Fig. 4b).
Fig. 3.
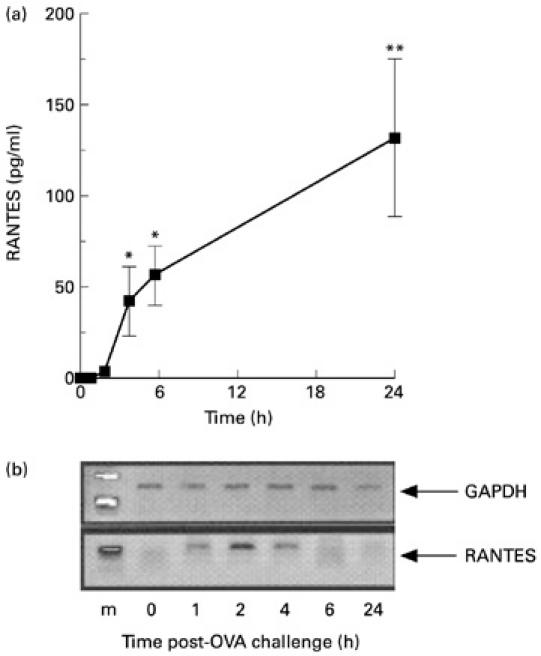
Time course of RANTES protein and gene expression following challenge with ovalbumin (OVA). (a,b) ELISA values and reverse transcriptase-polymerase chain reaction (RT-PCR) products in the supernatants of lavage fluids and cell pellets, respectively. Samples were generated at the reported time points following i.p. challenge with OVA. Data in (a) are mean ± s.e.m. of four to six mice per group. *P < 0.05; **P < 0.01 compared with non-injected mice at 0 h. (b) Lane 1 shows the markers (m), lanes 2–6 show chemokine expression at 1, 2, 4, 6 and 24 h post-OVA injection, respectively. Amplification of GAPDH served as a control for the preparation of the cDNA and to provide an indication of the relative levels of cDNA loaded in each lane. Representative data from two separate experiments (three mice per experiment) are shown.
Fig. 4.
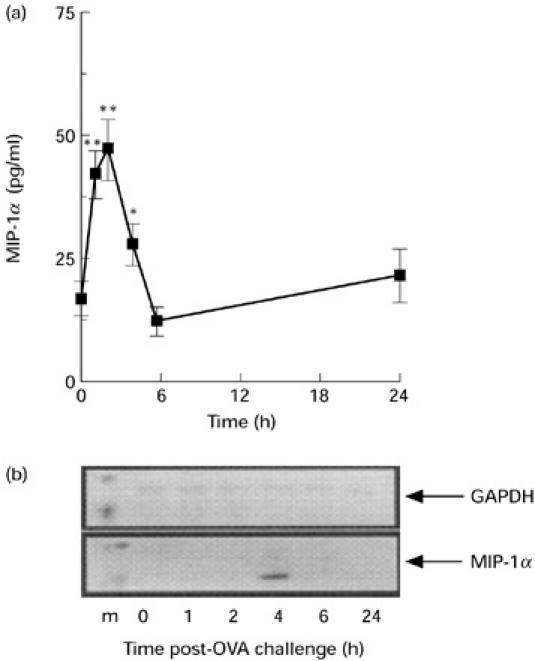
Time course of MIP-1α protein and gene expression following challenge with ovalbumin (OVA). (a) ELISA values and (b) reverse transcriptase-polymerase chain reaction (RT-PCR) products in the supernatants of lavage fluids and cell pellets, respectively. Samples were generated at the reported time points following i.p. challenge with OVA. (a) Data are mean ± s.e.m. of four to six mice per group. *P < 0.05; **P < 0.01 compared with non-njected mice at 0 h. (b) Lane 1 shows the markers (m), lanes 2–6 show chemokine expression at 1, 2, 4, 6 and 24 h post-OVA injection, respectively. Amplification of GAPDH served as a control for the preparation of the cDNA and to provide an indication of the relative levels of cDNA loaded in each lane. Representative data from two separate experiments (three mice per experiment) are shown.
The role of endogenous chemokines in eosinophil infiltration after OVA challenge
To investigate the in vivo role of RANTES and MIP-1α in eosinophil infiltration in response to OVA challenge, OVA-sensitized mice were injected intraperitoneally with either neutralizing anti-chemokine antibodies (IgG-purified) or control IgG together with OVA and cellular infiltration was quantified 6 h and 24 h later. At the 6-h time point, control IgG co-injected with OVA did not modify eosinophil accumulation into the peritoneal cavity (compare fig. 2 with fig. 5a). injection with anti-mip-1[agr] antibodies did not significantly affect the eosinophil infiltration. however, in the group of mice treated with the anti-rantes antibodies, a significant (> 60%) reduction in the number of eosinophils was observed. the effect of the antibodies on the neutrophil influx at 6 h post-challenge was also analysed (Fig. 5b). Treatment with anti-MIP-1α, but not anti-RANTES, antibodies reduced the neutrophil migration by almost 70%. At the 24-h time point there was a marked eosinophil influx in control/IgG-treated mice (Fig. 5c). As observed at 6 h post-challenge, co-treatment with anti-MIP-1α antibodies did not modulate the accumulation of eosinophils in response to OVA challenge. However, when anti-RANTES antibodies were co-administered with OVA, there was a significant (approx. 50%) inhibition of the eosinophil infiltration.
Fig. 5.
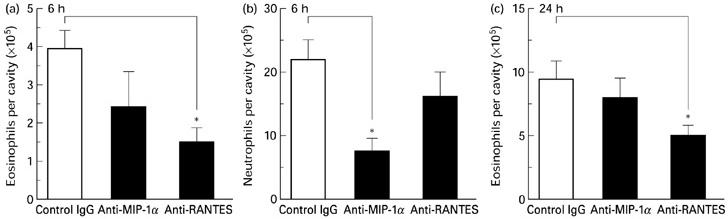
Effect of neutralization of mRANTES and mMIP-1α on ovalbumin (OVA)-induced eosinophil and neutrophil infiltration. Mice were injected intraperitoneally with 200 μg of either anti-RANTES, anti-MIP-1α or rabbit IgG (control), together with OVA. The number of eosinophils (a) and neutrophils (b) were quantified at 6 h post-challenge, and the number of eosinophils was also quantified at 24 h post-challenge (c). The data are expressed as the mean ± s.e.m. of 9–11 mice. *P < 0.05 compared with control IgG/OVA-treated mice.
Effect of exogenous RANTES
The effect of administration of murine RANTES in OVA-sensitized mice was then investigated. Injection of 500 ng of RANTES into the peritoneal cavity did not induce any detectable infiltration of leucocytes when compared with mice receiving vehicle injections. The total number of cells in the peritoneal cavity were 6.1 ± 0.5 × 106 (mean ± s.e.m.; n = 6) in mice injected with vehicle and 7.04 ± 0.7 × 106 (n = 7) in mice injected with RANTES. The number of eosinophils in mice injected with vehicle and RANTES were 2.2 ± 0.5 × 105 and 1.9 ± 0.3 × 105, respectively. The number of neutrophils and mononuclear cells were 4.3 ± 1.3 × 105 and 5.4 ± 0.5 × 106, respectively, in mice injected with vehicle, and the numbers in mice injected with RANTES were 6.0 ± 1.7 × 105 and 6.1 ± 0.6 × 106, respectively.
DISCUSSION
Significant in vitro data collected over the last decade suggest that members of the chemokine gene superfamily are likely to play a critical role in regulating inflammatory reactions and immune responses [3,4,22]. These in vitro data are now being supported in some cases by the results of studies in vivo [14,15,18,23–25]. However, significantly more work is required to understand the specific roles for these molecules in different inflammatory conditions in vivo. In the present study we have investigated the role of two CC chemokines, namely RANTES and MIP-1α, in leucocyte recruitment in a model of allergic inflammation. The results of this study demonstrate an important role for RANTES in mediating the influx of eosinophils and for MIP-1α in neutrophil migration after antigen challenge in the mouse.
The sensitization/challenge protocol used in the present study has been validated previously. This procedure induces a blood and bone marrow eosinophilia [9,19] and, following OVA challenge in subcutaneous air-pouches, a marked eosinophil infiltrate is observed at the site of antigen injection [19]. Further, injection of eotaxin into the peritoneal cavity, but not subcutaneous air-pouches, of sensitized mice leads to an eosinophil infiltration [9,10]. In the present study, we have extended these previous findings by investigating the role of endogenous chemokines in the generation of allergic inflammation in vivo.
OVA challenge in the peritoneal cavity of OVA-sensitized mice elicited an inflammatory cell infiltrate that was characterized by the presence of neutrophils and eosinophils. The number of neutrophils was increased from as early as 4 h after antigen challenge but subsided to the baseline level by 24 h post-challenge. In contrast, significant eosinophil infiltration was observed within 6 h post-challenge, with the eosinophilia increasing up to 24 h. No significant alterations in the number of mononuclear cells were observed at any of the time points assessed. This profile of leucocyte influx into the peritoneal cavity in response to OVA challenge is similar to that reported previously [26].
Expression of the CC chemokines RANTES and MIP-1α in response to antigen challenge was assessed at the level of both mRNA and protein. RANTES mRNA was observed from as early as 1 h following OVA injection, with levels remaining elevated for up to 4 h post-challenge. The expression of RANTES mRNA correlated well with the time course of RANTES production as measured by ELISA. RANTES protein was only detectable from 4 h post-challenge, with significant levels persisting for up to 24 h. A similar time course of production of RANTES protein has been observed in a murine model of antigen-induced pulmonary inflammation [17]. However, a different mRNA profile for RANTES has been reported in the murine lung [16]. In the latter study, constitutive expression of RANTES mRNA was observed with no changes after antigen challenge. The discrepancies in these data could be due to differences in sensitization and challenge protocols, or to the differing abilities of resident lung and peritoneal cavity cells (e.g. macrophages) to synthesize chemokines [27]. However, it must be noted that in the present study significant levels of RANTES protein were still measurable in the lavage fluid hours after mRNA for the protein in the cell pellets was no longer detectable. This indicates that cells or tissues other than those present in the cells obtained by peritoneal lavages may be synthesizing RANTES at later time points, or that secretion of RANTES protein continues for some time after mRNA levels have returned to baseline.
Neutralization of RANTES by administration of specific antibodies demonstrated an important role for RANTES in mediating both the early and late (6 h and 24 h) eosinophil influx following OVA challenge. A number of studies have demonstrated a correlation between RANTES protein levels and eosinophil infiltration following antigen challenge in mice [17] and humans [7,28]. However, in the present study, i.p. injection of RANTES failed to induce any significant eosinophil migration into the peritoneal cavity. The 6-h time point was chosen for this experiment, as our previous studies have demonstrated the ability of eotaxin [9] and MCP-1 [13] to induce eosinophil and monocyte influx, respectively, within this time frame. While intradermal injection of RANTES has been shown to induce eosinophil-rich lesions in dogs [29] and humans [30], direct injection of certain C-C chemokines into either murine subcutaneous air-pouches [14] or skin [31] does not induce leucocyte accumulation. Potential differences in CCR3 expression between species may be at the basis of this discrepancy. Alternatively, RANTES may contribute to eosinophil attraction during an allergic reaction by acting in concert with other cofactor(s) or mediator(s) which are obviously absent when exogenous RANTES is injected alone, without challenge with OVA. This is not unique to RANTES, since an identical example is provided by MCP-1 with respect to accumulation of mouse neutrophils in experimental systems [13,14,21].
MIP-1α mRNA expression was observed only at 4 h post-challenge. In contrast, MIP-1α protein was detected in the peritoneal washes of sensitized but unchallenged mice, and the level of MIP-1α protein increased from 1 h after OVA injection, peaked at 2 h, after which protein levels returned to those observed in non-injected mice. We have detected similar levels of MIP-1α protein under basal conditions in murine air-pouches [14,15]. Similar time courses of MIP-1α mRNA [16] and protein levels [17] have been reported in the lungs of sensitized and challenged mice. The presence of MIP-1α protein in the cavity washes prior to antigen challenge in the absence of mRNA in the cellular exudate is interesting, and suggests that resident cavity cells which are present in the washes prior to antigen challenge, such as macrophages and mast cells [10], do not express mRNA for the chemokine under basal conditions. It is also possible that MIP-1α protein is stored preformed in some resident cells. These cells are very likely to be the mast cells. In fact, there is evidence that a human mast cell leukaemia line can express multiple chemokine genes [32] and that IL-8 is then stored in granules and released upon activation [33]. We have similar indications for the murine chemokines KC and MCP-1, where mast cell depletion is associated with a reduced release of these two chemokines during a murine experimental peritonitis [34]. Alternatively, cells present in the cavity lining, such as mesothelial cells and fibroblasts, may also be the initial source of the protein. Neutrophils, which have been shown to express MIP-1α [35,36], may be responsible for the increase in mRNA detected in the exudate population at 4 h post-challenge. However, these cells may not release sufficient quantities of MIP-1α protein to maintain the high levels observed at 2–4 h post-challenge. Of relevance, stimulation of neutrophils by phagocytosis is required for maximal secretion of chemokines [35,36].
Surprisingly, MIP-1α was not found to be involved in mediating eosinophil migration at 6 h or 24 h, even though MIP-1α has been shown to recruit murine eosinophils when injected into skin sites [31,37] and in murine lungs after antigen challenge [17]. However, in the present study, a role for MIP-1α in neutrophil recruitment was observed. Previously, it has been shown that injection of MIP-1α into the footpads of mice induces a marked neutrophil infiltration [38] that is dependent on resident mast cells. Although in vitro MIP-1α has been shown to have chemotactic properties for monocytes [39], eosinophils [6] and lymphocytes [38], but not neutrophils, this chemokine might induce the generation of other neutrophil-specific chemotactic mediators in vivo, by a similar mechanism observed for the recruitment of lymphocytes induced by IL-8 [40]. Or, more likely, there is a species specificity for the biological actions ascribed to MIP-1α: since murine neutrophils express CCR1, it is not surprising that these cells respond to this chemokine both in vitro and in vivo [41]. In line with this, pretreatment of mice with neutralizing antibodies directed against MIP-1α significantly inhibits neutrophil recruitment in response to tumour necrosis factor-alpha (TNF-α) and superantigens in acute subcutaneous inflammation, even though direct injection of MIP-1α is without effect on neutrophil recruitment [14,15]. Taken together, these observations suggest an indirect, but critical role for MIP-1α in neutrophil recruitment in vivo.
In conclusion, the present study has demonstrated a functional relationship between RANTES expression and eosinophil accumulation, and MIP-1α expression and neutrophil recruitment, in an experimental model of allergic inflammation. In view of the central role that the chemokine RANTES plays in allergic inflammatory conditions, this model may be important for developing potential therapeutic targets directed against chemokines for effective control of human eosinophilia.
Acknowledgments
This work was supported by the Wellcome Trust and the National Health and Medical Research Council of Australia. M.N.A. is supported by a grant from the Joint Research Board of the St Bartholomew's Trust, R.J.F. is a Principal Research Fellow of the Wellcome Trust, M.P. is a Post-doctoral Fellow of the Arthritis Research Campaign (UK).
REFERENCES
- 1.Martin LB, Kita H, Leiferman KM, Gleich GJ. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int Arch Allergy Immunol. 1996;109:207–15. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira MM, Williams TJ, Hellewell PG. Mechanisms and pharmacological manipulation of eosinophil accumulation in vivo. Trends Pharmacol Sci. 1995;16:418–23. doi: 10.1016/s0165-6147(00)89092-6. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 4.Gale LM, McColl SR. Chemokines; extracellular messengers for all occasions? Bioessays. doi: 10.1002/(SICI)1521-1878(199901)21:1<17::AID-BIES3>3.0.CO;2-4. in press. [DOI] [PubMed] [Google Scholar]
- 5.Ebisawa M, Yamada T, Bickel C, Klunk D, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. III. Effect of the chemokine RANTES. J Immunol. 1994;153:2153–60. [PubMed] [Google Scholar]
- 6.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1α induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–95. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teran LM, Noso N, Carroll M, Davies DE, Holgate S, Schroder J-M. Eosinophil recruitment following allergen challenge is associated with the release of the chemokine RANTES into asthmatic airways. J Immunol. 1996;157:1806–12. [PubMed] [Google Scholar]
- 8.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1α, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–83. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 9.Das AM, Flower RJ, Perretti M. Eotaxin-induced eosinophil migration in the peritoneal cavity of ovalbumin-sensitized mice. Mechanism of action. J Immunol. 1997;159:1466–73. [PubMed] [Google Scholar]
- 10.Das AM, Flower RJ, Perretti M. Resident mast cells are important for eotaxin-induced eosinophil accumulation in vivo. J Leukocyte Biol. 1998;64:156–62. doi: 10.1002/jlb.64.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Perretti M, Harris JG, Flower RJ. A role for endogenous histamine in interleukin-8-induced neutrophil infiltration into mouse air-pouch: investigation of the modulatory action of systemic and local dexamethasone. Br J Pharmacol. 1994;112:801–8. doi: 10.1111/j.1476-5381.1994.tb13150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris JG, Flower RJ, Watanabe K, Tsurufuji S, Wolitzky BA, Perretti M. Relative contribution of the selectins in the neutrophil recruitment caused by the chemokine cytokine-induced neutrophil chemoattractant (CINC) Biochem Biophys Res Commun. 1996;221:692–6. doi: 10.1006/bbrc.1996.0658. [DOI] [PubMed] [Google Scholar]
- 13.Ajuebor MN, Flower RJ, Hannon R, et al. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63:108–16. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo. Involvement of CXC and CC chemokines in neutrophil extravasation in vivo in response to TNF-α. J Immunol. 1997;159:3595–602. [PubMed] [Google Scholar]
- 15.Tessier PA, Naccache PH, Diener KR, et al. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1 and TNF-α. J Immunol. 1998;161:1202–11. [PubMed] [Google Scholar]
- 16.MacLean JA, Ownbey R, Luster AD. T cell-dependent regulation of eotaxin in antigen-induced pulmonary eosinophilia. J Exp Med. 1996;184:1461–9. doi: 10.1084/jem.184.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alterations of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–404. [PubMed] [Google Scholar]
- 18.Gonzalo J-A, Lloyd CM, Wen D, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das AM, Flower RJ, Hellewell PG, Teixeira MM, Perretti M. A novel murine model of allergic inflammation to study the effect of dexamethasone on eosinophil recruitment. Br J Pharmacol. 1997;121:105–17. doi: 10.1038/sj.bjp.0701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark-Lewis I, Dewald B, Loetscher M, Moser B, Baggiolini M. Structural requirements for interleukin-8 function identified by design of analogs and CXC chemokine hybrids. J Biol Chem. 1994;269:16075–81. [PubMed] [Google Scholar]
- 21.Ajuebor MN, Gibbs L, Flower RJ, Das AM, Perretti M. Investigation of the functional role played by the chemokine monocyte chemoattractant protein-1 in interleukin-1-induced murine peritonitis. Br J Pharmacol. 1998;125:319–26. doi: 10.1038/sj.bjp.0702071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. New Engl J Med. 1998;338:346–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 23.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–10. [PubMed] [Google Scholar]
- 24.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 25.Rand ML, Warren JS, Mansour MK, Newman W, Ringler DJ. Inhibition of T cell recruitment and cutaneous delayed-type hypersensitivity-induced inflammation with antibodies to monocyte chemoattractant protein-1. Am J Pathol. 1996;148:855–64. [PMC free article] [PubMed] [Google Scholar]
- 26.Zuany-Amorim C, Leduc D, Vargaftig BB, Pretolani M. Characterisation and pharmacological modulation of antigen-induced peritonitis in actively sensitised mice. Br J Pharmacol. 1993;110:917–24. doi: 10.1111/j.1476-5381.1993.tb13900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Otteren GM, Standiford TJ, Kunkel SL, et al. Expression and regulation of macrophage inflammatory protein-1α by murine alveolar and peritoneal macrophages. Am J Respir Cell Mol Biol. 1994;10:8–15. doi: 10.1165/ajrcmb.10.1.8292385. [DOI] [PubMed] [Google Scholar]
- 28.Venge J, Lampinen M, Hakansson L, Rak S, Venge P. Identification of IL-5 and RANTES as the major eosinophil chemoattractants in the asthmatic lung. J Allergy Clin Immunol. 1996;97:1110–5. doi: 10.1016/s0091-6749(96)70265-8. [DOI] [PubMed] [Google Scholar]
- 29.Meurer R, Van Riper G, Feeney W, et al. Formation of eosinophilic and monocytic intradermal inflammatory sites in the dog by injection of human RANTES but not human monocyte chemoattractant protein 1, human macrophage inflammatory protein 1α, or human interleukin 8. J Exp Med. 1993;178:1913–21. doi: 10.1084/jem.178.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck LA, Dalke S, Eiferman KM, et al. Cutaneous injection of RANTES causes eosinophil recruitment. Comparison of nonallergic and allergic human subjects. J Immunol. 1997;159:22962–72. [PubMed] [Google Scholar]
- 31.Teixeira MM, Wells TNC, Lukacs NW, et al. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an allergy model in mouse skin. J Clin Invest. 1997;100:1657–66. doi: 10.1172/JCI119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvan RS, Butterfiled JH, Krangel MS. Expression of multiple chemokine genes by a human mast cell leukemia. J Biol Chem. 1994;269:13893–8. [PubMed] [Google Scholar]
- 33.Möller A, Lippert U, Lessmann D, et al. Human mast cells produce IL-8. J Immunol. 1993;151:3261–6. [PubMed] [Google Scholar]
- 34.Ajuebor MN, Das AM, Virág L, et al. Role of peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–91. [PubMed] [Google Scholar]
- 35.Hachicha M, Naccache PH, McColl SR. Inflammatory microcrystals differentially regulate the secretion of macrophage inflammatory protein 1 and interleukin 8 by human neutrophils: a possible mechanism for neutrophil recruitment to sites of inflammation in synovitis. J Exp Med. 1995;182:2019–25. doi: 10.1084/jem.182.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hachicha M, Rathanaswami P, Naccache PH, McColl SR. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J Immunol. 1997;160:449–54. [PubMed] [Google Scholar]
- 37.Teixeira MM, Das AM, Miotla JM, Perretti M, Hellewell PG. The role of lipocortin-1 in the inhibitory action of dexamethasone on eosinophil trafficking in cutaneous inflammatory reactions in the mouse. Br J Pharmacol. 1998;123:538–44. doi: 10.1038/sj.bjp.0701625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam R, Kumar D, Anderson-Walters D, Forsythe PA. Macrophage inflammatory protein-1α and monocyte chemoattractant peptide-1 elicit immediate and late cutaneous reactions and activate murine mast cells in vivo. J Immunol. 1994;152:1298–303. [PubMed] [Google Scholar]
- 39.Schall TJ, Bacon K, Camp RDR, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein α (MIP-1α) and MIP-1β chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taub DD, Anver M, Oppenheim JJ, Longo DL, Murphy WJ. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. 1996;97:1931–41. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao JL, Wynn TA, Chang Y, et al. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1–type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–68. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]


