Abstract
To investigate the regulation of Fcγ receptor (FcγR) expression on circulating phagocytes in Kawasaki disease (KD), we analysed the expressions of FcγRI, II and III on neutrophils and monocytes in 20 patients with KD, 10 with a bacterial infection (BI), 10 with a viral infection (VI), and 10 healthy controls (HC) using flow cytometric analysis. The KD patients had a significantly higher level of FcγRI expression on neutrophils, but not on monocytes, than the BI, VI and HC patients. FcγRII expression on neutrophils was significantly higher in KD, BI and VI than HC, but there was no significant difference in FcγRII expression among KD, BI and VI. FcγRIII expression on neutrophils in KD was significantly lower than in VI and HC, but was higher on monocytes. A kinetic analysis of FcγR expression in KD demonstrated the expression of FcγRI and II on neutrophils to decline, but no remarkable change was observed in the monocytes, from the subacute phase through the convalescent phase. In addition, FcγRIII expression on neutrophils increased, while FcγRIII expression on monocytes decreased during the time course of KD. FcγR expression in the acute phase of KD is thus characterized by markedly increased expression of FcγRI on neutrophils, followed by a subsequent decrease, and decreased expression of FcγRIII on neutrophils and increased expression of FcγRIII on monocytes followed by a reverse kinetics during the clinical course. These findings are thus considered to reflect the functional up-regulation of neutrophils and monocytes in KD.
Keywords: Kawasaki disease, Fcγ receptor, monocytes, neutrophils
INTRODUCTION
Phagocytes (neutrophils and monocytes/macrophages) play an important role in the recognition and elimination of pathogens which invade the human body. After an opsonin such as IgG attaches to microorganisms and their particles in the serum, they can be recognized by the receptors binding to the Fc domain of IgG (FcγR) on their surfaces. A heterogeneous group of receptors functions as a key molecule for phagocytosis, endocytosis, the clearance of immune complexes, antibody-dependent cell-mediated cytotoxicity (ADCC), and triggering of the release of inflammatory mediators [1,2]. Three distinct classes of FcγR have been reported on human leucocytes [1,2]. FcγRI (CD64) is expressed constitutively and primarily on monocytes, and cytokines such as interferon-gamma (IFN-γ) and granulocyte colony-stimulating factor (G-CSF) induce an expression of FcγRI on neutrophils and also induce an increase in FcγRI expression on monocytes [3,4]. FcγRII (CD32) is widely found on blood leucocytes, including neutrophils and monocytes [1,2], and its expression is not consistently modified by cytokines [5]. FcγRIII (CD16) is expressed abundantly on neutrophils, but less densely on monocytes [1,2]. FcγRIIIa, which is expressed on the subpopulation of monocytes, is a transmembrane protein, while FcγRIIIb, expressed on neutrophils and eosinophils, is linked to the outer leaflet of the plasma membrane via a glycosylphosphatidylinositol (GPI) anchor. IFN-γ and G-CSF cause a decrease in FcγRIIIb expression on neutrophils [5], mainly due to receptor shedding [6]. Although multiple biological functions can be triggered via these three FcγRs, little has so far been elucidated on their exact functional role.
Kawasaki disease (KD) is an acute type of systemic vasculitis in children [7]. Immunological abnormalities during the acute phase of KD are characterized by marked activation of the immune system, including the functional activation of neutrophils and monocytes and their excess production in such inflammatory mediators as cytokines (IL-1, IFN-γ and tumour necrosis factor-alpha (TNF-α)), proteases (neutrophil elastase and myeloperoxidase) and toxic oxygen radicals [8–11]. Although a strong response via FcγR on phagocytes is expected in the acute phase of KD, there has so far been no report on the up- or down-regulation of FcγR expression on their surfaces in this disease. The aims of the present study were to investigate the expression of FcγRI, II and III on circulating neutrophils and monocytes in patients with KD, patients with other diseases (bacterial or viral infection), and healthy children, and to investigate the kinetics of FcγR expression during the clinical course of KD.
PATIENTS AND METHODS
Patients and sample preparations
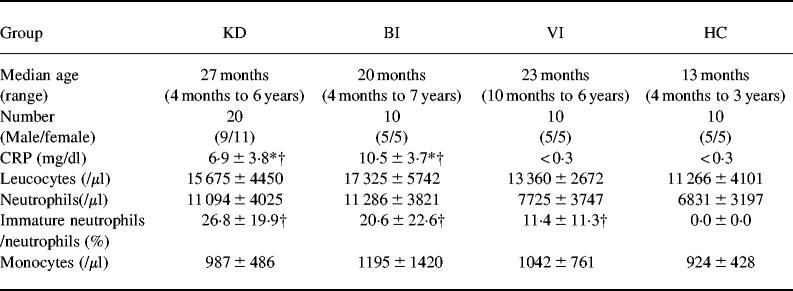
The patients and healthy controls enrolled in this study were classified into four groups consisting of patients with KD, bacterial infection (BI), viral infection (VI), and healthy controls (HC). The patient profiles and laboratory findings are shown in Table 1. The patients were hospitalized at the National Defence Medical College Hospital between March 1996 and July 1998. Twenty KD patients were enrolled within 7 days of the onset of illness, with day 1 defined as the first day of fever, and all 20 met the diagnostic criteria for KD established by the Japanese Kawasaki Disease Research Committee [12]. No bacterial species were identified in blood cultures from the KD patients. All patients were scheduled to receive both aspirin (30 mg/kg per day) and intravenous immunoglobulin (IVIG, 1 g/kg or 2 g/kg). No patients had a coronary aneurysm. Serial blood samples were obtained from all KD patients in the acute phase (pre-IVIG, before IVIG therapy on days 3–7; post-IVIG, within 24 h after completing IVIG therapy on days 8–12), in the afebrile subacute phase (days 15–20), and in the convalescent phase (days 21–30), when the C-reactive protein (CRP) of each patient was < 0.3 mg/dl. The BI group included 10 children: five microbiologically documented and five clinically documented. Four had a urinary tract infection with Escherichia coli, one had meningitis and sepsis with Haemophilus influenzae. These organisms were isolated from urine, spinal fluid and blood cultures. Five patients had pneumonia with high CRP levels of > 5.0 mg/dl, although the causative microorganisms were not identified. The VI group included 10 patients, consisting of four with an upper respiratory infection, three with bronchitis, and one each with enterocolitis, aseptic meningitis or croup. Although a viral culture was not carried out, the CRP levels were < 0.3 mg/dl in all patients in this group. The HC group consisted of 10 healthy children serving as controls. They neither had any underlying diseases nor were receiving any medications. Informed consent was obtained from the parents of all children. Peripheral blood neutrophils and mononuclear cells were immediately isolated by density gradient centrifugation using a Mono-Poly Resolving Medium (ICN Biochemicals, Costa Mesa, CA) and washed with PBS containing 1% bovine serum albumin (BSA).
Table 1.
Patient characteristics and laboratory findings
KD, Kawasaki disease; BI, bacterial infection; VI, viral infection; HC, healthy controls; CRP, C-reactive protein. *P < 0.05 versus VI; †P < 0.05 versus HC.
Monoclonal antibody and reagents
The FITC-conjugated anti-FcγRI MoAb (clone 22), PE-conjugated anti-FcγRII MoAb (clone 2E1) and PE-conjugated anti-FcγRIII MoAb (clone 3G8) were purchased from Immunotech (Marseille, France). FITC- or PE-conjugated isotype-matched control MoAbs (IgG1 and IgG2a) were purchased from Dako (Glostrup, Denmark).
Staining procedure and flow cytometric analysis
The cells (5 × 105/ml) were suspended in PBS containing 1% BSA and incubated with FcγRI, FcγRII, FcγRIII or isotype MoAb at 4°C for 30 min. All samples were analysed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). After setting the gates around the neutrophil and monocyte populations, the data were obtained using CellQuest software (Becton Dickinson). After the fluorescence intensity in > 95% of cells stained with isotype MoAb was set at less than 10 arbitrary units, the mean fluorescence intensity (MFI) of each type of FcγR was estimated.
Statistical analysis
All data are expressed as mean ± s.d. Differences in the MFI levels of neutrophils and monocytes between the acute and convalescent phases in the same group were assessed by the Wilcoxon signed rank test. Intergroup differences were analysed by the Mann–Whitney test. P < 0.05 was considered significant.
RESULTS
FcγRI, II, III expression on neutrophils and monocytes
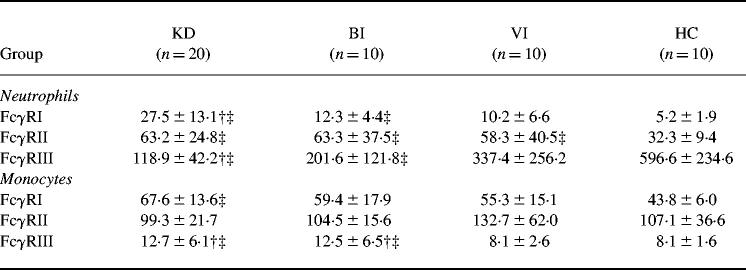
KD patients had a significantly higher MFI of FcγRI expression on the neutrophils than the patients with BI, VI and HC (Table 2). The MFI of FcγRI expression on the monocytes in KD, but not in BI and VI, were significantly higher than that in HC. Although the MFI of FcγRII on the neutrophils were significantly higher in KD, BI and VI than in HC, there was no significant difference in FcγRII expression on either neutrophils or monocytes among KD, BI and VI. The MFI of FcγRIII expression on neutrophils in KD was significantly lower than in VI and HC, and that on the neutrophils in BI was significantly lower than only in HC. On the other hand, the MFI of FcγRIII expression on monocytes was significantly higher in KD and BI than in VI and HC.
Table 2.
FcγR expression on neutrophils and monocytes
KD, Kawasaki disease; BI, bacterial infection; VI, viral infection; HC, healthy controls. *P < 0.05 versus BI; †P < 0.05 versus VI; ‡P < 0.05 versus HC.
Kinetic analysis of FcγRI, II and III expression on neutrophils and monocytes in the clinical course of KD
Figure 1 demonstrates the time course of each FcγR expression on neutrophils and monocytes in KD. FcγRI and FcγRII expression on neutrophils decreased from the subacute though the convalescent phase. On the other hand, FcγRI and FcγRII expression on monocytes did not show any significant change. FcγRIII expression on neutrophils increased from the subacute phase through the convalescent phase, while FcγRIII expression on monocytes declined during the time course of KD.
Fig. 1.
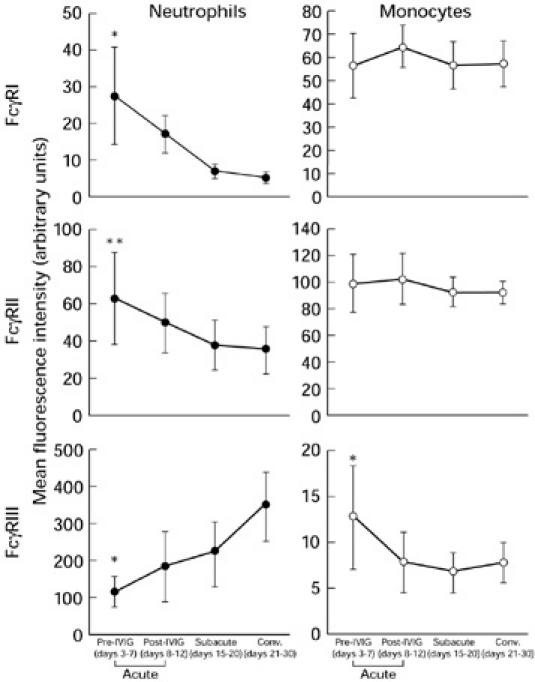
Serial changes of FcγRI, II and III expressions on neutrophils and monocytes in Kawasaki disease (KD). *P < 0.05 versus post-IVIG, subacute phase, and convalescent (conv.) phase; **P < 0.05 versus subacute phase and conv. phase.
DISCUSSION
In the present study, we demonstrate that FcγRI expression on neutrophils was significantly higher in the acute phase of KD than in BI, VI and HC, and that FcγRIII expression on neutrophils in KD was significantly lower, but higher on monocytes, than in VI and HC. Furthermore, in the clinical course of KD FcγRI and II expression on neutrophils and FcγRIII expression on monocytes in the acute phase declined from the subacute phase through the convalescent phase, whereas FcγRIII expression on neutrophils showed an increase during the time course.
Neutrophils and monocytes/macrophages play an important role in the host defence against infection due to microorganisms. Once IgG or immune complexes bind to the FcγR on their surfaces in circulation, biological responses via the receptor are triggered, thus contributing to the activation of the immune system [1,2]. The cause of KD is still unknown, but the acute phase of KD is characterized by marked activation of the immune system [8–11]. According to our expectations, FcγR expression showed dynamic changes for KD in the present study. The expression patterns in KD also mimicked those in BI rather than VI. Furthermore, FcγRI expression on neutrophils in KD increased significantly more than in any other group, including BI. Takata et al. reported serum IFN-γ levels in patients with KD to be significantly higher than in those with BI [13]. Since IFN-γ is the representative cytokine which strongly induces FcγRI expression on neutrophils [1–4], the marked up-regulation of FcγRI expression in KD may be at least partly explained by the elevated level of IFN-γ in serum. On the other hand, we and others reported that activated neutrophils in the acute phase of KD secreted an excess of enzymes such as proteases compared with sepsis [10] and an increased release of toxic oxygen radicals [11], thus suggesting the presence of neutrophil-mediated endothelial injury. Therefore, FcγRI may possibly be a key receptor which induces biological responses in neutrophils during the acute phase of KD.
Although FcγRII expression on neutrophils showed a significant increase in KD, BI and VI compared with HC, no significant difference was observed in FcγRII expression on both neutrophils and monocytes among these three groups in the present study. Leino et al. reported that BI was characterized by increased levels of both FcγRI and FcγRII on neutrophils, while VI was characterized by an increased FcγRI expression with a normal or decreased level of FcγRII in adult febrile patients, thus indicating that FcγRII expression in BI was distinct from that in VI [14]. These discrepancies might be caused by the differences in the age and number of patients enrolled, MoAbs used, and a variety of diseases included in the VI group between our and their studies. Reportedly, the expression of FcγRII is not affected by cytokines [1,2]. However, as previously pointed out by other investigators [1,14], either unknown cytokines or some hormones may also help to enhance FcγRII expression. In any case, since the number of BI and VI groups in the present study was not large and the clinical characterization of each set of patients was not uniform, the comparison of FcγR expression between KD and other infectious diseases in children should be further investigated.
The present kinetics of FcγRIII expression on neutrophils contrasted with that on monocytes in KD and BI. Furthermore, FcγRIII expression on neutrophils appeared to be slightly lower in KD than in BI in the acute phase. The decreased expression of FcγRIII on neutrophils may indicate the shedding of these molecules as a result of their activation [5,6]. The increased expression of FcγRIII on monocytes may indicate their maturation, because the circulating CD16 (FcγRIII)+ monocytes seen in sepsis are reported to be considered as circulating macrophages [15,16]. Therefore, either neutrophils with a decreased expression of FcγRIII or monocytes with an increased expression of FcγRIII seen in the present study may reflect their functional activation. A reduced expression of FcγRIII on neutrophils was also found in advanced AIDS with a bacterial infection [17] and severe trauma [18]. There have so far been no reports in which FcγRIII expression on both neutrophils and monocytes was simultaneously evaluated. In the present study, FcγRIII expression showed the reciprocal kinetics between neutrophils and monocytes during the clinical course of KD, thus reflecting the inflammatory process in the disease with an activated immune system.
Although therapy with IVIG has a marked effect on KD patients [19,20], its mechanism of action remains obscure. As one possible mechanism, infused IVIG is thought to bind to FcγR on phagocytes, thus resulting in the down-regulation of the immune system, including the secretion of cytokines [20], and thereby causing an alteration in FcγR-mediated phagocytosis [21]. Since the present kinetic analysis of FcγR in KD demonstrated that the expression of FcγRI and II on neutrophils and FcγRIII on monocytes decreased from the pre-IVIG through the post-IVIG, the infused IVIG might therefore partially block these FcγRs. However, it is difficult to prove that the infused IVIG blocks FcγR on circulating neutrophils and monocytes in vivo. IVIG also rapidly suppresses marked immune activation in KD, probably due to the neutralization by immunoglobulin of a causative microbial toxin or due to a significant increase in the number of suppressor T cells and a decrease in helper T cells [20,22,23]. It is therefore likely that IVIG down-regulates FcγR expression via an indirect mechanism of immune suppression.
In conclusion, FcγRI expression on neutrophils was markedly up-regulated in the acute phase of KD, and thereafter decreased from the subacute through the convalescent phase. FcγRIII expression on neutrophils was down-regulated, while that on monocytes was up-regulated in the acute phase of KD, followed by a reversal in the kinetics during the time course of KD. These findings indicate that the expression of FcγR on circulating phagocytes may thus be an inflammatory marker of KD, a systemic disease which activates the immune system.
REFERENCES
- 1.van de Winkel JGJ, Anderson CL. Biology of human immunoglobulin G Fc receptors. J Leuk Biol. 1991;49:511–24. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- 2.van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implication. Immunol Today. 1993;14:215–21. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 3.Guyre PM, Campbell S, Kniffin WD, Fanger MW. Monocytes and polymorphonuclear neutrophils of patients with streptococcal pharyngitis express increased numbers of type I IgG Fc receptors. J Clin Invest. 1990;86:1892–6. doi: 10.1172/JCI114921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan L, Mendel DB, Zurlo J, Guyre PM. Regulation of the steady state level of FcγRI mRNA by IFN-γ and dexamethasone in human monocytes, neutrophils, and U-937 cells. J Immunol. 1990;145:267–75. [PubMed] [Google Scholar]
- 5.Buckle A-M, Hogg N. The effect of IFN-γ and colony-stimulating factors on the expression of neutrophil cells membrane receptors. J Immunol. 1989;143:2295–301. [PubMed] [Google Scholar]
- 6.Leino L, Lilius E-M. The up- and down-regulation of immunoglobulin G Fc receptors and complement receptors on activated human neutrophils depends on the nature of activator. J Leukoc Biol. 1992;51:157–63. doi: 10.1002/jlb.51.2.157. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Clinical observation of 50 cases. Jpn J Allergy. 1967;16:178–222. [PubMed] [Google Scholar]
- 8.Leung DYM, Cotran RS, Kurt-Jone E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;2:1298–302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-γ in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 10.Takeshita S, Nakatani K, Kawase H, et al. The role of bacterial lipopolysaccharide-bound neutrophils in the pathogenesis of Kawasaki disease. J Infect Dis. 1999;179:508–12. doi: 10.1086/314600. [DOI] [PubMed] [Google Scholar]
- 11.Niwa Y, Sohmiya K. Enhanced neutrophilic function in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984;104:56–60. doi: 10.1016/s0022-3476(84)80589-2. [DOI] [PubMed] [Google Scholar]
- 12.Diagnostic guidelines of Kawasaki disease. 4. Tokyo: Japan Kawasaki Disease Research Committee; 1984. Japan Kawasaki Disease Research Committee. [Google Scholar]
- 13.Takata Y, Seki S, Dobashi H, et al. Inhibition of IL-12 synthesis of peripheral blood mononuclear cells (PBMC) stimulated with a bacterial superantigen by pooled human immunoglobulin: implication of its effect on Kawasaki disease (KD) Clin Exp Immunol. 1998;114:311–9. doi: 10.1046/j.1365-2249.1998.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leino L, Sorvajärvi K, Katajisto J, et al. Febrile infection changes the expression of IgG Fc receptors and complement receptors in human neutrophils in vivo. Clin Exp Immunol. 1997;107:37–43. doi: 10.1046/j.1365-2249.1997.d01-899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HWL. The novel subset of CD14+/CD16+ blood monocytes is expanded in septic patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 16.Ziegler-Heitbrock HWL, Fingerle G, Ströbel M, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 17.Boros P, Gardos E, Bekesi G, Unkeless JC. Change in expression of FcγR (CD64) on neutrophils from human deficiency virus-infected individuals. Clin Immunol Immunopathol. 1990;54:281–9. doi: 10.1016/0090-1229(90)90089-9. [DOI] [PubMed] [Google Scholar]
- 18.White-Owen C, Alexander JW, Babcock GF. Reduced expression of neutrophil CD11b and CD16 after severe traumatic injury. J Surg Res. 1992;52:22–26. doi: 10.1016/0022-4804(92)90273-3. [DOI] [PubMed] [Google Scholar]
- 19.Furusho K, Kamiya T, Nakano H, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–7. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 20.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. New Engl J Med. 1986;315:341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 21.Newland AC, Macey MG. Immune thrombocytopenia and Fc receptor-mediated phagocyte function. Ann Hematol. 1994;69:61–67. doi: 10.1007/BF01698483. [DOI] [PubMed] [Google Scholar]
- 22.Leung DYM, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;2:1298–302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 23.Leung DYM. Immunomodulation by intravenous immune globulin in Kawasaki disease. J Allergy Clin Immunol. 1989;84:588–92. doi: 10.1016/0091-6749(89)90195-4. [DOI] [PubMed] [Google Scholar]